Introducing a Simple Method to Investigate Relative Volatilities of Flavour Compounds in Fruit Brandies
Abstract
1. Introduction
1.1. Thermodynamic Fundamentals
1.2. Previous Studies on VLEs
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Distillation
2.3. Gas Chromatography Headspace Analysis
2.4. Statistical Analysis
3. Results and Discussion
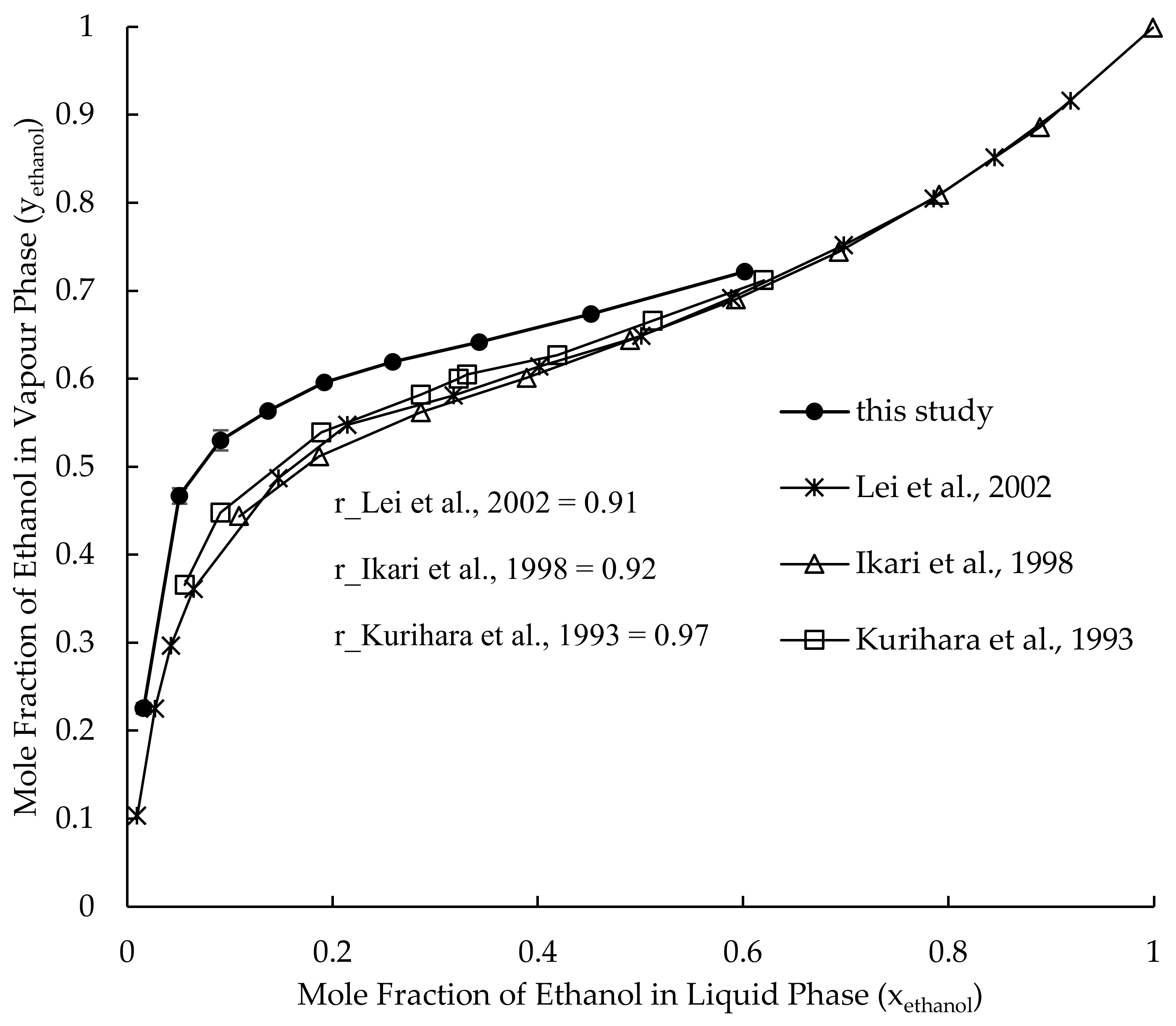
3.1. Vapour–Liquid Equilibrium of Ethanol–Water System
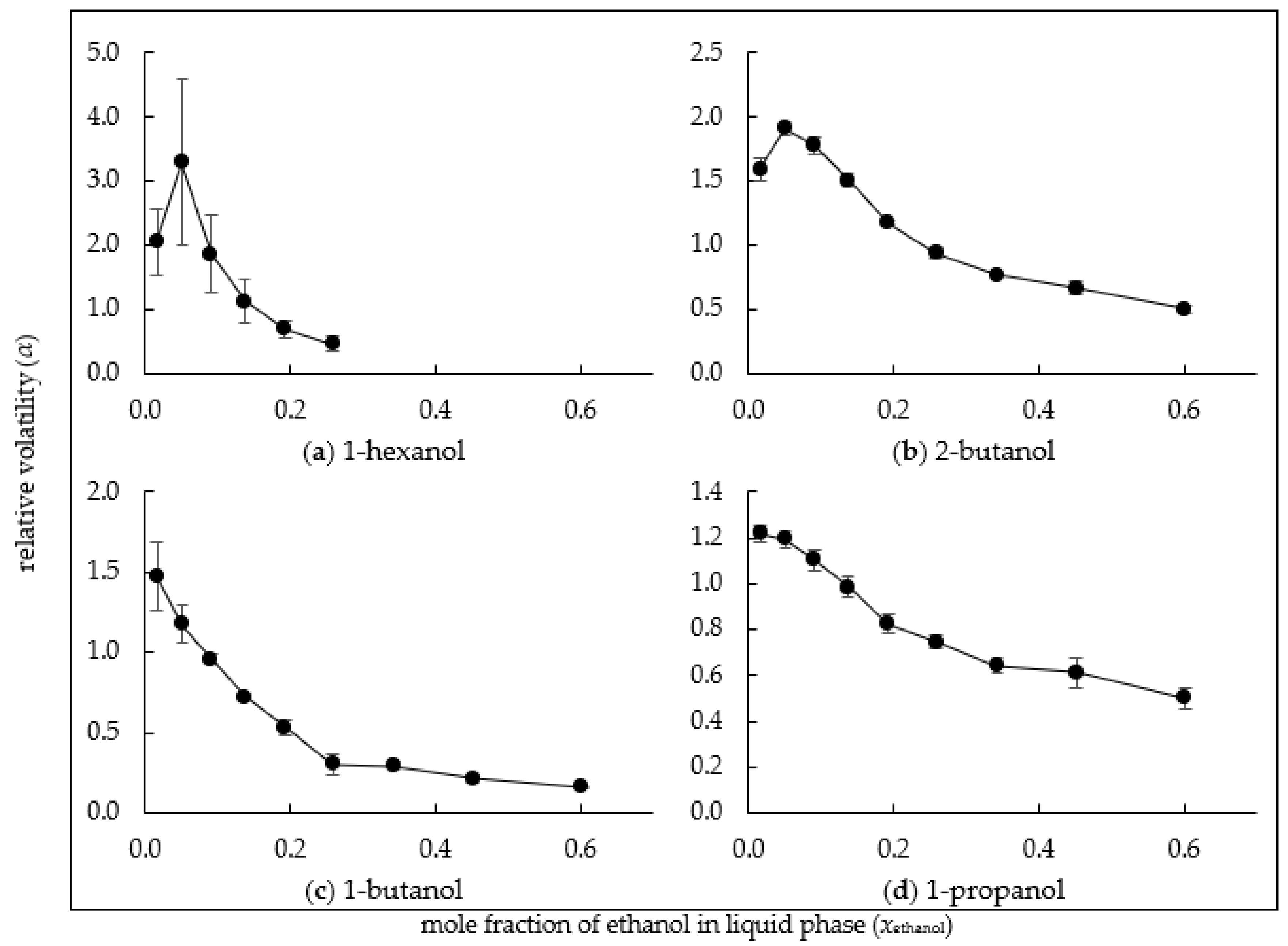
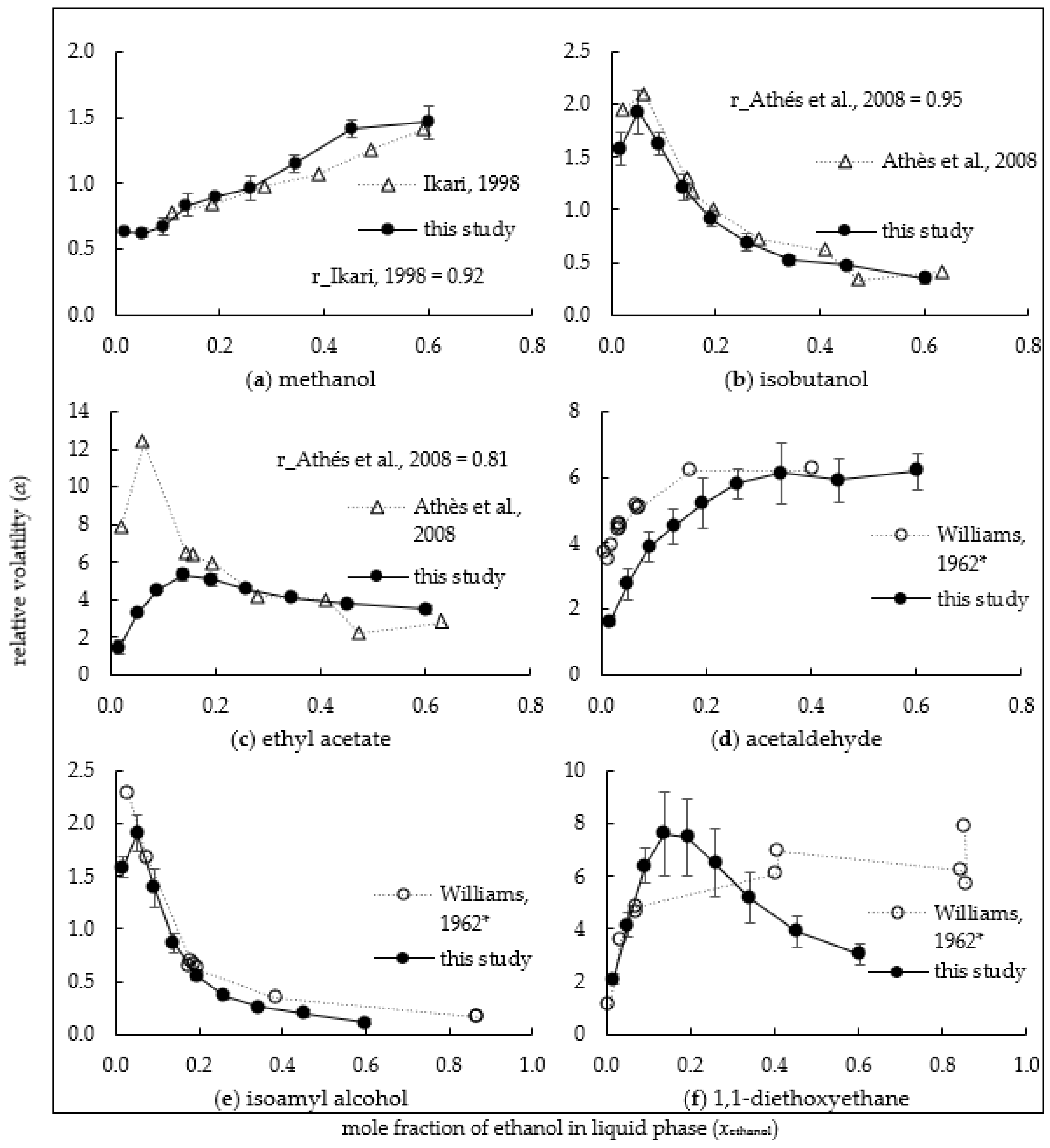
3.2. Relative Volatility of Alcohols
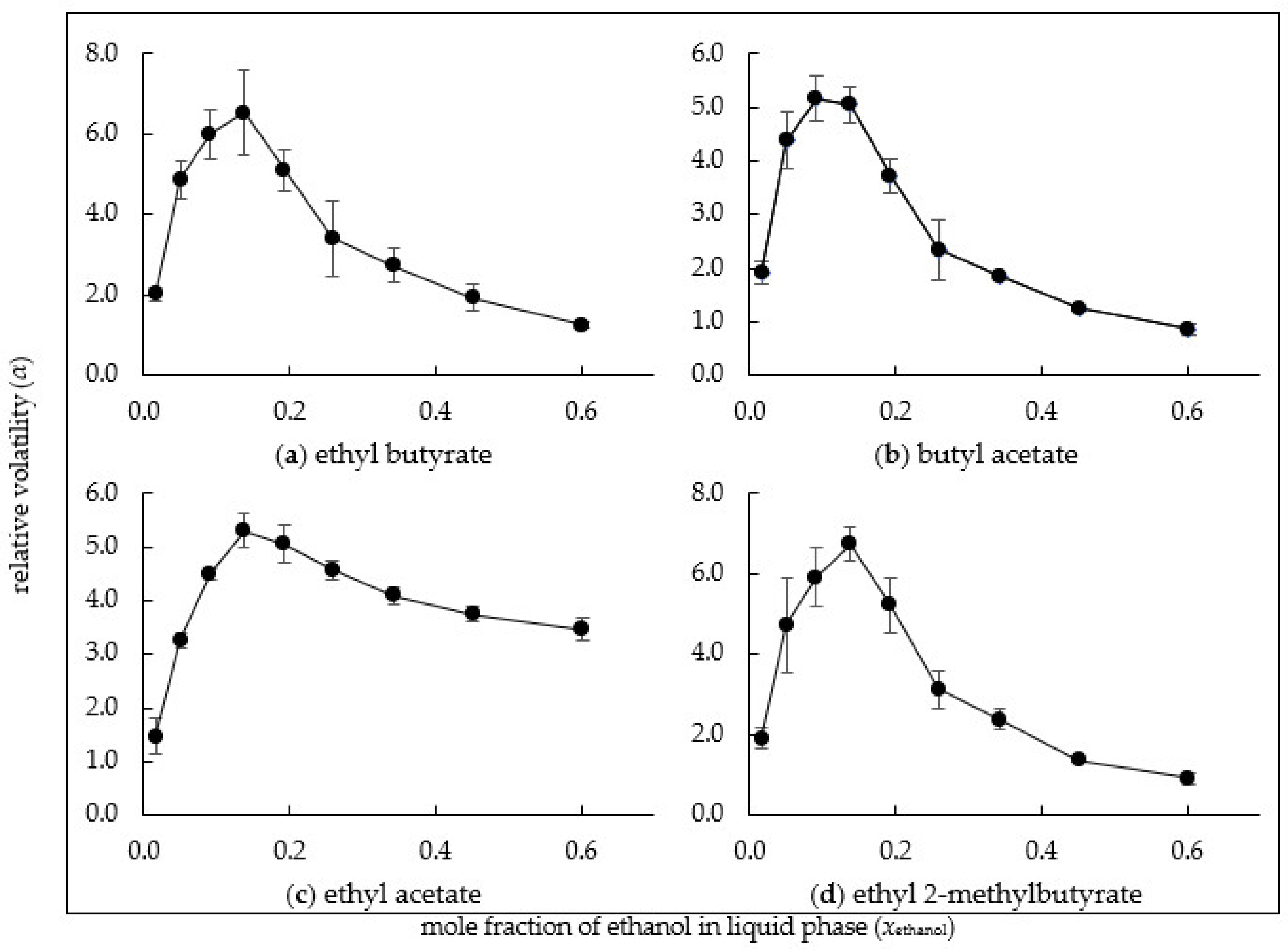
3.3. Relative Volatility of Esters
3.4. Relative Volatility of Aldehydes
3.5. Relative Volatility of β-Damascenone and 1,1-Diethoxyethane
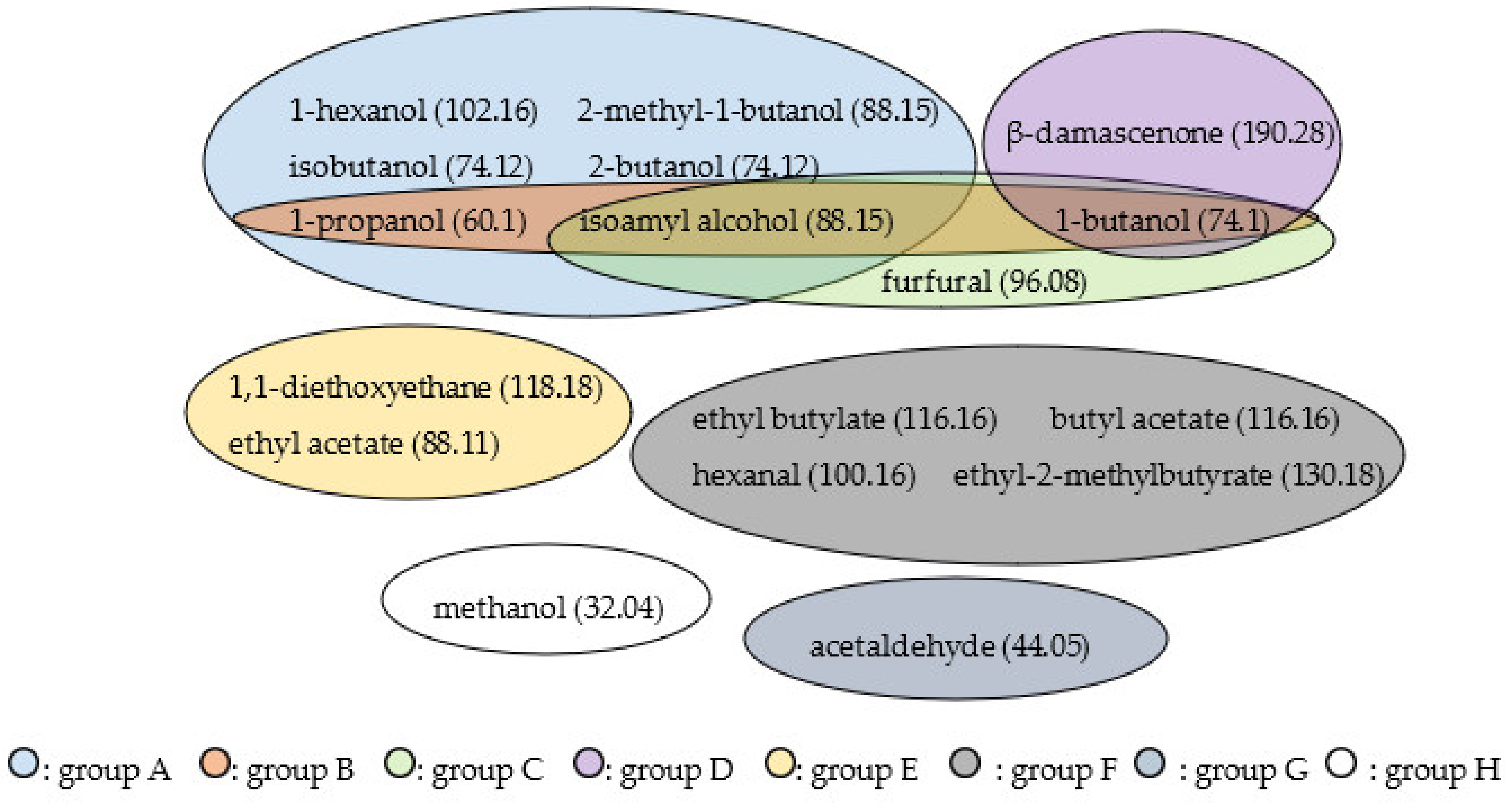
3.6. Results of Statistical Analysis
3.7. Validation of Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 1-Hexanol | 1,1-Diethoxyethane | 2-Methyl-1-Butanol | Acetaldehyde | Isobutanol | 2-Butanol | 1-Propanol | Isoamyl Alcohol | |
|---|---|---|---|---|---|---|---|---|
| 1,1-diethoxyethane | −0.382 | |||||||
| 2-methyl-1-butanol | 0.964 *** | −0.096 | ||||||
| acetaldehyde | −0.767 *** | 0.171 | −0.883 *** | |||||
| isobutanol | 0.927 *** | 0.029 | 0.985 *** | −0.864 *** | ||||
| 2-butanol | 0.835 *** | 0.161 | 0.936 *** | −0.821 *** | 0.976 *** | |||
| 1-propanol | 0.842 *** | 0.013 | 0.939 *** | −0.918 *** | 0.962 *** | 0.959 *** | ||
| isoamyl alcohol | 0.861 *** | −0.119 | 0.970 *** | −0.905 *** | 0.963 *** | 0.946 *** | 0.950 *** | |
| furfural | 0.633 ** | −0.384 * | 0.778 *** | −0.904 *** | 0.726 *** | 0.682 *** | 0.789 *** | 0.840 *** |
| methanol | −0.686 ** | −0.205 | −0.824 *** | 0.796 *** | −0.878 *** | −0.925 *** | −0.927 *** | −0.865 *** |
| ethyl acetate | −0.424 | 0.874 *** | −0.347 | 0.535 ** | −0.234 | −0.106 | −0.289 | −0.393 * |
| 1-butanol | 0.592 ** | −0.248 | 0.797 *** | −0.830 *** | 0.798 *** | 0.758 *** | 0.831 *** | 0.840 *** |
| ethyl butylate | 0.156 | 0.763 *** | 0.423 * | −0.260 | 0.522 ** | 0.636 ** | 0.478 * | 0.409 * |
| hexanal | 0.372 | 0.571 ** | 0.594 ** | −0.374 | 0.687 *** | 0.763 *** | 0.624 ** | 0.567 ** |
| butyl acetate | 0.282 | 0.522 ** | 0.541** | −0.299 | 0.644 ** | 0.732 *** | 0.589 ** | 0.530 ** |
| ethyl-2-methylbutyrate | 0.164 | 0.669 ** | 0.426 * | −0.184 | 0.541 ** | 0.623 ** | 0.483 * | 0.367 |
| β-damascenone | 0.130 | −0.750 ** | 0.224 | −0.548 * | 0.161 | −0.010 | 0.326 | 0.327 |
| Furfural | Methanol | Ethyl Acetate | 1-Butanol | Ethyl Butylate | Hexanal | Butyl Acetate | Ethyl-2-Methylbutyrate | |
|---|---|---|---|---|---|---|---|---|
| 1,1-diethoxyethane | ||||||||
| 2-methyl-1-butanol | ||||||||
| acetaldehyde | ||||||||
| isobutanol | ||||||||
| 2-butanol | ||||||||
| 1-propanol | ||||||||
| isoamyl alcohol | ||||||||
| furfural | ||||||||
| methanol | −0.679 *** | |||||||
| ethyl acetate | −0.708 *** | 0.112 | ||||||
| 1-butanol | 0.789 *** | −0.709 *** | −0.527 ** | |||||
| ethyl butylate | 0.034 | −0.580 ** | 0.591 ** | 0.266 | ||||
| hexanal | 0.156 | −0.661 ** | 0.430 * | 0.482 * | 0.937 *** | |||
| butyl acetate | 0.095 | −0.644 ** | 0.445 * | 0.452 * | 0.892 *** | 0.979 *** | ||
| ethyl-2-methylbutyrate | −0.057 | −0.544 ** | 0.599 ** | 0.298 | 0.895 *** | 0.947 *** | 0.950 *** | |
| β-damascenone | 0.527 * | −0.152 | −0.808 ** | 0.883 *** | −0.757 ** | −0.699 ** | −0.672 ** | −0.749 ** |
Appendix B
| Control | Flavour Category 1 | ||||||
|---|---|---|---|---|---|---|---|
| Ethanol Concentration% (v/v) | xethanol | D.T. (sec) | Temp. 1 (°C) | Temp. 2 (°C) | D.T. (sec) | Temp. 1 (°C) | Temp. 2 (°C) |
| 5 | 0.0159 | 227 ± 2.94 | 87.4 ± 0.704 | 93.6 ± 0.704 | 221 ± 13.3 | 90.8 ± 0.327 | 94.4 ± 0.492 |
| 15 | 0.0509 | 139 ± 10.5 | 82.2 ± 0.579 | 86.8 ± 0.579 | 131 ± 4.78 | 84.4 ± 4.99 | 86.2 ± 0.432 |
| 25 | 0.0910 | 116 ± 19.4 | 79.1 ± 4.24 | 83.4 ± 4.235 | 113 ± 8.99 | 80.2 ± 0.340 | 82.3 ± 0.294 |
| 35 | 0.1371 | 107 ± 14.5 | 79.7 ± 0.262 | 80.4 ± 0.262 | 105 ± 3.68 | 79.2 ± 0.535 | 80.6 ± 0.464 |
| 45 | 0.1921 | 107 ± 4.24 | 77.7 ± 0.665 | 78.3 ± 0.665 | 102 ± 5.44 | 78.3 ± 0.356 | 79.3 ± 0.455 |
| 55 | 0.2588 | 103 ± 6.02 | 78.4 ± 0.216 | 78.9 ± 0.216 | 101 ± 0.94 | 77.4 ± 0.531 | 78.5 ± 0.082 |
| 65 | 0.3430 | 112 ± 10.9 | 76.8 ± 0.450 | 77.4 ± 0.450 | 101 ± 6.48 | 76.7 ± 0.478 | 77.6 ± 0.386 |
| 75 | 0.4518 | 102 ± 2.05 | 77.3 ± 0.082 | 77.7 ± 0.082 | 96 ± 5.6 | 75.5 ± 0.330 | 77.2 ± 0.787 |
| 85 | 0.6016 | 96.3 ± 0.943 | 75.4 ± 0.163 | 76.4 ± 0.163 | 91 ± 7.6 | 73.4 ± 1.67 | 75.3 ± 0.957 |
| Flavour Category 2 | Flavour Category 3 | ||||||
|---|---|---|---|---|---|---|---|
| Ethanol Concentration % (v/v) | xethanol | D.T. (sec) | Temp. 1 (°C) | Temp. 2 (°C) | D.T. (sec) | Temp. 1 (°C) | Temp. 2 (°C) |
| 5 | 0.0159 | 218 ± 10.8 | 85.5 ± 0.368 | 95.8 ± 0.929 | 263 ± 29.1 | 85.7 ± 0.838 | 93.1 ± 0.732 |
| 15 | 0.0509 | 144 ± 8.60 | 77.6 ± 0.655 | 82.2 ± 0.613 | 155 ± 17.5 | 83.5 ± 0.464 | 87.0 ± 0.368 |
| 25 | 0.0910 | 114 ± 7.36 | 76.4 ± 0.386 | 78.0 ± 0.668 | 128 ± 12.0 | 78.1 ± 0.300 | 80.0 ± 0.386 |
| 35 | 0.1371 | 113 ± 3.74 | 75.3 ± 0.411 | 77.8 ± 0.249 | 121 ± 16.4 | 78.2 ± 0.249 | 81.4 ± 0.492 |
| 45 | 0.1921 | 106 ± 6.68 | 74.5 ± 1.14 | 78.2 ± 0.249 | 119 ± 9.03 | 77.6 ± 0.386 | 79.0 ± 0.400 |
| 55 | 0.2588 | 104 ± 4.71 | 73.8 ± 0.249 | 77.5 ± 0.478 | 118 ± 10.8 | 77.4 ± 0.829 | 79.2 ± 0.125 |
| 65 | 0.3430 | 102 ± 3.68 | 73.5 ± 0.287 | 75.4 ± 0.638 | 98 ± 0.47 | 77.1 ± 0.556 | 78.1 ± 0.497 |
| 75 | 0.4518 | 90 ± 8.8 | 73.9 ± 0.535 | 75.1 ± 1.20 | 87 ± 3.1 | 76.0 ± 1.558 | 78.2 ± 0.125 |
| 85 | 0.6016 | 94 ± 3.4 | 70.8 ± 0.759 | 72.7 ± 1.31 | 84 ± 12 | 75.3 ± 0.873 | 76.9 ± 0.125 |
References
- Puentes, C.; Joulia, X.; Athès, V.; Esteban-Decloux, M. Review and Thermodynamic Modeling with NRTL Model of Vapor-Liquid Equilibria (VLE) of Aroma Compounds Highly Diluted in Ethanol-Water Mixtures at 101.3 kPa. Ind. Eng. Chem. Res. 2018, 57, 3443–3470. [Google Scholar] [CrossRef]
- Puentes, C.; Joulia, X.; Paricaud, P.; Giampaoli, P.; Athès, V.; Esteban-Decloux, M. Vapor-Liquid Equilibrium of Ethyl Lactate Highly Diluted in Ethanol-Water Mixtures at 101.3 kPa. Experimental Measurements and Thermodynamic Modeling Using Semiempirical Models. J. Chem. Eng. Data 2018, 63, 365–379. [Google Scholar] [CrossRef]
- Deterre, S.; Albet, J.; Joulia, X.; Baudouin, O.; Giampaoli, P.; Decloux, M.; Athès, V. Vapor-liquid equilibria measurements of bitter orange aroma compounds highly diluted in boiling hydro-alcoholic solutions at 101.3 kPa. J. Chem. Eng. Data 2012, 57, 3344–3356. [Google Scholar] [CrossRef]
- Williams, C.G. Vapor-liquid equilibria of organic homologues in ethanol-water solutions. Am. J. Enol. Vitic. 1962, 13, 169–182. [Google Scholar]
- Ikari, A.; Hatate, Y.; Fukumoto, T. Vapor-Liquid Equilibria of a Minute Amount of Methanol, Isovaleraldehyde and Diacetyl in Aqueous Ethanol Solution under Reduced Pressure. Kagaku Kogaku Ronbunshu 1998, 24, 111–115. [Google Scholar] [CrossRef]
- Ikari, A.; Kubo, R. Behavior of Various Impurities in Simple Distillation of Aqueous Solution of Ethanol. J. Chem. Eng. Jpn. 1975, 8, 294–299. [Google Scholar] [CrossRef]
- Ikari, A.; Hatate, Y.; Yanagida, M.; Eda, M. Vapor-Liquid Equilibria of a Minute Amount of n-Propyl, Isobutyl, and Isoamyl Alcohol in Aqueous Ethanol Solution under Reduced Pressure. Kagaku Kogaku Ronbunshu 1990, 16, 1101–1104. [Google Scholar] [CrossRef]
- Ikari, A.; Yasuo, H.; Seiichi, K.; Yukinori, S. Behavior of a Minute amount of Furfural Distillation of Aqueous Ethanol Solution in a simple mathematical model employing Murphree. J. Chem. Eng. Jpn. 1984, 17, 486–490. [Google Scholar] [CrossRef]
- Ikari, A.; Yasuo, H.; Aiko, R. Vapor-Liquid Equilibria of Trace Isobutyraldehyde, Ethyl Acetate and Isoamyl Acetate in Aqueous Ethanol Solution under Reduced Pressure. Kagaku Kogaku Ronbunshuu 1998, 24, 678–681. [Google Scholar] [CrossRef]
- Gillespie, C.T.D. Vapor-Liquid Equilibrium Still for Miscible Liquids. Ind. Eng. Chem. 1946, 18, 575–577. [Google Scholar] [CrossRef]
- Martín, A.; Carrillo, F.; Trillo, M.L.; Rosello, A. A quick method for obtaining partition factor of congeners in spirits. Eur. Food Res. Technol. 2009, 229, 697–703. [Google Scholar] [CrossRef]
- Athès, V.; Lillo, Y.P.M.; Bernard, C.; Pérez-Correa, R.; Souchon, I. Comparison of Experimental Methods for Measuring Infinite Dilution Volatilities of Aroma Compounds in Water/Ethanol Mixtures. J. Agric. Food Chem. 2004, 52, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, H.; Zhou, R.; Duan, Z. Influence of salt added to solvent on extractive distillation. Chem. Eng. J. 2002, 87, 149–156. [Google Scholar] [CrossRef]
- Athès, V.; Paricaud, P.; Ellaite, M.; Souchon, I.; Fürst, W. Vapour-liquid equilibria of aroma compounds in hydroalcoholic solutions: Measurements with a recirculation method and modelling with the NRTL and COSMO-SAC approaches. Fluid Phase Equilib. 2008, 265, 139–154. [Google Scholar] [CrossRef]
- Kurihara, K.; Nakamichi, M.; Kojima, K. Isobaric Vapor-Liquid Equilibria for Methanol + Ethanol + Water and the Three Constituent Binary Systems. J. Chem. Eng. Data 1993, 38, 446–449. [Google Scholar] [CrossRef]
- Heller, D.; Einfalt, D. Reproducibility of Fruit Spirit Distillation Processes. Beverages 2022, 8, 20. [Google Scholar] [CrossRef]
- Spaho, N. Distillation Techniques in the Fruit Spirits Production. In Distillation—Innovative Applications and Modeling, 1st ed.; Mendes, M., Ed.; IntechOpen: London, UK, 2017; pp. 129–152. [Google Scholar]
- de la Fuente-Blanco, A.; Saenz-Navajas, M.P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- García-Llobodanin, L.; Achaerandio, I.; Ferrando, M.; Güell, C.; López, F. Pear Distillates from Pear Juice Concentrate: Effect of Lees in the Aromatic Composition. J. Agric. Food Chem. 2007, 55, 3462–3468. [Google Scholar] [CrossRef]
- Zheng, J.; Liang, R.; Huang, J.; Zhou, R.P.; Chen, Z.J.; Wu, C.D.; Zhou, R.Q.; Liao, X.P. Volatile Compounds of Raw Spirits from Different Distilling Stages of Luzhou-flavor Spirit. Food Sci. Technol. Res. 2014, 20, 283–293. [Google Scholar] [CrossRef]
- Claus, J.M.; Berglund, A.K. Fruit Brandy production by batch column distillation with reflux. J. Food Process Eng. 2005, 28, 53–67. [Google Scholar] [CrossRef]
- Lukić, I.; Tomas, S.; Miličević, B.; Radeka, S.; Peršurić, Đ. Behaviour of Volatile Compounds during Traditional Alembic Distillation of Fermented Muscat Blanc and Muškat Ruža Porečki Grape Marcs. J. Inst. Brew. 2011, 117, 440–450. [Google Scholar] [CrossRef]
- Spaho, N.; Dürr, P.; Grba, S.; Velagić-Habul, E.; Blesić, M. Effects of distillation cut on the distribution of higher alcohols and esters in brandy produced from three plum varieties. J. Inst. Brew. 2013, 119, 48–56. [Google Scholar] [CrossRef]
- Tian, T.T.; Ruan, S.L.; Zhao, Y.P.; Li, J.M.; Yang, C.; Cao, H. Multi-objective evaluation of freshly distilled brandy: Characterisation and distribution patterns of key odour-active compounds. Food Chem. X 2022, 14, 100276. [Google Scholar] [CrossRef] [PubMed]



| Functional Group | Compound | Concentration in Solutions (mg/100 mL a.a.) | MW (g/mol) | Boiling Point (°C) | Supplier | Purity (≥%) | Flavour Category * |
|---|---|---|---|---|---|---|---|
| Alcohol | Methanol | 1300 | 32.04 | 64.7 | Roth | 99.9 | 2 |
| 1-Propanol | 350 | 60.10 | 97 | Merck | 99.5 | 2 | |
| Isobutanol | 70 | 74.12 | 108 | Fluka | 99.5 | 1 | |
| 1-Butanol | 5 | 74.10 | 117.7 | Fluka | 99 | 3 | |
| Isoamyl alcohol | 150 | 88.15 | 131 | Sigma-Aldrich | 98.5 | 2 | |
| 2-Methyl-1-butanol | 50 | 88.15 | 129 | Roth | 97.5 | 1 | |
| Phenethyl alcohol | 25 | 122.16 | 225 | Sigma-Aldrich | 99 | 1 | |
| 2-Butanol | 200 | 74.12 | 100 | Roth | 98.5 | 2 | |
| 1-Hexanol | 15 | 102.16 | 157 | Fluka | 99 | 1 | |
| Aldehyde | Acetaldehyde | 80 | 44.05 | 20.2 | Roth | 99.5 | 1 |
| Hexanal | 5 | 100.16 | 129 | Sigma-Aldrich | 95 | 3 | |
| Furfural | 100 | 96.08 | 162 | Merck | 98 | 2 | |
| Acetal | 1,1-Diethoxyethane | 50 | 118.18 | 102 | Sigma-Aldrich | 98 | 1 |
| Ester | Ethyl butyrate | 5 | 116.16 | 121 | Sigma-Aldrich | 99.5 | 3 |
| Butyl acetate | 5 | 116.16 | 126 | Sigma-Aldrich | 99 | 3 | |
| Ethyl 2-methylbutyrate | 5 | 130.18 | 133 | Sigma-Aldrich | 98 | 3 | |
| Ethyl acetate | 500 | 88.11 | 77.1 | Sigma-Aldrich | 99.9 | 2 | |
| Ketone | β-Damascenone | 5 | 190.28 | 116 | Sigma-Aldrich | 98 | 3 |
| df | Sum of Squares | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|
| Molecular Weight | 1 | 43.10 | 43.10 | 22.84 | <0.0001 |
| Functional Group | 2 | 177.31 | 88.66 | 46.98 | <0.0001 |
| Interaction | 2 | 86.40 | 43.20 | 22.89 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagishita, M.; Kölling, R.; Einfalt, D. Introducing a Simple Method to Investigate Relative Volatilities of Flavour Compounds in Fruit Brandies. Beverages 2023, 9, 32. https://doi.org/10.3390/beverages9020032
Yagishita M, Kölling R, Einfalt D. Introducing a Simple Method to Investigate Relative Volatilities of Flavour Compounds in Fruit Brandies. Beverages. 2023; 9(2):32. https://doi.org/10.3390/beverages9020032
Chicago/Turabian StyleYagishita, Manami, Ralf Kölling, and Daniel Einfalt. 2023. "Introducing a Simple Method to Investigate Relative Volatilities of Flavour Compounds in Fruit Brandies" Beverages 9, no. 2: 32. https://doi.org/10.3390/beverages9020032
APA StyleYagishita, M., Kölling, R., & Einfalt, D. (2023). Introducing a Simple Method to Investigate Relative Volatilities of Flavour Compounds in Fruit Brandies. Beverages, 9(2), 32. https://doi.org/10.3390/beverages9020032






