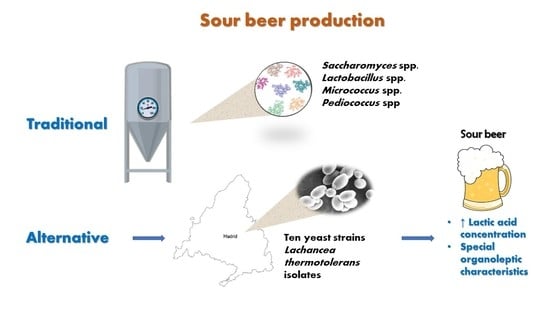
Lachancea thermotolerans, an Innovative Alternative for Sour Beer Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Selection of Yeast Strains
- Type I (white/cream): low–null production of H2S
- Type II (light brown): moderate production of H2S
- Type III (brown): high production of H2S
- Type IV (dark brown/black): very high production of H2S
2.3. Fermentation Conditions
2.4. Beer Analysis
2.5. Volatile Compounds Analysis
2.6. Melatonin Determination
2.7. Antioxidant Capacity
2.8. Sensory Analysis
2.9. Consumer Aceptability Test
2.10. Check-All-That-Apply Questions
2.11. Statistical Analysis
3. Results and Discussion
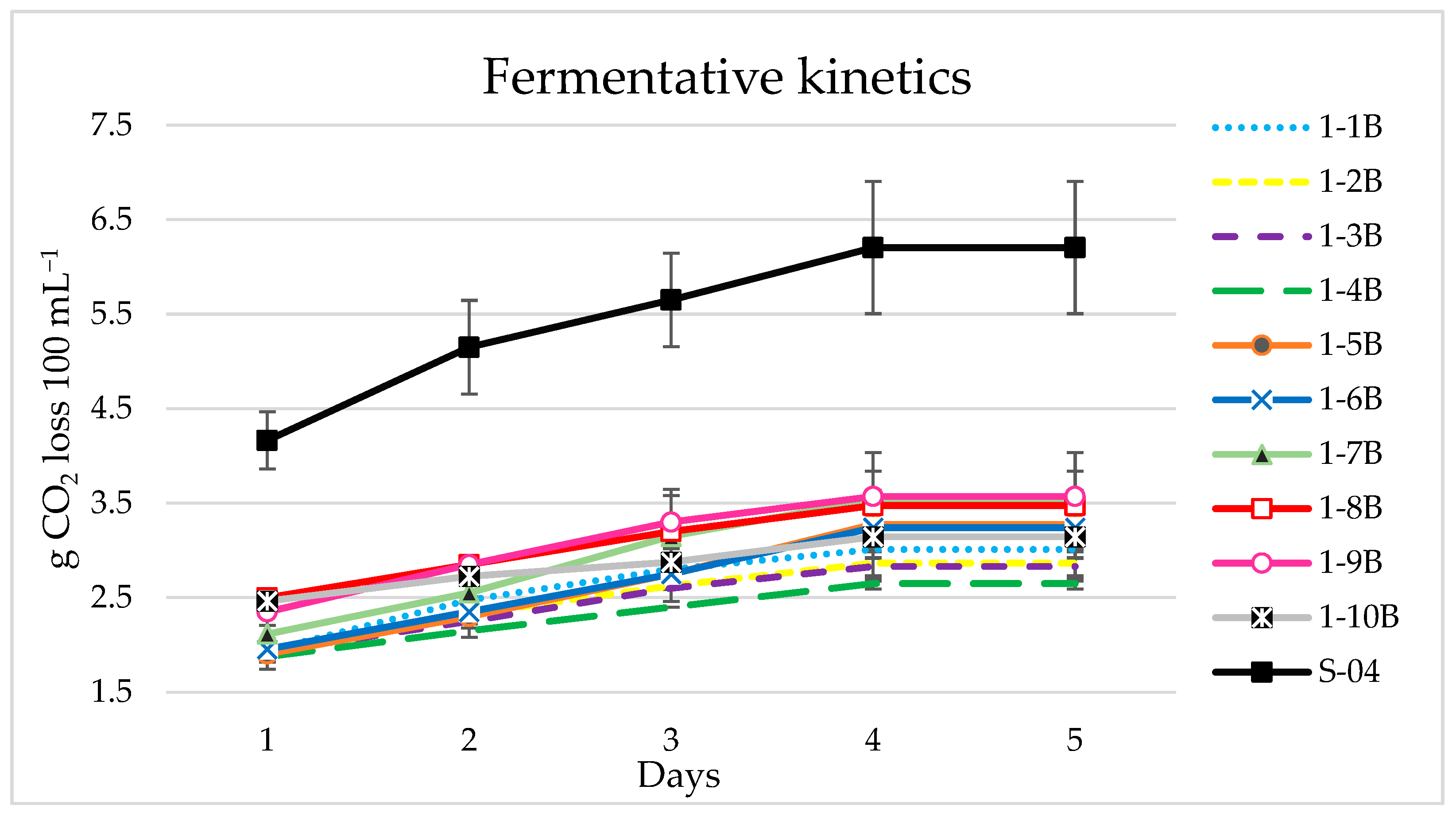
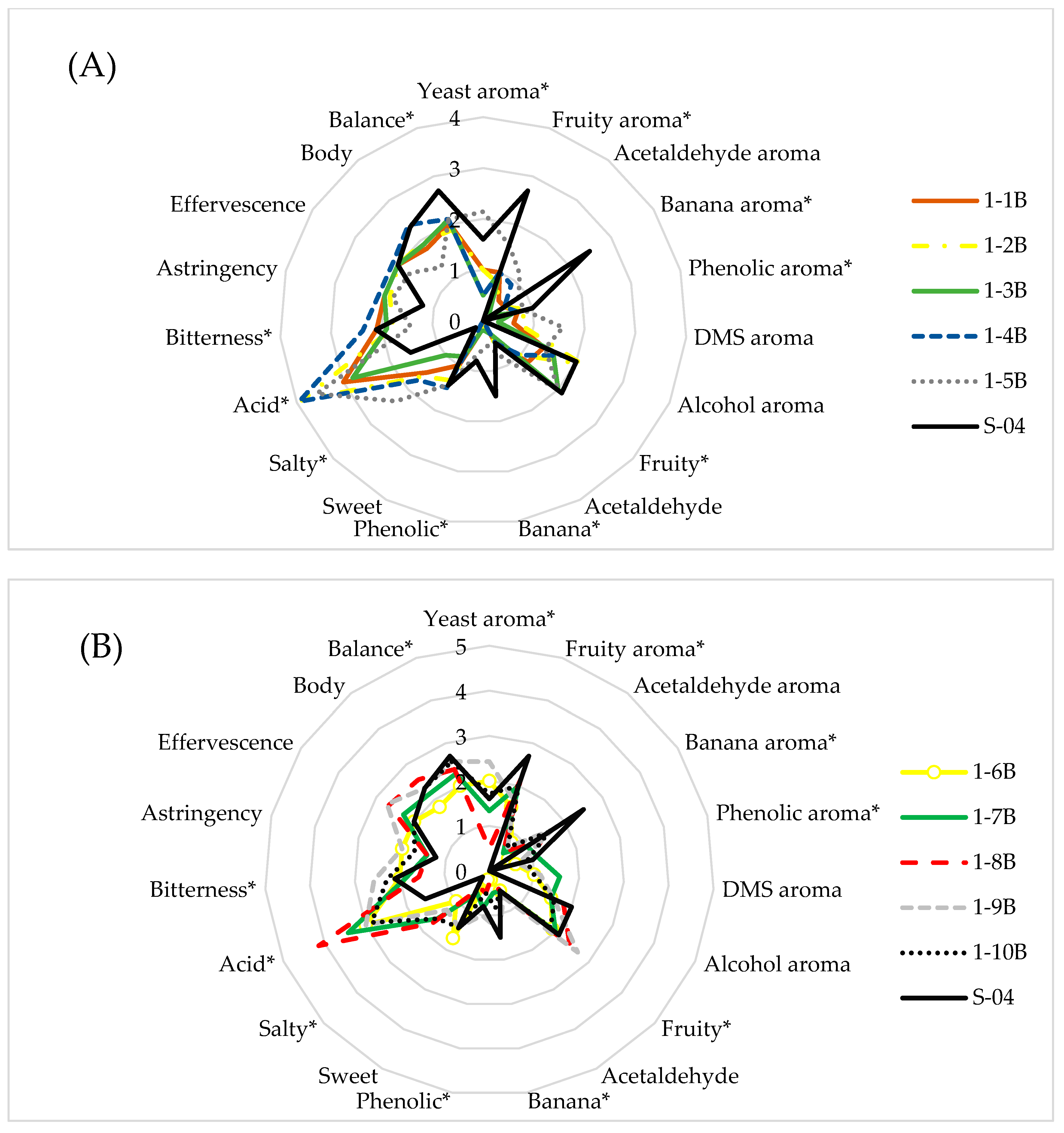
3.1. Yeast Screening
3.1.1. Beer Analyses
3.1.2. Volatile Compounds
| Yeast Strains | 1-1B | 1-2B | 1-3B | 1-4B | 1-5B | 1-6B | 1-7B | 1-8B | 1-9B | 1-10B | S-04 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher alcohols | |||||||||||
| Isobutanol | 12.54 ± 1.47 bc | 15.85 ± 1.76 bc | 12.46 ± 0.44 bc | 16.21 ± 1.84 bc | 14.43 ± 2.21 bc | 11.31 ± 0.66 c | 17.69 ± 2.38 abc | 18.65 ± 2.31 ab | 23.86 ± 5.28 a | 24.43 ± 2.95 a | 12.25 ± 2.60 bc |
| Isoamyl alcohol | 46.76 ± 5.61 cd | 49.95 ± 2.83 bcd | 41.92 ± 5.03 cd | 34.08 ± 21.96 d | 40.56 ± 4.16 cd | 41.66 ± 2.30 cd | 84.32 ± 11.81 a | 64.13 ± 7.03 abcd | 67.72 ± 8.36 abc | 79.21 ± 8.26 ab | 60.67 ± 18.16 abcd |
| Benzyl alcohol | nd | nd | nd | nd | 0.16 ± 0.14 | nd | 0.03 ± 0.05 | 0.18 ± 0.32 | nd | nd | nd |
| β-phenylethanol | 31.02 ± 3.97 b | 32.16 ± 1.26 ab | 28.54 ± 4.65 b | 26.93 ± 3.62 b | 30.62 ± 1.63 b | 29.49 ± 0.56 b | 26.90 ± 5.53 b | 30.25 ± 0.00 b | 31.11 ± 3.54 ab | 25.98 ± 5.97 b | 44.72 ± 10.75 a |
| Esters | |||||||||||
| Ethyl butyrate | 0.99 ± 0.11 cd | 1.04 ± 0.09 cd | 0.94 ± 0.04 cd | 1.43 ± 0.2 bc | 1.27 ± 0.14 c | 1.05 ± 0.05 cd | 2.71 ± 0.21 a | 2.16 ± 0.25 ab | 2.57 ± 0.64 a | 2.14 ± 0.62 ab | 0.45 ± 0.12 d |
| Ethyl hexanoate | 0.18 ± 0.04 ab | 0.14 ± 0.03 ab | 0.16 ± 0.06 ab | 0.15 ± 0.04 ab | 0.02 ± 0.04 ab | nd | 0.34 ± 0.29 ab | 0.21 ± 0.21 ab | 0.32 ± 0.05 ab | nd | 0.06 ± 0.04 ab |
| Ethyl lactate | nd | nd | 7.37 ± 6.38 a | nd | nd | nd | nd | nd | nd | nd | 0.58 ± 0.83 a |
| Ethyl octanoate | 0.10 ± 0.02 | 0.05 ± 0.04 | 0.06 ± 0.05 | nd | 0.23 ± 0.39 | nd | nd | nd | 0.18 ± 0.16 | nd | nd |
| Diethyl succinate | nd | nd | nd | nd | nd | nd | 1.31 ± 0.51 a | 0.70 ± 0.02 b | nd | nd | nd |
| Fatty Acids | |||||||||||
| Butyric acid | nd | nd | nd | nd | nd | nd | nd | nd | 1.66 ± 2.87 | 2.70 ± 4.67 | nd |
| Isovaleric acid | 0.14 ± 0.08 c | 0.26 ± 0.06 c | 0.16 ± 0.01 c | 0.39 ± 0.16 bc | 0.19 ± 0.22 c | 0.13 ± 0.03 c | nd | nd | 0.78 ± 0.21 ab | 0.85 ± 0.34 a | nd |
| Hexanoic acid | 1.62 ± 1.14 | 0.31 ± 0.14 | 0.02 ± 0.04 | 0.97 ± 1.06 | 0.49 ± 0.43 | 0.08 ± 0.02 | 2.21 ± 2.30 | nd | 1.15 ± 0.69 | nd | 0.45 ± 0.23 |
| Octanoic acid | nd | nd | nd | nd | nd | nd | 0.86 ± 0.81 ab | nd | nd | 0.07 ± 0.13 b | 1.05 ± 0.59 a |
| Decanoic acid | nd | nd | nd | 0.14 ± 0.24 | nd | nd | 1.06 ± 0.94 | nd | nd | 0.28 ± 0.48 | 1.85 ± 3.07 |
| Aldehydes/Ketones | |||||||||||
| Acetoin | 6.19 ± 0.63 a | 5.15 ± 0.74 ab | 5.02 ± 0.38 ab | 1.62 ± 2.81 bc | nd | nd | nd | 6.19 ± 1.22 a | nd | nd | 3.81 ± 2.63 ab |
| Guaiacol | nd | nd | 0.02 ± 0.04 b | 0.04 ± 0.07 b | nd | nd | nd | 0.86 ± 0.75 a | nd | nd | 0.03 ± 0.04 b |
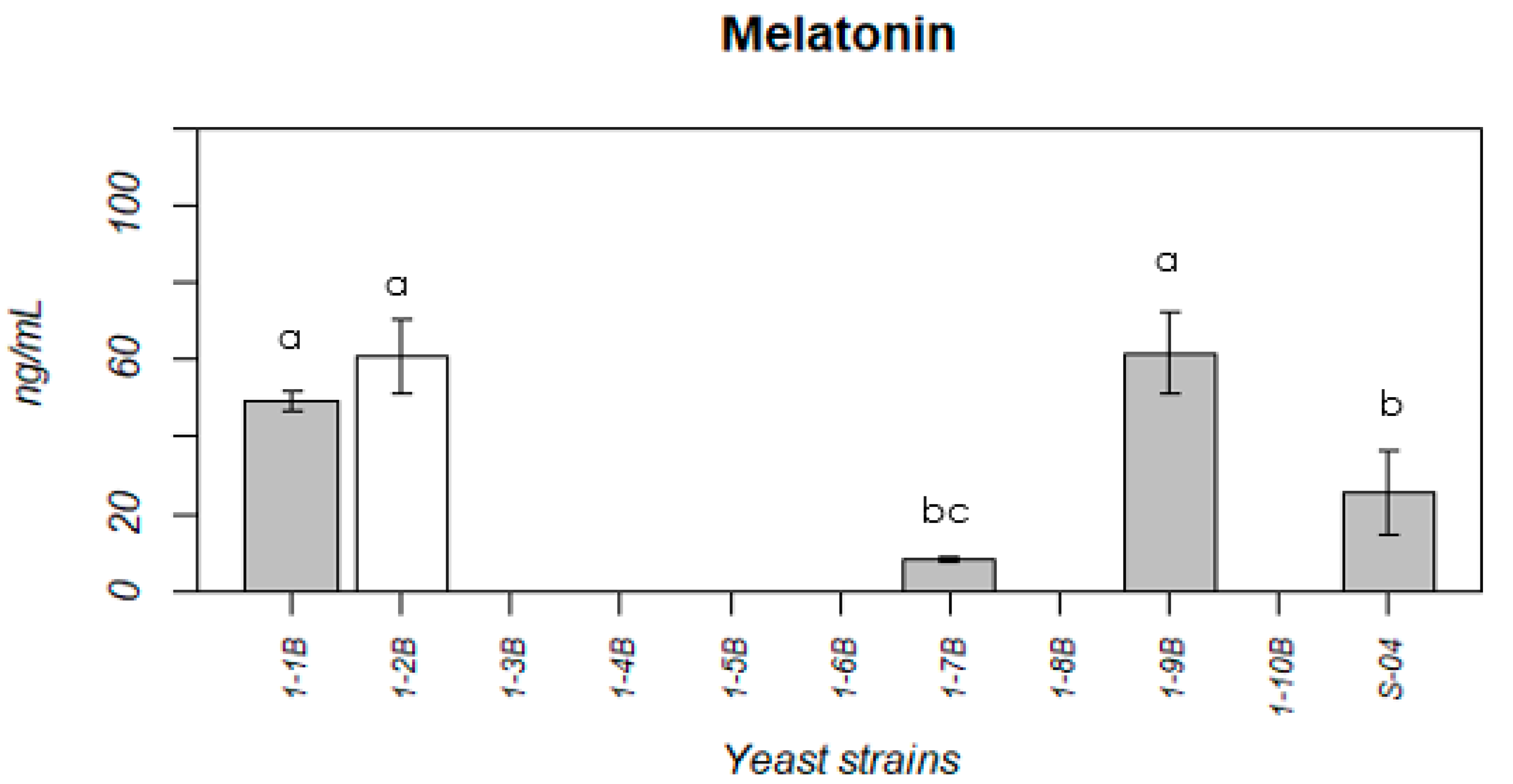
3.1.3. Melatonin Production
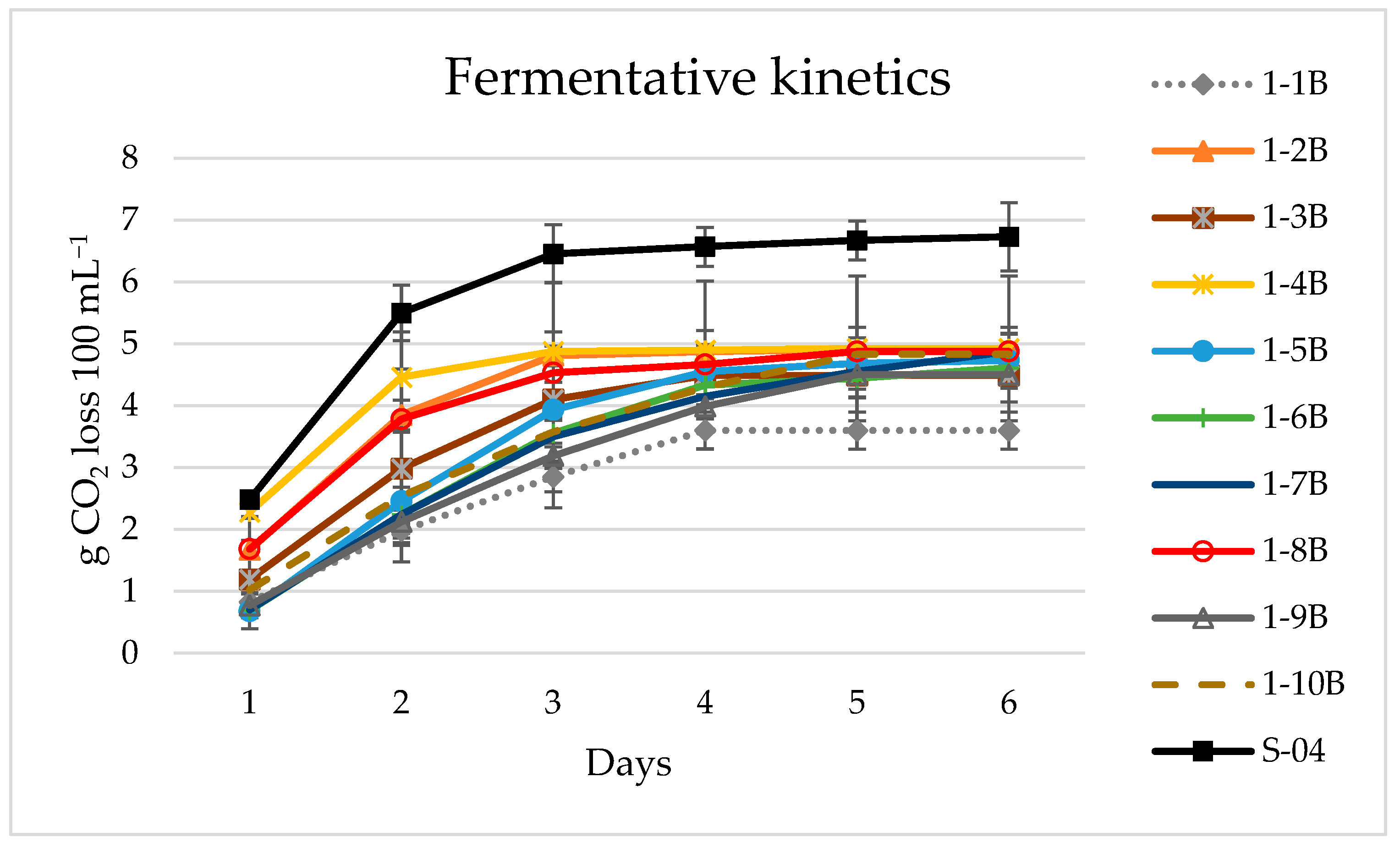
3.2. Lab Scale Fermentation in 1 L
3.2.1. Sensory Analysis
3.2.2. Beer Analysis
3.2.3. Volatile Compounds
3.2.4. Melatonin Content
3.2.5. Antioxidant Capacity
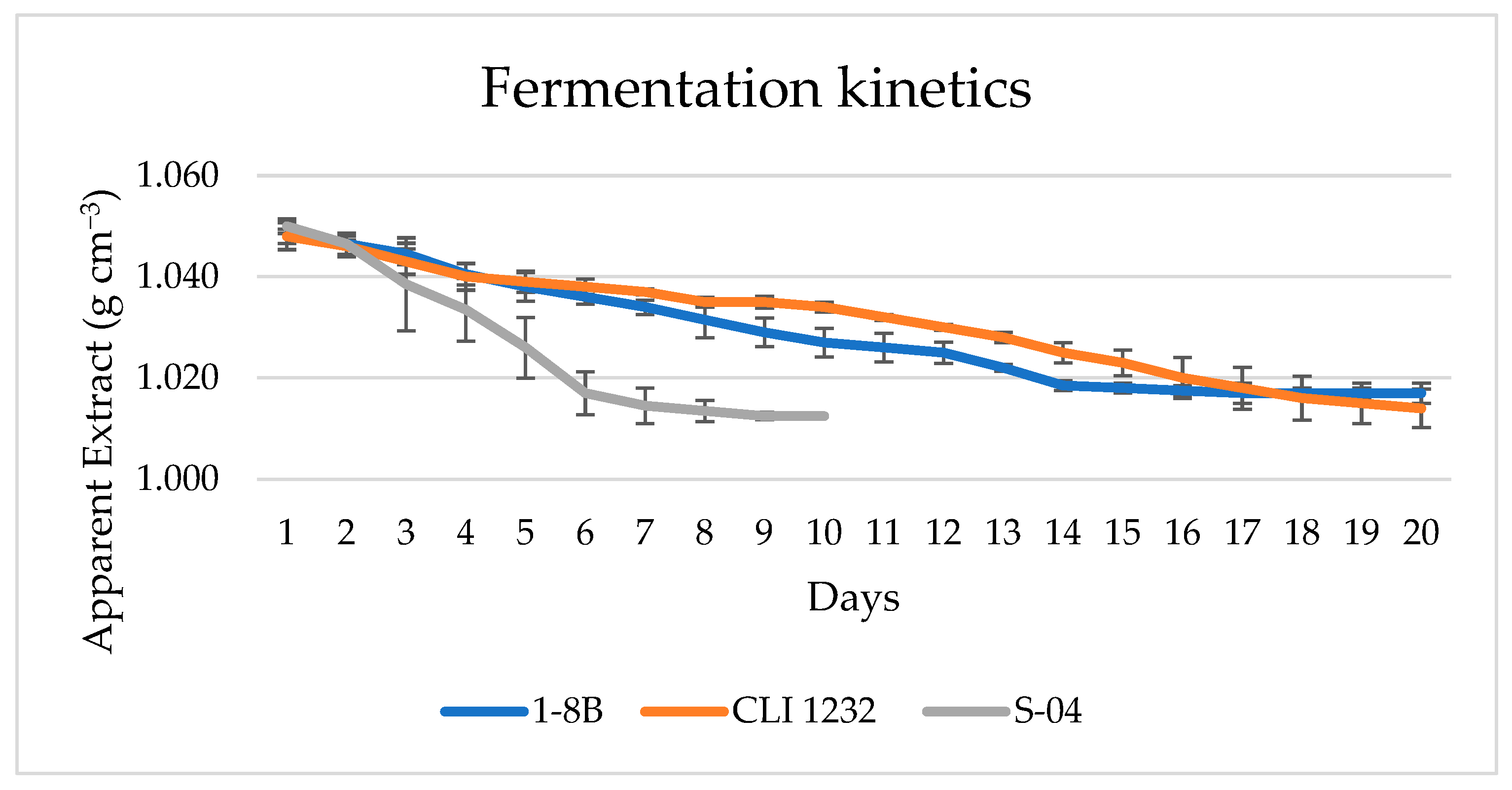
3.3. Industrial Scale: 100 L
3.3.1. Beer Analysis in 100 L
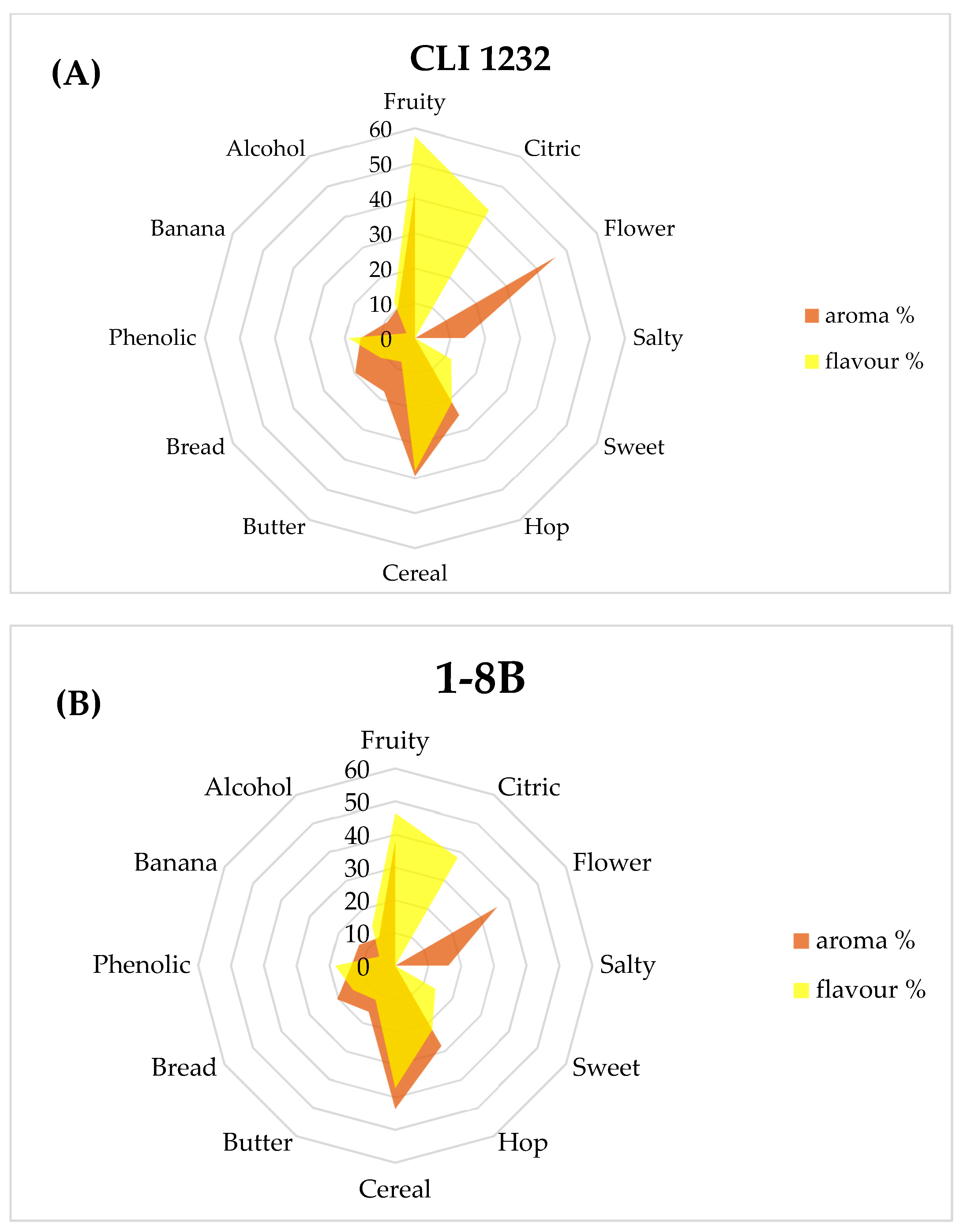
3.3.2. Consumer Analysis
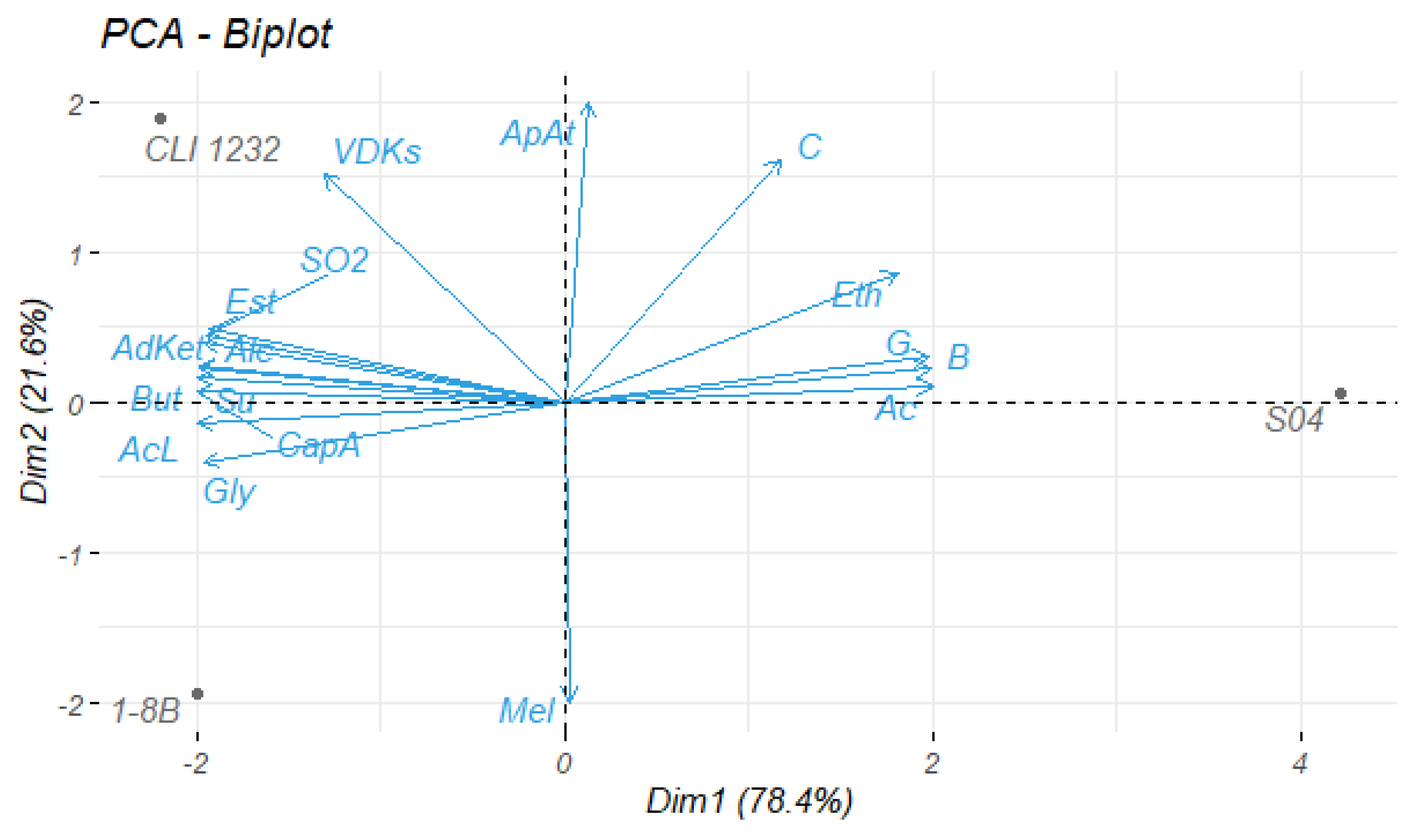
3.3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer Choice and Consumption Determinants When Craft Beers Are Tasted: An Exploratory Study of Consumer Preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Brungard, M. Calcium and Magnesium in Brewing Water. New Brew. 2014, 31, 80–88. [Google Scholar]
- So, A. Developing New Barely Varities: A Work in Progress. New Brew. 2014, 31, 60–68. [Google Scholar]
- Carbone, K.; Bianchi, G.; Petrozziello, M.; Bonello, F.; Macchioni, V.; Parisse, B.; De Natale, F.; Alilla, R.; Cravero, M.C. Tasting the Italian Terroir through Craft Beer: Quality and Sensory Assessment of Cascade Hops Grown in Central Italy and Derived Monovarietal Beers. Foods 2021, 10, 2085. [Google Scholar] [CrossRef] [PubMed]
- Tusha, K.; Nešpor, J.; Jelínek, L.; Vodičková, H.; Kinčl, T.; Dostálek, P. Effect of Czech Hop Varieties on Aroma of Dry-Hopped Lager Beer. Foods 2022, 11, 2520. [Google Scholar] [CrossRef]
- Osburn, K.; Ahmad, N.N.; Bochman, M.L. Bio-Prospecting, Selection, and Analysis of Wild Yeasts for Ethanol Fermentation. Zymurgy 2016, 39, 81–88. [Google Scholar]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the Brewing Process: A New Approach to Enhance Bioflavour and to Reduce Ethanol Content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Kochlánová, T.; Kij, D.; Kopecká, J.; Kubizniaková, P.; Matoulková, D. Non-Saccharomyces Yeasts and Their Importance in the Brewing Industry. Part II. Kvas. Prum. 2016, 62, 206–214. [Google Scholar] [CrossRef]
- Johansson, L.; Nikulin, J.; Juvonen, R.; Krogerus, K.; Magalhães, F.; Mikkelson, A.; Nuppunen-Puputti, M.; Sohlberg, E.; de Francesco, G.; Perretti, G.; et al. Sourdough Cultures as Reservoirs of Maltose-Negative Yeasts for Low-Alcohol Beer Brewing. Food Microbiol. 2021, 94, 103629. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Bamforth, C.W.; Mills, D.A. Brewhouse-Resident Microbiota Are Responsible for Multi-Stage Fermentation of American Coolship Ale. PLoS ONE 2012, 7, e35507. [Google Scholar] [CrossRef] [PubMed]
- Tonsmeire, M. American Sour Beer: Innovative Techniques for Mixed Fermentations; Brewers Publications: Boulder, CO, USA, 2014. [Google Scholar]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Daniel, H.-M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The Microbial Diversity of Traditional Spontaneously Fermented Lambic Beer. PLoS ONE 2014, 9, e95384. [Google Scholar] [CrossRef] [PubMed]
- Hornsey, I.S. A History of Beer and Brewing; Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Esmaeili, S.; Mogharrabi, M.; Safi, F.; Sohrabvandi, S.; Mortazavian, A.M.; Bagheripoor-Fallah, N. The common spoilage microorganisms of beer: Occurrence, defects, and determination—A review. Carpathian J. Food Sci. Technol. 2015, 7, 68–73. [Google Scholar]
- Lee, Y.-J.; Choi, Y.-R.; Lee, S.-Y.; Park, J.-T.; Shim, J.-H.; Park, K.-H.; Kim, J.-W. Screening Wild Yeast Strains for Alcohol Fermentation from Various Fruits. Mycobiology 2011, 39, 33. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, J.P.; Gonçalves, P. Natural Populations of Saccharomyces kudriavzevii in Portugal Are Associated with Oak Bark and Are Sympatric with S. cerevisiae and S. paradoxus. Appl. Environ. Microbiol. 2008, 74, 2144–2152. [Google Scholar] [CrossRef]
- Marongiu, A.; Zara, G.; Legras, J.-L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel Starters for Old Processes: Use of Saccharomyces cerevisiae Strains Isolated from Artisanal Sourdough for Craft Beer Production at a Brewery Scale. J. Ind. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for Brewers: Exploiting Natural Diversity for Naturally Diverse Beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Evaluation of Saccharomyces cerevisiae Strains Isolated from Non-Brewing Environments in Beer Production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Domizio, P.; House, J.F.; Joseph, C.M.L.; Bisson, L.F.; Bamforth, C.W. Lachancea thermotolerans as an Alternative Yeast for the Production of Beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Osburn, K.; Amaral, J.; Metcalf, S.R.; Nickens, D.M.; Rogers, C.M.; Sausen, C.; Caputo, R.; Miller, J.; Li, H.; Tennessen, J.M.; et al. Primary Souring: A Novel Bacteria-Free Method for Sour Beer Production. Food Microbiol. 2018, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Postigo, V.; Sánchez, A.; Cabellos, J.M.; Arroyo, T. New Approaches for the Fermentation of Beer: Non-Saccharomyces Yeasts from Wine. Fermentation 2022, 8, 280. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of the Potential of Commercial Non-Saccharomyces Yeast Strains of Torulaspora delbrueckii and Lachancea thermotolerans in Beer Fermentation. Int. J. Food Sci. Technol. 2020, 55, 2049–2059. [Google Scholar] [CrossRef]
- Vaquero, C.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Marchante-Cuevas, L.; Heras, J.M.; Morata, A. Use of Lachancea thermotolerans for Biological vs. Chemical Acidification at Pilot-Scale in White Wines from Warm Areas. Fermentation 2021, 7, 193. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Bañuelos, M.A.; Heras, J.M.; Cuerda, R.; Morata, A. Industrial Performance of Several Lachancea thermotolerans Strains for PH Control in White Wines from Warm Areas. Microorganisms 2020, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea thermotolerans as a Tool to Improve PH in Red Wines from Warm Regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Morera, A.L.; Abreu, P. Seasonality of Psychopathology and Circannual Melatonin Rhythm. J. Pineal Res. 2006, 41, 279–283. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D. Melatonin in Walnuts: Influence on Levels of Melatonin and Total Antioxidant Capacity of Blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Manfredi, M.; Ribas-Serna, J.; Garcia-Moreno, H.; Calvo, J.R. Melatonin Administrated Immediately before an Intense Exercise Reverses Oxidative Stress, Improves Immunological Defenses and Lipid Metabolism in Football Players. Physiol. Behav. 2012, 105, 1099–1103. [Google Scholar] [CrossRef]
- Poeggeler, B.; Reiter, R.J.; Tan, D.-X.; Chen, L.; Manchester, L.C. Melatonin, Hydroxyl Radical-Mediated Oxidative Damage, and Aging: A Hypothesis. J. Pineal Res. 1993, 14, 151–168. [Google Scholar] [CrossRef]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-Related Changes in Melatonin Synthesis in Rat Extrapineal Tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Mora-Santos, M.; Naji, L.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Calvo, J.R. Evidence of Melatonin Synthesis and Release by Mast Cells. Possible Modulatory Role on Inflammation. Pharmacol. Res. 2010, 62, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Motilva, V.; García-Mauriño, S.; Talero, E.; Illanes, M. New Paradigms in Chronic Intestinal Inflammation and Colon Cancer: Role of Melatonin. J. Pineal Res. 2011, 51, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, S.J.; Youn, J.-P.; Hwang, S.Y.; Park, C.-S.; Park, Y.S. MicroRNA and Gene Expression Analysis of Melatonin-Exposed Human Breast Cancer Cell Lines Indicating Involvement of the Anticancer Effect. J. Pineal Res. 2011, 51, 345–352. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Reiter, R.J. Melatonin Uses in Oncology: Breast Cancer Prevention and Reduction of the Side Effects of Chemotherapy and Radiation. Expert Opin. Investig. Drugs 2012, 21, 819–831. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Siu, A.W.; Sánchez-Hidalgo, M.; Acuna-Castroviejo, D.; Escames, G. Melatonin and Lipid Uptake by Murine Fibroblasts: Clinical Implications. Neuroendocrinol. Lett. 2006, 27, 601–608. [Google Scholar]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin Treatment Improves Blood Pressure, Lipid Profile, and Parameters of Oxidative Stress in Patients with Metabolic Syndrome. J. Pineal Res. 2011, 50, 261–266. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could Non-Saccharomyces Yeasts Contribute on Innovative Brewing Fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Montrocher, R.; Verner, M.C.; Briolay, J.; Gautier, C.; Marmeisse, R. Phylogenetic Analysis of the Saccharomyces cerevisiae Group Based on Polymorphisms of RDNA Spacer Sequences. Int. J. Syst. Bacteriol. 1998, 48, 295–303. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of Yeasts by RFLP Analysis of the 5.8S RRNA Gene and the Two Ribosomal Internal Transcribed Spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae Var. Boulardii in Co-Fermentations with S. cerevisiae for the Production of Craft Beers with Potential Healthy Value-Added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Marconi, O.; Rossi, S.; Galgano, F.; Sileoni, V.; Perretti, G. Influence of Yeast Strain, Priming Solution and Temperature on Beer Bottle Conditioning. J. Sci. Food Agric. 2016, 96, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H. Bioflavoring and Beer Refermentation. Appl. Microbiol. Biotechnol. 2003, 62, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast Analysis of Important Wine Volatile Compounds—Development and Validation of a New Method Based on Gas Chromatographic-Flame Ionisation Detection Analysis of Dichloromethane Microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin Is Synthesised by Yeast during Alcoholic Fermentation in Wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Kocadaǧli, T.; Yilmaz, C.; Gökmen, V. Determination of Melatonin and Its Isomer in Foods by Liquid Chromatography Tandem Mass Spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Hevia, D.; Mayo, J.C.; Tan, D.-X.; Rodriguez-Garcia, A.; Sainz, R.M. Melatonin Enhances Photo-Oxidation of 2′,7′-Dichlorodihydrofluorescein by an Antioxidant Reaction That Renders N1-Acetyl-N2-Formyl-5-Methoxykynuramine (AFMK). PLoS ONE 2014, 9, e109257. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mazza, G. Simultaneous Analysis of Serotonin, Melatonin, Piceid and Resveratrol in Fruits Using Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.-M.; Porumb, R.; Epure, P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.0. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2004. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.4. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.12. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.10. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- Varela, P.; Ares, G. Sensory Profiling, the Blurred Line between Sensory and Consumer Science. A Review of Novel Methods for Product Characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the Overall Quality of Wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Callejo, M.J.; García Navas, J.J.; Alba, R.; Escott, C.; Loira, I.; González, M.C.; Morata, A. Wort Fermentation and Beer Conditioning with Selected Non-Saccharomyces Yeasts in Craft Beers. Eur. Food Res. Technol. 2019, 245, 1229–1238. [Google Scholar] [CrossRef]
- Zheng, X.; D’Amore, T.; Russell, I.; Stewart, G.G. Transport Kinetics of Maltotriose in Strains of Saccharomyces. J. Ind. Microbiol. 1994, 13, 159–166. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Satora, P.; Pater, A.; Bogacz, S. Low Lactic Acid-Producing Strain of Lachancea thermotolerans as a New Starter for Beer Production. Biomolecules 2020, 10, 256. [Google Scholar] [CrossRef]
- Dysvik, A.; Liland, K.H.; Myhrer, K.S.; Westereng, B.; Rukke, E.O.; de Rouck, G.; Wicklund, T. Pre-Fermentation with Lactic Acid Bacteria in Sour Beer Production. J. Inst. Brew. 2019, 125, 342–356. [Google Scholar] [CrossRef]
- Clapperton, J.F.; Brown, D.G.W. Caprylic Flavour As a Feature of Beer Flavour. J. Inst. Brew. 1978, 84, 90–92. [Google Scholar] [CrossRef]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The Chemical Profiling of Fatty Acids during the Brewing Process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef]
- Viana, A.C.; Pimentel, T.C.; Borges do Vale, R.; Clementino, L.S.; Januario Ferreira, E.T.; Magnani, M.; dos Santos Lima, M. American Pale Ale Craft Beer: Influence of Brewer’s Yeast Strains on the Chemical Composition and Antioxidant Capacity. LWT 2021, 152, 112317. [Google Scholar] [CrossRef]
- Dysvik, A.; La Rosa, S.L.; Buffetto, F.; Liland, K.H.; Myhrer, K.S.; Rukke, E.-O.; Wicklund, T.; Westereng, B. Secondary Lactic Acid Bacteria Fermentation with Wood-Derived Xylooligosaccharides as a Tool To Expedite Sour Beer Production. J. Agric. Food Chem. 2020, 68, 301–314. [Google Scholar] [CrossRef]
- Alcine Chan, M.Z.; Chua, J.Y.; Toh, M.; Liu, S.Q. Survival of Probiotic Strain Lactobacillus Paracasei L26 during Co-Fermentation with S. cerevisiae for the Development of a Novel Beer Beverage. Food Microbiol. 2019, 82, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.E. Brewing Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781782423317. [Google Scholar]
- Bamforth, C.W. Beer and Health. In Beer; Elsevier: Amsterdam, The Netherlands, 2009; pp. 229–253. ISBN 9780126692013. [Google Scholar]
- Laws, D.R.J.; McGuinness, J.D.; Rennie, H. The Losses of Bitter Substances during Fermentation. J. Inst. Brew. 1972, 78, 314–321. [Google Scholar] [CrossRef]
- Popescu, V.; Soceanu, A.; Dobrinas, S.; Stanciu, G. A Study of Beer Bitterness Loss during the Various Stages of the Romanian Beer Production Process. J. Inst. Brew. 2013, 119, 111–115. [Google Scholar] [CrossRef]
- Hughes, P.S.; Baxter, E.D. Beer: Quality, Safety and Nutritional Aspects; Royal Society of Chemistry: Cambridge, UK, 2001; ISBN 0854045880. [Google Scholar]
- Langstaff, S.A.; Lewis, M.J. The mouthfeel of beer—A review. J. Inst. Brew. 1993, 99, 31–37. [Google Scholar] [CrossRef]
- Mcfeeters, R.F. Fermentation Microorganisms and Flavor Changes in Fermented Foods. J. Food Sci. 2004, 69, FMS35–FMS37. [Google Scholar] [CrossRef]
- Nsogning Dongmo, S.; Sacher, B.; Kollmannsberger, H.; Becker, T. Key Volatile Aroma Compounds of Lactic Acid Fermented Malt Based Beverages—Impact of Lactic Acid Bacteria Strains. Food Chem. 2017, 229, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Peyer, L. Lactic Acid Bacteria Fermentation of Wort as a Tool to Add Functionality in Malting, Brewing and Novel Beverages. Ph.D. Thesis, University College Cork, Cork, Ireland, 2017. [Google Scholar]
- Ripari, V.; Tomassetti, M.; Cecchi, T.; Enrico, B. Recipe, Volatiles Profile, Sensory Analysis, Physico-Chemical and Microbial Characterization of Acidic Beers from Both Sourdough Yeasts and Lactic Acid Bacteria. Eur. Food Res. Technol. 2018, 244, 2027–2040. [Google Scholar] [CrossRef]
- Engan, S. Organoleptic Threshold Values of Some Alcohols and Esters in Beer. J. Inst. Brew. 1972, 78, 33–36. [Google Scholar] [CrossRef]
- Meilgaard, M. Flavor Chemistry of Beer: Part II: Flavor and Threshold of 239 Aroma Volatiles. Master Brew. Assoc. Am. Tech. Q. 1975, 12, 151–168. [Google Scholar]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by Non-Conventional Yeasts in Sequential Beer Fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Tan, Y.; Siebert, K.J. Quantitative Structure-Activity Relationship Modeling of Alcohol, Ester, Aldehyde, and Ketone Flavor Thresholds in Beer from Molecular Features. J. Agric. Food Chem. 2004, 52, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M. Flavor Chemistry of Beer. I. Flavor Interaction between Principal Volatiles. Master Brew. Assoc. Am. Tech. Q. 1975, 12, 107–117. [Google Scholar]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- Thompson Witrick, K.; Duncan, S.E.; Hurley, K.E.; O’keefe, S.F. Acid and Volatiles of Commercially-Available Lambic Beers. Beverages 2017, 3, 51. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of Chemical and Sensory Properties during Aging of Top-Fermented Beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.C. Prediction of Flavor Differences between Beers from Their Chemical Composition. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Sterckx, F.L.; Missiaen, J.; Saison, D.; Delvaux, F.R. Contribution of Monophenols to Beer Flavour Based on Flavour Thresholds, Interactions and Recombination Experiments. Food Chem. 2011, 126, 1679–1685. [Google Scholar] [CrossRef]
- Ciosek, A.; Fulara, K.; Hrabia, O.; Satora, P.; Poreda, A. Chemical Composition of Sour Beer Resulting from Supplementation the Fermentation Medium with Magnesium and Zinc Ions. Biomolecules 2020, 10, 1599. [Google Scholar] [CrossRef]
- Brányik, T.; Vicente, A.A.; Dostálek, P.; Teixeira, J.A. A Review of Flavour Formation in Continuous Beer Fermentations. J. Inst. Brew. 2008, 114, 3–13. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zarnkow, M.; Jacob, F.; De Schutter, D.P.; Arendt, E.K. Sour Brewing: Impact of Lactobacillus Amylovorus FST2.11 on Technological and Quality Attributes of Acid Beers. J. Am. Soc. Brew. Chem. 2017, 75, 207–216. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin Present in Beer Contributes to Increase the Levels of Melatonin and Antioxidant Capacity of the Human Serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef]
- Vigentini, I.; Gardana, C.; Fracassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast Contribution to Melatonin, Melatonin Isomers and Tryptophan Ethyl Ester during Alcoholic Fermentation of Grape Musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef]
- Morcillo-Parra, M.Á.; Beltran, G.; Mas, A.; Torija, M.-J. Effect of Several Nutrients and Environmental Conditions on Intracellular Melatonin Synthesis in Saccharomyces cerevisiae. Microorganisms 2020, 8, 853. [Google Scholar] [CrossRef]
- Dysvik, A.; La Rosa, S.L.; Liland, K.H.; Myhrer, K.S.; Østlie, H.M.; De Rouck, G.; Rukke, E.O.; Westereng, B.; Wicklund, T. Co-Fermentation Involving Saccharomyces cerevisiae and Lactobacillus Species Tolerant to Brewing-Related Stress Factors for Controlled and Rapid Production of Sour Beer. Front. Microbiol. 2020, 11, 279. [Google Scholar] [CrossRef]
- Burini, J.A.; Eizaguirre, J.I.; Loviso, C.; Libkind, D. Levaduras No Convencionales Como Herramientas de Innovación y Diferenciación en la Producción de Cerveza. Rev. Argent. Microbiol. 2021, 53, 359–377. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Delvaux, F.; Daenen, L.; Verachtert, H.; Delvaux, F.R. Aging Characteristics of Different Beer Types. Food Chem. 2007, 103, 404–412. [Google Scholar] [CrossRef]
- Piddocke, M.P.; Kreisz, S.; Heldt-Hansen, H.P.; Nielsen, K.F.; Olsson, L. Physiological Characterization of Brewer’s Yeast in High-Gravity Beer Fermentations with Glucose or Maltose Syrups as Adjuncts. Appl. Microbiol. Biotechnol. 2009, 84, 453–464. [Google Scholar] [CrossRef]
- Meilgaard, M.; Elizondo, A.; Moya, E. A Study of Carbonyl Compounds in Beer, Part II. Flavor and Flavor Thresholds of Aldehydes and Ketones Added to Beer. Tech. Q. Master Brew. Assoc. Am. 1970, 7, 143–149. [Google Scholar]
- Meilgaard, M.C. Individual Differences in Sensory Threshold for Aroma Chemicals Added to Beer. Food Qual. Prefer. 1993, 4, 153–167. [Google Scholar] [CrossRef]
- Dvořak, J.; Dostálek, P.; Štěrba, K.; Čejka, P.; Kellner, V.; Čulík, J.; Beinrohr, E. Determination of Total Sulphur Dioxide in Beer Samples by Flow-through Chronopotentiometry. J. Inst. Brew. 2006, 112, 308–313. [Google Scholar] [CrossRef]
- Guido, L.F. Sulfites in Beer: Reviewing Regulation, Analysis and Role. Sci. Agric. 2016, 73, 189–197. [Google Scholar] [CrossRef]
- Bourbon-Melo, N.; Palma, M.; Rocha, M.P.; Ferreira, A.; Bronze, M.R.; Elias, H.; Sá-Correia, I. Use of Hanseniaspora guilliermondii and Hanseniaspora opuntiae to Enhance the Aromatic Profile of Beer in Mixed-Culture Fermentation with Saccharomyces cerevisiae. Food Microbiol. 2021, 95, 103678. [Google Scholar] [CrossRef]
- Haukeli, A.D.; Lie, S. Formation and removal of acetoin during yeast fermentation. J. Inst. Brew. 1975, 81, 58–64. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon 2020, 6, e03648. [Google Scholar]
- Gerhäuser, C. Beer Constituents as Potential Cancer Chemopreventive Agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant Effectiveness of Selected Wines in Comparison with (+)-Catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Faria, J.D.A.F.; Cruz, A.G. Characterization of Brazilian Lager and Brown Ale Beers Based on Color, Phenolic Compounds, and Antioxidant Activity Using Chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.J.; Wagner, R.S.; Hutzler, M. Review: Pure Non-Saccharomyces Starter Cultures for Beer Fermentation with a Focus on Secondary Metabolites and Practical Applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Sannino, C.; Mezzasoma, A.; Buzzini, P.; Turchetti, B. Non-Conventional Yeasts for Producing Alternative Beers. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 361–388. ISBN 978-3-030-21109-7. [Google Scholar]
- Ahmad, M.N.; Holland, C.R.; McKay, G. Mass Transfer Studies in Batch Fermentation: Mixing Characteristics. J. Food Eng. 1994, 23, 145–158. [Google Scholar] [CrossRef]
- Díaz, M.; García, A.I.; García, L.A. Mixing Power, External Convection, and Effectiveness in Bioreactors. Biotechnol. Bioeng. 1996, 51, 131–140. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Daenen, L.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Furfuryl Ethyl Ether: Important Aging Flavor and a New Marker for the Storage Conditions of Beer. J. Agric. Food Chem. 2004, 52, 1661–1668. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Bamforth, C.W. Assessing Color Quality of Beer. ACS Symp. Ser. 2008, 983, 192–202. [Google Scholar] [CrossRef]
- Hudson, J.R.; Birtwistle, S.E. Wort-boiling in relation to beer quality. J. Inst. Brew. 1966, 72, 46–50. [Google Scholar] [CrossRef]
- Eßlinger, H.M. Handbook of Brewing Processes Technology Markets-Wiley; Wiley: Weinheim, Germany, 2009; ISBN 9783527314065. [Google Scholar]
| Yeast Strains | Lactic Acid mg L−1 | Colour EBC | Bitterness IBU | Ethanol % (v/v) | Residual Sugars g L−1 |
|---|---|---|---|---|---|
| 1-1B | 1877.50 ± 31.82 cd | 10.00 ± 0.00 b | 11.00 ± 0.00 c | 3.85 ± 0.35 b | 19.00 ± 0.00 b |
| 1-2B | 2084.50 ± 111.02 bc | 10.50 ± 0.71 b | 11.00 ± 0.00 c | 4.30 ± 0.57 b | 18.00 ± 0.00 b |
| 1-3B | 1925.00 ± 77.78 bcd | 10.00 ± 0.00 b | 13.65 ± 0.78 b | 4.10 ± 0.56 b | 18.00 ± 0.00 b |
| 1-4B | 2115.00 ± 70.71 b | 10.00 ± 0.00 b | 14.80 ± 0.99 b | 4.15 ± 0.21 b | 18.00 ± 0.00 b |
| 1-5B | 1593.00 ± 43.84 e | 10.50 ± 0.71 b | 16.10 ± 1.13 ab | 3.95 ± 0.21 b | 19.00 ± 0.00 b |
| 1-6B | 2093.00 ± 9.90 bc | 12.00 ± 0.00 b | 10.00 ± 0.00 c | 4.20 ± 0.28 b | 18.25 ± 1.06 b |
| 1-7B | 2402.00 ± 53.74 a | 11.50 ± 0.71 b | 10.00 ± 0.00 c | 5.00 ± 0.57 ab | 17.50 ± 0.71 b |
| 1-8B | 2554.00 ± 65.05 a | 13.50 ± 0.71 ab | 10.80 ± 0.35 c | 4.70 ± 0.28 b | 18.00 ± 0.00 b |
| 1-9B | 1925.00 ± 49.50 bcd | 13.50 ± 0.71 ab | 11.00 ± 0.00 c | 5.35 ± 0.49 a | 17.50 ± 0.71 b |
| 1-10B | 1694.00 ± 62.22 de | 13.50 ± 0.71 ab | 11.10 ± 0.17 c | 4.00 ± 0.14 ab | 18.00 ± 0.00 b |
| S-04 | 263.33 ± 37.29 f | 14.33 ± 1.53 a | 21.10 ± 3.32 a | 5.57 ± 0.59 a | 15.57 ± 3.19 a |
| Yeast Strains | Lactic Acid (mg L−1) | Colour EBC | Bitterness IBU | VDKs (mg L−1) | SO2 (mg L−1) | Ethanol %(v/v) | Residual Sugars (g L−1) |
|---|---|---|---|---|---|---|---|
| CLI 1232 | 1942.00 ± 82.02 a | 10.5 ± 2.12 | 12.75 ± 2.90 b | 0.11 ± 0.08 | 1.15 ± 0.21 a | 4.05 ± 0.49 b | 16.80 ± 1.70 a |
| 1-8B | 1639.00 ± 39.60 b | 11.00 ± 0.00 | 16.85 ± 2.33 a | 0.16 ± 0.04 | 1.25 ± 0.07 a | 4.35 ± 0.35 b | 18.00 ± 0.00 a |
| S-04 | 321.00 ± 21.00 c | 13.00 ± 0.00 | 16.50 ± 0.30 a | 0.11 ± 0.03 | ≤1 b | 5.40 ± 0.10 a | 5.90 ± 0.50 b |
| Yeast Strains | CLI 1232 | 1-8B | S-04 |
|---|---|---|---|
| Isobutanol | 17.39 ± 2.00 ab | 12.76 ± 1.10 b | 23.54 ± 2.78 a |
| 1-butanol | nd | 0.02 ± 0.00 b | 0.03 ± 0.00 a |
| Isoamyl alcohol | 33.33 ± 4.54 b | 46.04 ± 0.73 b | 68.73 ± 5.45 a |
| 1-hexanol | 0.02 ± 0.04 | 0.07 ± 0.00 | 0.06 ± 0.00 |
| Benzyl alcohol | 0.01 ± 0.00 ab | 0.01 ± 0.00 a | nd |
| β-phenyl ethanol | 31.11 ± 0.95 b | 29.80 ± 1.95 b | 45.90 ± 4.09 a |
| Total higher alcohols | 82.22 ± 7.53 | 89.58 ± 3.78 | 138.26 ± 12.32 |
| Ethyl isobutyrate | 0.06 ± 0.07 b | 2.11 ± 0.29 a | 0.40 ± 0.15 b |
| Ethyl butyrate | 1.51 ± 0.56 a | 1.90 ± 0.25 a | 0.13 ± 0.00 b |
| Ethyl isovalerate | nd | nd | 0.19 ± 0.00 a |
| Isoamyl acetate | 0.10 ± 0.04 b | 0.10 ± 0.00 b | 1.81 ± 0.26 a |
| Ethyl hexanoate | 0.04 ± 0.05 | 0.09 ± 0.01 | 0.03 ± 0.00 |
| Ethyl lactate | 11.02 ± 1.43 a | 10.93 ± 0.07 a | 0.19 ± 0.01 b |
| Ethyl octanoate | 0.08 ± 0.01 b | 0.08 ± 0.00 b | 0.13 ± 0.00 a |
| Diethyl succinate | 0.36 ± 0.05 b | 0.88 ± 0.07 a | 0.82 ± 0.09 a |
| Total esters | 12.83 ± 2.22 | 15.21 ± 0.69 | 3.71 ± 0.51 |
| Isovaleric acid | 3.39 ± 0.78 b | 4.45 ± 0.25 b | 9.82 ± 0.59 a |
| Hexanoic acid | 0.19 ± 0.05 | 0.39 ± 0.04 | 1.56 ± 0.07 |
| Octanoic acid | 0.17 ± 0.01 b | 0.17 ± 0.01 b | 5.50 ± 0.35 a |
| Decanoic acid | 0.46 ± 0.21 | 0.29 ± 0.10 | 0.17 ± 0.02 |
| Total fatty acids | 4.22 ± 1.05 | 5.31 ± 0.4 | 17.05 ± 1.03 |
| Acetoin | 1.62 ± 0.55 | 1.31 ± 0.23 | 0.67 ± 0.43 |
| Furfural | 0.02 ± 0.03 | nd | nd |
| Total aldehydes/ ketones | 1.65 ± 0.58 | 1.32 ± 0.23 | 0.67 ± 0.43 |
| γ-Butyrolactone | 0.46 ± 0.03 a | 0.45 ± 0.03 a | 0.27 ± 0.00 b |
| Guaiacol | 0.06 ± 0.00 b | 0.05 ± 0.00 c | 0.13 ± 0.00 a |
| Yeast Strains | Q1 | Q2 | Qt |
|---|---|---|---|
| CLI 1232 | 3.52 ± 0.07 a | 8.88 ± 0.93 a | 12.41 ± 0.85 a |
| 1-8B | 3.58 ± 0.51 a | 8.92 ± 0.12 a | 12.50 ± 0.64 a |
| S-04 | 3.79 ± 0.00 a | 8.37 ± 0.21 a | 12.15 ± 0.21 a |
| Parameters | 1 L Fermentation | 100 L Fermentation | ||||
|---|---|---|---|---|---|---|
| CLI 1232 | 1-8B | S-04 | CLI 1232 | 1-8B | S-04 | |
| Ethanol (% v/v) | 4.00 ± 0.16 bc | 4.23 ± 0.25 bc | 5.53 ± 0.05 a | 3.88 ± 0.69 bc | 3.38 ± 0.44 c | 4.62 ± 0.43 ab |
| Residual sugars (g L−1) | ||||||
| Maltotriose | 14.22 ± 1.78 a | 15.67 ± 0.04 a | 1.43 ± 0.08 d | 11.48 ± 1.07 b | 13.43 ± 2.06 ab | 7.74 ± 0.91 c |
| Maltose | 1.61 ± 0.22 c | 2.77 ± 1.10 c | 1.45 ± 0.01 c | 2.33 ± 0.84 c | 8.06 ± 1.02 a | 2.29 ± 0.20 b |
| Fructose | 0.18 ± 0.05 | 0.14 ± 0.00 | 0.18 ± 0.02 | 0.06 ± 0.04 | 0.14 ± 0.02 | 0.49 ± 0.17 |
| Glucose | 0.10 ± 0.00 c | 0.11 ± 0.01 c | 0.16 ± 0.00 b | 0.19 ± 0.04 b | 0.17 ± 0.03 b | 0.30 ± 0.10 a |
| Glycerol (g L−1) | 3.82 ± 0.43 bc | 5.01 ± 0.14 a | 3.43 ± 0.01 bc | 3.97 ± 0.53 b | 4.20 ± 0.32 ab | 2.93 ± 0.46 c |
| Lactic acid (mg L−1) | 1942.00 ± 58.00 ab | 1639.00 ± 28.00 b | 321.00 ± 21.00 c | 1930 ± 29.00 ab | 1785.00 ± 60.00 b | 337.00 ± 17.00 c |
| Colour (EBC) | 10.50 ± 1.50 a | 11.00 ± 0.00 a | 13.00 ± 0.00 a | 5.50 ± 1.50 b | 4.50 ± 0.50 b | 5.67 ± 1.15 b |
| Bitterness (IBU) | 12.75 ± 2.05 | 16.85 ± 1.65 | 16.50 ± 0.30 | 13.75 ± 1.95 | 14.75 ± 1.45 | 23.40 ± 3.33 |
| SO2 (mg L−1) | 1.15 ± 0.15 a | 1.25 ± 0.05 a | nd | 1.45 ± 0.45 a | 1.35 ± 0.05 a | 1.07 ± 0.12 a |
| VDKs (mg L−1) | 0.11 ± 0.06 b | 0.16 ± 0.03 b | 0.37 ± 0.11 a | 0.35 ± 0.05 a | 0.16 ± 0.11 b | 0.12 ± 0.03 b |
| Total higher alcohols (mg L−1) | 82.22 ± 4.01 d | 89.58 ± 1.06 cd | 139.08 ± 12.40 a | 110.97 ± 7.82 b | 106.14 ± 10.62 bc | 80.41 ± 2.39 d |
| Total esters (mg L−1) | 12.83 ± 0.63 a | 15.21 ± 0.34 a | 2.89 ± 0.11 d | 11.62 ± 0.71 a | 8.70 ± 0.54 b | 1.12 ± 0.10 e |
| Total fatty acids (mg L−1) | 4.22 ± 0.68 d | 5.31 ± 0.07 cd | 17.05 ± 0.15 a | 6.64 ± 0.64 b | 6.30 ± 0.20 bc | 17.01 ± 0.66 a |
| T. aldehyde/ketones (mg L−1) | 1.65 ± 0.41 a | 1.32 ± 0.16 ab | 0.67 ± 0.43 b | 1.67 ± 0.20 a | 1.51 ± 0.36 a | 1.01 ± 0.04 ab |
| γ-Butyrolactone (mg L−1) | 0.46 ± 0.02 a | 0.45 ± 0.02 a | 0.27 ± 0.00 bc | 0.31 ± 0.01 b | 0.29 ± 0.11 b | 0.15 ± 0.04 c |
| Guaiacol (mg L−1) | 0.06 ± 0.00 bc | 0.05 ± 0.00 c | 0.13 ± 0.00 a | 0.06 ± 0.01 bc | 0.05 ± 0.01 bc | 0.07 ± 0.02 b |
| Melatonin (ng mL−1) | nd | 47.78 ± 12.69 a | 20.41 ± 5.25 b | nd | 47.13 ± 7.91 a | 22.88 ± 3.08 b |
| Antioxidant capacity (Qt) (mmol TE L−1) | 12.41 ± 0.60 b | 12.50 ± 0.45 b | 12.15 ± 0.21 b | 12.51 ± 1.35 b | 11.91 ± 0.72 b | 10.05 ± 0.52 c |
| Sensory Attributes | Response (n = 142) | |
|---|---|---|
| Appearance | CLI 1232 | 1-8B |
| Foam consistency | ||
| Light | 82 | 55 |
| Fine | 3 | 15 |
| Medium | 6 | 21 |
| Persistent | 6 | 6 |
| Creamy | 3 | 3 |
| Visual impression | ||
| Very hazy | 15 | 16 |
| Hazy | 56 | 50 |
| Dull | 16 | 17 |
| Clear | 12 | 11 |
| Bright | 1 | 6 |
| Aroma | ||
| Aroma intensity | ||
| Low | 14 | 16 |
| Low–medium | 32 | 29 |
| Medium | 28 | 33 |
| Medium–high | 25 | 20 |
| High | 1 | 2 |
| Taste | ||
| Acidity | ||
| Low | 15 | 19 |
| Low–medium | 27 | 20 |
| Medium | 24 | 29 |
| Medium–high | 27 | 17 |
| High | 7 | 15 |
| Bitterness | ||
| Low | 45 | 47 |
| Low–medium | 25 | 26 |
| Medium | 18 | 13 |
| Medium–high | 10 | 8 |
| High | 2 | 6 |
| Mouthfeel body | ||
| Light | 13 | 16 |
| Light–medium | 42 | 40 |
| Medium | 34 | 32 |
| Medium–full | 9 | 10 |
| Full | 2 | 2 |
| Aftertaste intensity | ||
| Short | 2 | 1 |
| Short–medium | 16 | 18 |
| Medium | 30 | 31 |
| Medium–long | 40 | 36 |
| Long | 12 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postigo, V.; Esteban, S.; Arroyo, T. Lachancea thermotolerans, an Innovative Alternative for Sour Beer Production. Beverages 2023, 9, 20. https://doi.org/10.3390/beverages9010020
Postigo V, Esteban S, Arroyo T. Lachancea thermotolerans, an Innovative Alternative for Sour Beer Production. Beverages. 2023; 9(1):20. https://doi.org/10.3390/beverages9010020
Chicago/Turabian StylePostigo, Vanesa, Sergio Esteban, and Teresa Arroyo. 2023. "Lachancea thermotolerans, an Innovative Alternative for Sour Beer Production" Beverages 9, no. 1: 20. https://doi.org/10.3390/beverages9010020
APA StylePostigo, V., Esteban, S., & Arroyo, T. (2023). Lachancea thermotolerans, an Innovative Alternative for Sour Beer Production. Beverages, 9(1), 20. https://doi.org/10.3390/beverages9010020