Abstract
Excessive sugar consumption is a risk factor for becoming overweight. Due to the increase in consumer nutritional awareness, functional beverages with reduced caloric content have attracted great interest in recent years. The current state of knowledge on the feasibility of using low- and no-calorie sweeteners (LNCS) as substitutes for traditional sugar in the production of functional beverages while maintaining their osmolality properties is limited. Selected sweeteners were examined for the presence of five selected macronutrients (calcium, potassium, magnesium, sodium, and sulfur) and iron by ICP-OES, osmolality, total dissolved solids (TDS), and electrical conductivity (EC) in their solutions. The obtained results formed the basis for evaluating the applicability of the studied sweeteners in the production of functional beverages.
1. Introduction
Osmolality is the phenomenon of the flow of a solvent across a semi-permeable membrane from an area of lower concentration to an area of higher concentration. This leads to an equalisation of the concentrations in the two solutions. This phenomenon describes the total concentration of solutes in a solution. A solution of low osmolality has fewer molecules of solute per litre of solution, while a solution of high osmolality has more molecules of solute per litre of solution. When solutions of different osmolality are separated by a membrane that is permeable to water but not to other solutes, water will move from the lower osmolality side to the higher osmolality side [1,2]. It is expressed as the number of moles of osmotically active substances in 1 kg of solvent (mOsm/kg H2O). It is widely used in industry for, among other things, the production and design of tonic drinks, the purpose of which, depending on their intended use, may be to quickly or optimally hydrate the body, replenish electrolytes lost with sweat during exercise, and provide rapid energy [3]. Tonicity is the ability of the extracellular solution to move water into or out of the cell by osmosis. Tonicity differs from osmolality in that it takes into account both the relative concentrations of solutes and the permeability of the cell membrane to these solutes [4].
A special place in the food segment is given to foods designed for athletes, including all kinds of functional beverages. The functional beverage division provides ample opportunity to create new products dedicated to virtually any audience. The most well-known group of functional beverages are tonic drinks, including energy drinks and isotonic drinks. Vitamin drinks, shots, functional waters, probiotic milk drinks, and protein drinks are also popular [5].
Tonic drinks can be classified as hypotonic, isotonic, and hypertonic [6]. These three terms are used to describe whether the solution causes water to move into or out of the cell: If the cell is placed in a hypertonic solution, water will leave the cell to equalise the relative concentrations of solutes on either side of the cell membrane. The water, acting as a solvent, will move from the lower concentration region to the higher concentration region, causing the cell to shrink. In an isotonic environment, the relative concentrations of solute and water are the same on both sides of the membrane, so there is no net movement of water and no change in cell size. When a cell is placed in a hypotonic environment, water enters the cell, and the cell swells. Hypotonic drinks are drinks that contain concentrations of electrolytes and sugars that are lower than the concentration of fluids in the body. As a result, they are very easily absorbed by the body and quench thirst quickly. Hypertonic drinks are beverages that contain a concentration of electrolytes and sugars greater than the concentration of fluids in the body. They are used to replenish energy stores in a short period of time [7,8].
Isotonic drinks have an osmolality similar to that of human body fluids, in the range of 270 to 330 mOsm/kg. As a result, they enable optimal hydration of the body [9,10,11]. According to the literature, the content of simple carbohydrates in isotonic drinks is 4–8 g/100 mL [10]. Their consumption is recommended to replenish fluids and electrolytes lost through sweating during increased physical activity [11]. According to the common division of sweeteners, a distinction is made between natural sweeteners, semi-synthetic sweeteners, and synthetic sweeteners [12,13,14].
White sugar extracted from sugar beets is almost pure sucrose. Its energy value is 4 kcal in 1 g. Cane sugar is made from sugar cane. Its energy value and sweetness are equivalent to traditional sugar. Coconut sugar is also available on the market and is obtained from the sap of the flower buds of the coconut palm. Its energy value and sweetness are similar to white sugar. It has a characteristic caramel aftertaste. Coconut sugar consists mainly of sucrose but also glucose and fructose [15]. Natural sweeteners also include fructose, known as fruit sugar. Fructose is a simple candy found naturally in fruits, showing 40% more sweetening power than sucrose, so even small amounts of fructose give a sweet taste sensation. The energy value of fructose is 4 kcal in 1 g [16]. Sugar is a substance that stands out for its high caloric content, which can contribute to the development of lifestyle diseases, weight gain, and obesity.
Polyols are sugar substitutes of semi-synthetic origin. They are aliphatic polyols that contain at least two hydroxyl groups in their molecule. They are in demand because of their numerous health benefits. Unlike sucrose, they are tooth-friendly, less caloric and low-glycemic [17]. Well-known and commercially available polyols include xylitol, isomalt, and erythrol, among others.
Erythritol is a four-carbon sugar alcohol used as a sugar substitute (C4H10O4) with a molecular weight of 122.12 g/mol and a glycemic index of 0. Its advantage is that it has no effect on blood glucose levels, so it is considered a good alternative for diabetics. Erythritol is produced by biotechnological methods.
Erythritol does not provide us with calories because the body is unable to digest it, which means it does not undergo internal metabolic processes and is excreted with urine from our body. Significant consumption of erythritol can cause side effects such as abdominal pain and diarrhoea. In addition, erythrol shows health-promoting effects on the body through its antioxidant properties and reducing the risk of developing dental caries, thanks to stopping the fermentation of sugars by the bacteria responsible for the formation of dental cavities [18].
Another sweetener classified as a polyol is xylitol (E967), also known as birch sugar. It provides about 2.4 kcal in 1 g. Its sweetness corresponds to about 90% of that of sucrose. It is known for its positive effects on teeth and anti-corrosive effects [19].
Isomalt (E953) is a synthetically derived, low-calorie sweetener that has a sweetening power of about 50% that of sucrose. Isomalt does not occur naturally in nature; it is obtained from sucrose through two main processes. In the first stage, sucrose is converted to isomaltulose through the action of appropriate enzymes. In the second stage, the previously obtained isomaltulose undergoes hydrogenation attachment of hydrogen atoms.
Artificial sweeteners are also distinguished. The use of sugar substitutes makes it possible to obtain products with lower calories. Artificial sweeteners include aspartame and cyclamate [20,21] and other substitutes, including stevia, inulin, and tagatose. Studies show that a major source of dietary calories is the consumption of sweetened beverages. Excessive amounts of energy consumed regularly in this way contribute to being overweight and obesity, which are considered risk factors for cardiovascular disease or type 2 diabetes. Epidemiological and experimental studies suggest there is an association between the consumption of sweetened beverages and weight gain and the occurrence of the above-mentioned diseases [22,23,24,25].
Given the continued high consumption of isotonic and energy drinks and the huge growth potential of the energy-reduced beverage sector, a review of selected sweeteners used as an osmolated base for functional beverages was prepared. The aim of this manuscript was to analyse the available sweeteners that could be used in the production of functional beverages in terms of their ionic composition and osmolality. The authors aimed to analyse the ionic richness of 13 sweeteners and their potential impact on the tonic properties of future beverages. They also considered literature data relating to the sweetness levels of the analysed sweeteners.
2. Materials and Methods
Widely available sweeteners of commercial origin were selected for the study. In order to accurately compare the osmolality of the tested sweeteners, all dilutions were tested at a concentration of 1 g/100 mL. The samples were analysed in five replicates.
2.1. Determination of Mineral Content by ICP-OES Analysis
The composition of micro and macro elements with toxic elements as well in the macerate was determined using ICP-OES apparatus (Schaumburg, IL, USA), Thermo iCAP Dual 6500 with horizontal plasma, and capacity for detection along and across the plasma flame (radial and axial). Before measuring each batch of samples, calibration was performed using certified (Merck) models, with concentrations of 10,000 ppm for Ca, Mg, K, and P and 1000 ppm for Na, S, and Fe.
In each case, a three-point calibration curve was used for each element, with optical adjustment applying the method of internal models in the form of yttrium and ytterbium ions at concentrations of 2 mg/L and 5 mg/L, respectively. The analytical methods were validated with two independent tests. Certified Reference Material (NIST CRM—Tea Leaves) was used, and the recovery obtained for specific elements is shown in Table 1. In order to identify the relevant measurement lines and avoid possible interferences, the method of adding a model with known concentration was applied (Environmental analysis, Method 200.7, US EPA, Drinking water).

Table 1.
Three-point calibration curve.
2.2. EC TDS Determination Procedure
The samples were assessed using a TDS&EC meter. It is applied to measure such factors as Total Dissolved Solids (TDS) and electrical conductivity (EC). The value of TDS makes it possible to determine the degree of overall water mineralisation and is expressed in ppm. TDS < 50 corresponds to water with a very low level of mineralisation, TDS 50–500 to water with a low level of mineralisation, TDS 500–1500 to water with a moderate level of mineralisation, and TDS > 1500 to water with a high level of mineralisation. Electrical conductivity (EC) is a numerical value reflecting the ability of an aqueous solution to carry an electrical current. By measuring EC, it is possible to assess the degree of water mineralisation or salinity of water. The EC value depends on the mobility, valence and concentration of the specific ions present in a given solution. Clean water is a very poor electrical conductor, and its electrical conductivity increases proportionally to the quantity of ions dissolved in it. The electrical conductivity of water is most commonly expressed in microsiemens per centimetre (µS/cm).
2.3. Osmometer Determination Procedure
Osmolality was measured in an automated digital osmometer 800 CLG (TRIDENT MED., Warsaw, Poland), which allows for its determination by freezing temperature measurement with single-point user calibration. The test sample was pipetted into the measuring device, which was then inserted into the measuring head and cooling chamber. The measurement was performed automatically as soon as the head was gripped by the electromagnet. Each determination was performed in duplicate. The osmolality of the drinks was read directly on the display in units of mOsm/kg H2O.
3. Results
A total of 13 sweeteners were analyzed for osmolality, as well as 5 selected macronutrients (calcium, potassium, magnesium, sodium, sulfur) and iron by ICP-OES, total dissolved solids (TDS) and electrical conductivity (EC) in their solutions. Figure 1 shows the osmolality of selected sweeteners in 5 g/100 mL solutions expressed in mOsm/kg H2O. The lowest osmolality among the tested sweeteners was found in aspartame (61 mOsm/kg H2O) and the highest in sodium cyclamate (521 mOsm/kg H2O). Erythritol (447 mOsm/kg H2O), stevia (439 mOsm/kg H2O), and xylitol (mOsm/kg H2O mOsm/kg H2O) also showed high osmolality. The magnitude of the tested parameter for sucrose was 157 mOsm/kg H2O. Similar values were found for inulin (154 mOsm/kg H2O) and isomalt (160 mOsm/kg H2O). Carbohydrates in isotonic beverages come mainly from sucrose, glucose, and fructose. They are present in amounts ranging from 4–8 g/100 mL of beverages [26]. The content of carbohydrates is one of the main parameters that shape the osmolality of isotonic drinks. Knowing the osmolality of sweeteners with an energy value lower than traditional sugar or richer in ions makes it possible to create an isotonic beverage with reduced caloric content or, in a healthier version, richer in ions of natural origin.
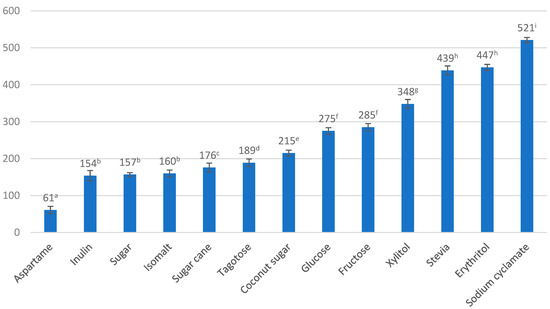
Figure 1.
Osmolality results of selected sweeteners for a weight of 5 g sweetener/100 mL demineralised water. a, b—averages denoted by different letters in the row are significantly different at α = 0.05 (Tukey’s test), n = 3.
Figure 1 shows the osmolality results of selected sweeteners for a weight of 5 g sweetener/100 mL demineralised water.
Taking into account the results of osmolality, each time the formulation of the beverage is analysed, nominal amounts of sweetener should be used. Taking into account the above in the Table 2. The sweetness of the selected sweeteners in relation to sugar and the osmolality of solutions after dissolving 1 g in 100 mL of distilled water are presented. Based on the data presented, it is possible to calculate the potential amount of sweetener in grams needed to achieve an osmolality of 250 mOsm/kg H2O * and the sweetness of the resulting solutions. The data show that it is not possible to produce an isotonic beverage sweetened only with aspartame, stevia, or sodium cyclamate because using these sweeteners in the amount needed to achieve the osmolality characteristic of isotonic beverages would produce drinks that are too sweet. The data presented in the table indicate that inulin is also not a good sweetener for isotonic beverages, as it gives too low of a sweetness sensation. The polyols studied, i.e., xylitol, erythrol, and isomalt, are characterised by both lower calories and sweetness. Their use in the production of isotonic beverages in the amount needed to achieve adequate osmolality would give the possibility, as the literature indicates, of obtaining a product that is healthier for teeth, among other things, but less sweet. Based on the results obtained, it can be concluded that the greatest potential in the production of low-calorie isotonic drinks is shown by isomalt. It is a virtually calorie-free sugar substitute (0.2 kcal/g). The potential sweetness obtained, expressed in g sugar/100 mL, is closest to traditional isotonic drinks containing sucrose in the formulation. Another proposed solution could also be to combine the selected polyol with a calorie-free sweetener, which would result in a product with reduced caloric content, desired sweetness, and adequate tonicity.

Table 2.
Analysis of sweetness and osmolality of the tested sweeteners, expressed in terms of potential sweetness obtained with osmolality at a level representing the base for an isotonic drink.
Table 3 shows the results of the analysis of the chemical element content of the tested sweeteners.

Table 3.
Results of analysis of the content of selected chemical elements of the tested sweeteners.
The total dissolved substances (TDS) measurement is an indicator that tells, among other things, the compactness of minerals, salts, and metals dissolved in a given volume of water. TDS refers only to the components present in water that conduct electricity. Table 3 shows the TDS results expressed in ppm. The highest TDS values were found for cane sugar (101–105 ppm), aspartame (835–846 ppm) and sodium cyclamate (851–860 ppm). Often, a high TDS is associated with the presence of large amounts of chloride, sodium and potassium in the water, which is confirmed by the results of the analysis of the content of selected chemical elements of the tested sweeteners (Table 4), where both sodium cyclamate, aspartame and coconut sugar scored high compared to the other tested sweeteners. The high TDS may also have been influenced by the content of other elements.

Table 4.
Total dissolved solids (TDS) and electrical conductivity (EC) in 1 g/100 mL solutions.
4. Discussion
The functional beverage market has seen a steady increase in interest in recent years, linked to the emergence of increased physical activity and improved nutritional awareness among consumers. This trend is largely a result of the new lifestyle that is being promoted, which is based on taking care of one’s health, a healthy appearance, and physical fitness to ensure longer life in good mental and physical condition [28,29]. Today’s consumer expects food producers to provide high-quality products with high sensory qualities that are safe, minimally processed, and convenient to use, and also have health-promoting properties [30,31]. In the current model of the free market economy, the basic criterion determining the quality of the available food products is undoubtedly a requirement of the consumer. Foods that do not meet consumer expectations cannot function in the market. The market for functional foods, including functional beverages, has a huge potential for development, which is reflected in the fact that the range of such products is increasing year by year. [32,33,34,35,36].
Consumers are giving up sugar en masse. They are increasingly choosing low-calorie and healthier substitutes. When looking at this trend, it is worth noting that it is not the sugar itself that is the problem, but an excess of it in the diet. According to the World Health Organization (WHO), the energy provided by sugar should not exceed 10% of the total energy provided. With an average adult calorie requirement of 2000 kcal, this would be about 200 kcal, or 10–12 teaspoons of white sugar, as one teaspoon is about 20 kcal (5 g) [37,38]. Eating large amounts of simple sugars can even cause fluctuations in blood glucose levels involving rapid rises due to large amounts of insulin secretion, followed by rapid falls. When blood glucose levels rise, one feels a surge of energy, while a sudden drop can make one feel lethargic, unable to concentrate, and hungry. Excess glucose that the body does not use is converted into energy stores, including glycogen in the liver, but also in fat tissue stored under the skin and around internal organs, which can become one of the causes of overweight and obesity [39,40].
In their study, other authors [41] investigated the effect of switching from sugar-sweetened to artificially sweetened beverages for 12 weeks. They instructed participants to replace their consumption of sucrose-sweetened beverages with artificially sweetened beverages or water or to continue consuming them. At 12 weeks, there were no significant differences between groups in short-term verbal memory in the logical memory test, waist circumference in relation to height, body mass index or glucose tolerance. In participants who switched to water, a significant reduction in preference for high-sugar sucrose solutions was observed. The authors suggest that the duration of the study may have been too short to observe changes. High intake of added sugars is associated with excessive energy intake and poorer diet quality. Awareness of trends in added-sugar foods is useful in developing effective strategies to reduce the intake of added sugars in the population [42]. Wierzejska [43] analysed the composition of beverages available on the market before and after the introduction of the tax on sweetened beverages in Poland between 2020 and 2021. In the first year after the introduction of the tax on sweetened beverages in Poland, beneficial changes in the composition of 62% of the analysed beverages were found. After the introduction of the tax, the average sugar content in carbonated beverages decreased from 8.6 g to 6.9 g/100 mL and in non-carbonated beverages from 5.5 g to 4.8 g/100 mL. Across the beverage group as a whole, the percentage of beverages containing >5 g sugar/100 mL fell significantly from 70.2% in 2020 to 4.4% in 2021. As a result, manufacturers are looking for ways to reduce the tax rate and improve the nutritional value of their drinks, which would have likely not occurred to the same extent had the tax not been introduced. Reducing the sugar content of beverages can help to reduce dietary sugar intake, while the mere introduction of the tax and its media coverage can further raise awareness of the importance of good nutrition. The general trend in global food production is to reduce the calorie content of food products. In addition, the formulation of functional foods should demonstrate the potential to create products for those who wish to avoid consuming white sugar for reasons of conviction or health. The most significant changes in water osmolality are caused by carbohydrates, but knowledge in this area is still limited. A gap in the literature is the effect of mineral additive doses on the formation of the molecular index parameter in water.
5. Conclusions
The results of the above manuscript suggest that sugar substitutes, including both artificial and natural sweeteners, are a good source for optimising the osmolality of functional beverages if the aim is to reduce the calorie content of the finished beverage. This is due to the nominally lower number of grams of sweetener that can be achieved when formulating the finished functional beverage. This is beneficial in terms of reducing the overall calorific value of the beverage, although not without an impact on the ionic (including electrolyte) value of the tonic beverage. Therefore, when deciding on the use of a sugar substitute, it is important to consider both the sweetness of the sugar substitute used, its osmolality and its ionic potential.
Author Contributions
Conceptualisation, K.M. and G.Z.; methodology, G.Z.; software, B.S.; validation, A.S., B.S. and M.B.; formal analysis, K.M.; investigation, K.M.; resources, K.M.; data curation, K.M.; writing—original draft preparation, K.M.; writing—review and editing, G.Z.; visualisation, K.M.; supervision, C.P.; project administration, C.P.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
The project is financed by the program of the Minister of Education and Science named “Regional Initiative of Excellence” in the years 2019–2023, project number 026/RID/2018/19, the amount of financing PLN 9 542 500.00.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kramer, E.M.; Myers, D.R. Five Popular Misconceptions about Osmosis. Am. J. Phys. 2012, 80, 694–699. [Google Scholar] [CrossRef]
- Stasiuk, E.; Przybyłowski, P. Osmolality of Isotonic Drinks in the Aspect of Their Autenticity. Pol. J. Nat. Sci. 2017, 32, 161–168. [Google Scholar]
- Joachimiak, I.; Szoltysek, K. Stan Wiedzy Oraz Częstotliwość Spożycia Napojów Energetyzujących i Izoto-Nicznych Przez Osoby Młode, Czynne i Uprawiające Sport. Nauk. Inż. Technol. 2013, 1, 26–38. [Google Scholar]
- Kramer, E.M.; Myers, D.R.; Stasiuk, E.; Przybyłowski, P.; Bechke, E.E.; Zaplatosch, M.E.; Choi, J.-Y.; Adams, W.M. Utility of an Isotonic Beverage on Hydration Status and Cardiovascular Alterations. Nutrients 2022, 14, 1286. [Google Scholar]
- Sugajski, M.; Buszewska-Forajta, M.; Buszewski, B. Functional Beverages in the 21st Century. Beverages 2023, 9, 27. [Google Scholar] [CrossRef]
- Grzebisz, N. Żywność Funkcjonalna w Diecie Sportowców. Kwart. Naukowy Uczel. Vistula 2017, 2, 237–246. [Google Scholar]
- Waszkiewicz-Robak, B.; Chelminski, T. Charakterystyka Cisnienia Osmolalnego Roznych Napojow Rynkowych. Bromatol. Chem. Toksykol. 2005, 3, 307–311. [Google Scholar]
- Kalisz, Z. Składniki Odżywcze i Ich Rola w Diecie Sportowca = Nutrients and Their Role in Athlete’s Diet. J. Educ. Health Sport 2016, 8, 522–538. [Google Scholar]
- Stasiuk, E.; Przybyłowski, P. Elektrochemiczne Wskaźniki Jakości w Ocenie Napojów Izotonicznych. Probl. Hig. Epidemiol. 2015, 96, 827–829. [Google Scholar]
- Tomczyk, M.; Olesiuk, J.; Dżugan, M. Ocena Jakości Napojów Izotonicznych Przygotowantch Samodzielnie Na Bazie Natu-Ralnych Składników. Pol. J. Sports Med. 2019, 35, 169–177. [Google Scholar]
- Bonetti, D.L.; Hopkins, W. Effects of Hypotonic and Isotonic Sports Drinks on Endurance Performance and Physiology. Sportscience 2010, 14, 63–70. [Google Scholar]
- Bogacz, A.; Lewczuk, A. Intensywne Substancje Slodzace-Szansa Dla Polskiego Producenta i Konsumenta. Przem. Ferment. Owocowo-Warzywny 2002, 46, 15–16. [Google Scholar]
- Pietkiewicz, J.J.; Janczar, M. Podział i Ogólna Charakterystyka Substancji Słodzących. Prace Nauk. Akad. Ekon. Wrocławiu Technol. 2004, 1018, 7–35. [Google Scholar]
- Swierczek, U.; Borowiecka, A.; Feder-Kubis, J. Struktura, Właściwości i Przykłady Zastosowań Syntetycznych Substancji Słodzących. Żywn. Nauka Technol. Jakość 2016, 4, 15–25. [Google Scholar]
- Jessa, J.; Hozyasz, K.K. Wartość zdrowotna produktów kokosowych. Pediatr. Pol. 2015, 90, 415–423. [Google Scholar] [CrossRef]
- Ciok, J.; Tacikowski, T.; Wyrobek, I. Fruktoza Jako Czynnik Ryzyka Przewlekłych Chorób Metabolicznych. Żywn. Człowieka Metab. 2004, 31, 88–95. [Google Scholar]
- Livesey, G. Healthpotential of Polyols as Sugar Replacers, with Emphasis on Lowglycaemic Properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef]
- Seshadrinathan, S.; Chakraborty, S. Fermentative Production of Erythritol from Cane Molasses Using Candida Magnoliae: Media Optimization, Purification, and Characterization. Sustainability 2022, 14, 10342. [Google Scholar] [CrossRef]
- Milgrom, P.; Söderling, E.M.; Nelson, S.; Chi, D.L.; Nakai, Y. Clinical Evidence for Polyol Efficacy. Adv. Dent. Res. 2012, 24, 112–116. [Google Scholar] [CrossRef]
- Bortkun, O. Sacharydy i Substancje Słodzące w Produkcji Żywności. Przem. Ferment. Owocowo Warzywny 2004, 3, 7–35. [Google Scholar]
- Świąder, K.; Waszkiewicz-Robak, B.; Świderski, F. Substancje Intensywnie Słodzące-Korzyści i Zagrożenia. Probl. Hig. Epidemiol. 2011, 92, 392–396. [Google Scholar]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of Sugar-Sweetened Beverages and Weight Gain: A Systematic Review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef]
- Kavey, R.-E.W. How Sweet It Is: Sugar-Sweetened Beverage Consumption, Obesity, and Cardiovascular Risk in Childhood. J. Am. Diet. Assoc. 2010, 110, 1456–1460. [Google Scholar] [CrossRef]
- Berkey, C.S.; Rockett, H.R.H.; Field, A.E.; Gillman, M.W.; Colditz, G.A. Sugar-Added Beverages and Adolescent Weight Change. Obes. Res. 2004, 12, 778–788. [Google Scholar] [CrossRef]
- Fisberg, M.; Kovalskys, I.; Gómez, G.; Rigotti, A.; Sanabria, L.Y.C.; García, M.C.Y.; Torres, R.G.P.; Herrera-Cuenca, M.; Zimberg, I.Z.; Koletzko, B.; et al. Total and Added Sugar Intake: Assessment in Eight Latin American Countries. Nutrients 2018, 10, 389. [Google Scholar] [CrossRef]
- Kujałowicz, A. Charakterystyka Wybranych Cukrów i Słodzików. Tutoring Gedanensis 2022, 7, 25–40. [Google Scholar] [CrossRef]
- Kowalowski, P. Naturalne Srodki Slodzace w Swietle Dopuszczalnosci Ich Do Spozycia w Polsce i Krajach Unii Europejskiej. Herba Pol. 2003, 1, 105–114. [Google Scholar]
- Hafner, E.; Hribar, M.; Hristov, H.; Kušar, A.; Žmitek, K.; Roe, M.; Pravst, I. Trends in the Use of Low and No-Calorie Sweeteners in Non-Alcoholic Beverages in Slovenia. Foods 2021, 10, 387. [Google Scholar] [CrossRef]
- Cieślik, E.; Gębusia, A. Żywność Funkcjonalna z Dodatkiem Fruktanów. Żywn. Nauk. Technol. Jakosc 2011, 2, 27–37. [Google Scholar]
- Czajkowska-Mysłek, A. Ocena Zawartości Substancji Intensywnie Słodzących w Słodzikach w Tabletkach. Postępy Nauk. Technol. Przem. Rolno-Spoż. 2016, 1, 52–64. [Google Scholar]
- Piskuła, M.K.; Strączkowski, M.; Żmudzki, J. Charakterystyka Czynników Decydujących o Bezpieczeństwie Konsumentów i Jakości Prozdrowotnej Żywności. Pol. J. Agron. 2011, 7, 82–91. [Google Scholar]
- Saluk-Juszczak, J. Antocyjany Jako Składniki Żywności Funkcjonalnej Stosowanej w Profilaktyce Chorób Układu Krążenia. Postep. Hig. Med. Dosw. 2010, 64, 451–458. [Google Scholar]
- Kozłowska-Strawska, J.; Badora, B.; Chwil, S. Żywność Funkcjonalna i Tradycyjna-Właściwości i Wpływ Na Postawy Konsumentów. Probl. Hig. Epidemiol. 2017, 98, 212–216. [Google Scholar]
- Babicz-Zielińska, E. Postawy Konsumentów Wobec Nowej Żywności. Zesz. Nauk. Akad. Mor. Gdyni 2010, 65, 16–22. [Google Scholar]
- Gertig, H.; Stelmach-Mardas, M. O Bezpieczeństwie Żywności Tradycyjnej. Probl. Hig. Epidemiol. 2011, 92, 387–391. [Google Scholar]
- Lange, E. Produkty Owsiane Jako Żywność Funkcjonalna. Żywn. Nauk. Technol. Jakosc 2010, 17, 7–24. [Google Scholar]
- Szymecka-Wesołowska, A. Regulacja Oświadczeń Żywieniowych i Zdrowotnych w Stanach Zjednoczonych. Regulacja Oświadczeń Żywieniowych i Zdrowotnych w Stanach Zjednoczonych. Prz. Prawa Rol. 2011, 2, 199–223. [Google Scholar]
- Żakowska-Biemans, S. Bezpieczeństwo Żywności Jako Czynnik Determinujący Zachowania Konsumentów Na Rynku Żywności. Probl. Hig. Epidemiol. 2011, 92, 621–624. [Google Scholar]
- Joint WHO/FAO Expert Consultation. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Popkin, B.; Hawkes, C. Słodzenie Globalnej Diety, w Szczególności Napojów: Wzorce, Trendy i Reakcje Polityczne. Lancet Cukrzyca Endokrynol. 2016, 4, 174–186. [Google Scholar]
- Kendig, L.M.; Chow, J.Y.L.; Martire, S.I.; Rooney, K.B.; Boakes, R.A. Przejście z napojów zawierających cukier na napoje sztucznie słodzone: 12-tygodniowa próba. Składniki Odżywcze 2023, 15, 2191. [Google Scholar] [CrossRef]
- Bailey, K.P.; Fulgoni, V.L., III; Cowan, A.E.; Gaine, P.C. Źródła dodanych cukrów u małych dzieci, młodzieży i dorosłych z niskim i wysokim spożyciem dodanych cukrów. Składniki Odżywcze 2018, 10, 102. [Google Scholar]
- Wierzejska, R.E. Wpływ podatku od napojów słodzonych na ich reformę w Polsce–analiza składu napojów dostępnych w handlu przed i po wprowadzeniu podatku (2020 vs. 2021). Wewn. J. Śr. Rozdzielczość Zdr. Publiczne 2022, 19, 14464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).