Bottle Conditioning: Technology and Mechanisms Applied in Refermented Beers
Abstract
1. Introduction
2. Historical Aspects of Bottle Conditioning
3. Principles of Flavor Enrichment by Bottle Conditioning
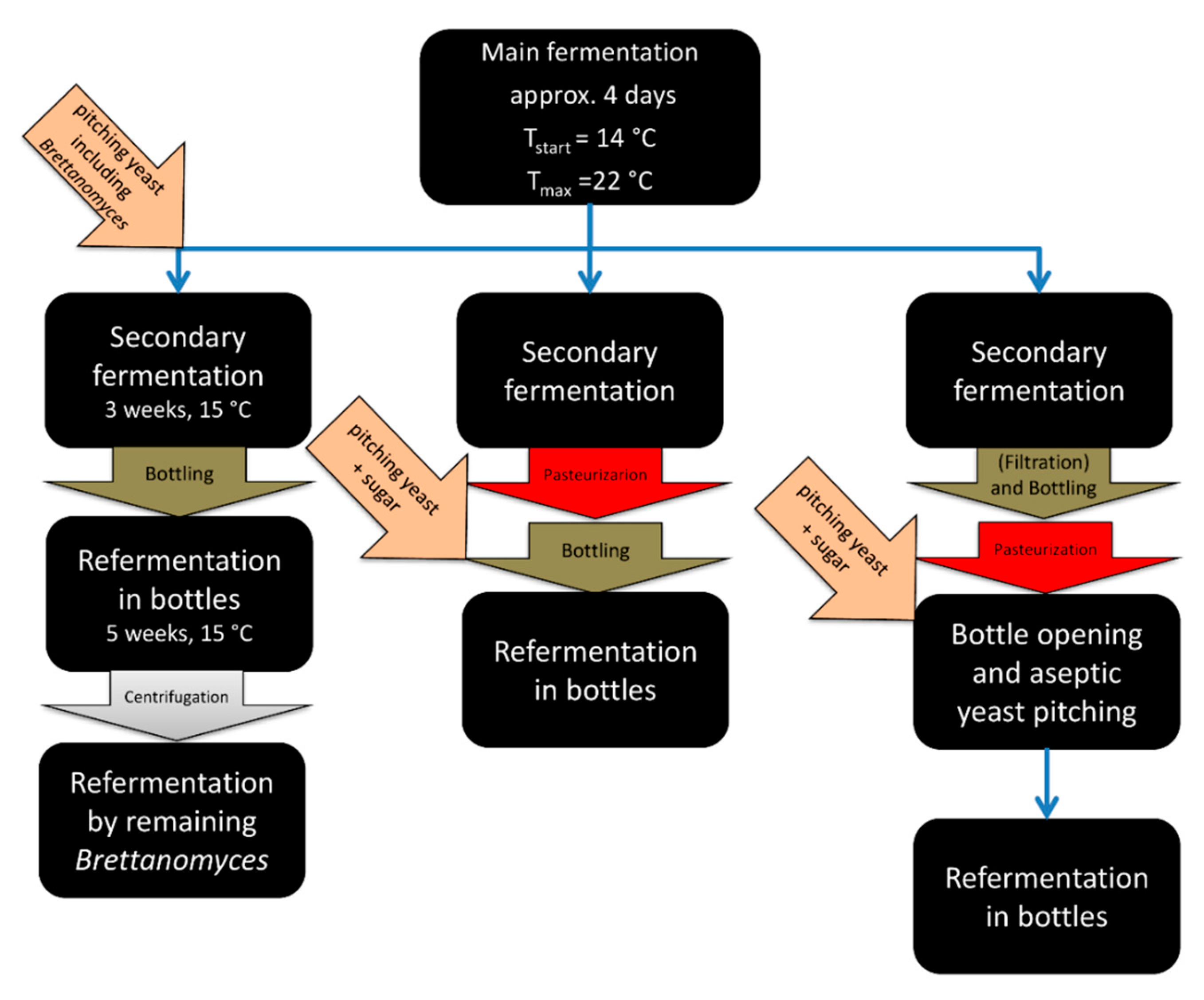
4. Conditioning Technology and Practices
5. Bottle Conditioning in the Presence of Mixed Microbial Cultures
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garavaglia, C.; Swinnen, J. Economics of the craft beer revolution: A comparative international perspective. In Economic Perspectives on Craft Beer, 1st ed.; Garavaglia, C., Swinnen, J., Eds.; Springer: New York, NY, USA, 2018; pp. 3–51. [Google Scholar]
- Elzinga, K.G.; Tremblay, C.H.; Tremblay, V.J. Craft beer in the United States: History, numbers, and geography. J. Wine Econ. 2015, 10, 242. [Google Scholar] [CrossRef]
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Daenen, L.; Saison, D.; De Schutter, D.; De Cooman, L.; Verstrepen, K.; Delvaux, F.; Derdelinckx, G.; Verachtert, H. Bioflavoring of beer through fermentation, refermentation and plant parts addition. In Beer in Health and Disease Prevention; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 33–49. [Google Scholar]
- Derdelinckx, G.; Neven, H.; Arnott, P.; Demeyer, I.; Delvaux, F. Belgian special beers: Refermented beers; white and wheat beers; amber and dark beers; spiced and hoppy beers. Cerevisia Biotechnol. 1995, 20, 67–73. [Google Scholar]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Improved flavor stability by aging beer in the presence of yeast. J. Am. Soc. Brew. Chem. 2011, 69, 50–56. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Berlowska, J.; Kregiel, D.; Rajkowska, K. Biodiversity of brewery yeast strains and their fermentative activities. Yeast 2015, 32, 289–300. [Google Scholar] [CrossRef]
- Liger-Belair, G. Wines: Champagne and sparkling wines–production and effervescence. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 526–533. [Google Scholar]
- Verachtert, H.; Derdelinckx, G. Belgian acidic beers: Daily reminiscences of the past. Cerevisia 2014, 38, 121–128. [Google Scholar] [CrossRef]
- Nelson, M. Celtic and Egyptian beer-production traditions and the origins of Western European monastic brewing. J. Mediev. Monast. Stud. 2018, 7, 47–77. [Google Scholar] [CrossRef]
- Moir, M. Hops—A millennium review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- Biendl, M.; Pinzl, C. Hops and health. MBAA TQ 2009, 46, 1–7. [Google Scholar] [CrossRef]
- DeLyser, D.Y.; Kasper, W.J. Hopped beer: The case for cultivation. Econ. Bot. 1994, 48, 166–170. [Google Scholar] [CrossRef]
- Wayens, B.; Van den Steen, I.; Ronveaux, M.-E. A short historical geography of beer. In Food and Environment: Geographies of Taste, 1st ed.; Montanari, A., Ed.; Società Geografica Italiana: Rome, Italy, 2002; pp. 93–114. [Google Scholar]
- Bamforth, C.W. BEERS|History and Types. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L., Finglas, P., Eds.; Academic Press: London, UK, 2003; pp. 418–422. [Google Scholar]
- Basařová, G. Profesor pražské techniky Carl Joseph Napoleon Balling (1805–1868). Kvas. Prum 2005, 51, 130–135. [Google Scholar] [CrossRef][Green Version]
- Šavel, J.; Košin, P.; Brož, A. Balling alcohol factors from the perspective of contemporary brewing. Kvas. Prum 2015, 61, 120–128. [Google Scholar] [CrossRef][Green Version]
- Pavsler, A.; Buiatti, S. Non-lager Beer. In Beer in Health and Disease Prevention; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 20. [Google Scholar]
- CAMRA’s Definition of Real Ale. Available online: https://s3-eu-west-1.amazonaws.com/www1-camra/wp-content/uploads/2019/04/14103840/CAMRA-Definition-of-Real-Ale-v.May2018.pdf (accessed on 6 June 2020).
- Basařová, G.; Šavel, J.; Basař, P.; Lejsek, T. Fermentation and Lagering. In Brewing, Theory and Practice of Beer Production, 1st ed.; VŠCHT Praha: Prague, Czech Republic, 2010; p. 385. [Google Scholar]
- Budvar Kroužek. Available online: https://www.budejovickybudvar.cz/sortiment/budvar-krouzek-3 (accessed on 25 July 2020).
- Twede, D. The cask age: The technology and history of wooden barrels. Packag. Technol. Sci. 2005, 18, 253–264. [Google Scholar] [CrossRef]
- Lowe, C.; Elkin, W. Beer packaging in glass and recent developments. J. Inst. Brew. 1986, 92, 517–528. [Google Scholar] [CrossRef]
- Chapman, N.G.; Lellock, J.S.; Lippard, C.D. Untapped: Exploring the Cultural Dimensions of Craft Beer; West Virginia University Press: Morgantown, WV, USA, 2017. [Google Scholar]
- Berning, J.; McCullough, M. Product line extension among New England craft breweries. Agric. Econ. Res. Rev. 2017, 46, 73–86. [Google Scholar] [CrossRef][Green Version]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Guido, L.; Rajendram, R.; Barros, A.A. Pitching Yeast and Beer Flavour. In Beer in Health and Disease Prevention; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 23–32. [Google Scholar]
- Hazelwood, L.A.; Daran, J.-M.; Van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Stewart, G.G. The production of secondary metabolites with flavour potential during brewing and distilling wort fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Individual differences in sensory threshold for aroma chemicals added to beer. Food Qual. Prefer. 1993, 4, 153–167. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Vanbeneden, N.; Daenen, L.; Delvaux, F.; Delvaux, F.R. Decrease of aged beer aroma by the reducing activity of brewing yeast. J. Agric. Food Chem. 2010, 58, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.; Kanauchi, M. Enzymology of vicinal diketone reduction in brewer’s yeast. J. Inst. Brew. 2004, 110, 83–93. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D. Brewing Yeasts and Fermentation; Blackwell: Oxford, UK, 2006; pp. 113–141. [Google Scholar]
- Nizet, S.; Gros, J.; Peeters, F.; Chaumont, S.; Robiette, R.; Collin, S. First evidence of the production of odorant polyfunctional thiols by bottle refermentation. J. Am. Soc. Brew. Chem. 2013, 71, 15–22. [Google Scholar] [CrossRef]
- Nizet, S.; Peeters, F.; Gros, J.; Collin, S. Odorant polyfunctional thiols issued from bottle beer refermentation. In Flavour Science; Elsevier: Amsterdam, The Netherlands, 2014; pp. 227–230. [Google Scholar]
- Belda, I.; Ruiz, J.; Navascués, E.; Marquina, D.; Santos, A. Improvement of aromatic thiol release through the selection of yeasts with increased β-lyase activity. Int. J. Food Microbiol. 2016, 225, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaegen, B.; Coghe, S.; Vanbeneden, N.; Van Landschoot, A.; Vanderhasselt, B. Yeasts as postfermentation agents in beer. Mon. Brauwiss. 2002, 55, 218–232. [Google Scholar]
- Daenen, L.; Saison, D.; De Cooman, L.; Derdelinckx, G.; Verachtert, H.; Delvaux, F. Flavour enhancement in beer: Hydrolysis of hop glycosides by yeast beta-glucosidase. Cerevisia 2007, 32, 24–36. [Google Scholar]
- Jin, H.; Rogers, P. Novel recovery of malt flavours from their glycosidically bound precursors. TQ MBAA 2000, 37, 79–83. [Google Scholar]
- Ferreira, C.S.; Bodart, E.; Collin, S. Why craft brewers should be advised to use bottle refermentation to improve late-hopped beer stability. Beverages 2019, 5, 39. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food. Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef]
- Lentz, M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef]
- Praet, T.; Van Opstaele, F.; Jaskula-Goiris, B.; Aerts, G.; De Cooman, L. Biotransformations of hop-derived aroma compounds by Saccharomyces cerevisiae upon fermentation. Cerevisia 2012, 36, 125–132. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.; Derdelinckx, G.; Verachtert, H. Bioflavoring and beer refermentation. Appl. Microbiol. Biotechnol. 2003, 62, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ormrod, I.; Lalor, E.; Sharpe, F. The release of yeast proteolytic enzymes into beer. J. Inst. Brew. 1991, 97, 441–443. [Google Scholar] [CrossRef]
- Chen, E.C.-H.; Jamieson, A.; Van Gheluwe, G. The release of fatty acids as a consequence of yeast autolysis. J. Am. Soc. Brew. Chem. 1980, 38, 13–18. [Google Scholar] [CrossRef]
- Neven, H.; Delvaux, F.; Derdelinckx, G. Flavor evolution of top fermented beers. TQ MBAA 1997, 34, 115–118. [Google Scholar]
- Vesely, P.; Volgyi, A.; Lusk, L.T.; Basarova, G.; Navarro, A. Impact of esterase activity in aseptically packaged, unpasteurized beer. TQ MBAA 2004, 41, 293–297. [Google Scholar]
- Lehnhardt, F.; Gastl, M.; Becker, T. Forced into aging: Analytical prediction of the flavor-stability of lager beer. A review. Crit. Rev. Food Sci. Nutr. 2004, 59, 2642–2653. [Google Scholar] [CrossRef]
- Kreim, J.; Stumpf, L.; Dobrick, S.; Hinrichs, J.; Pahl, R.; Brauer, J.; Schildbach, S. Enhancing flavour stability in beer using biological scavengers part 1: Methodology and preliminary trials. Mon. Brauwiss. 2018, 71, 12. [Google Scholar]
- Kuchel, L.; Brody, A.L.; Wicker, L. Oxygen and its reactions in beer. Packag. Technol. Sci. 2006, 19, 25–32. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Muller, R.; Walker, M. Oxygen and oxygen radicals in malting and brewing: A review. J. Am. Soc. Brew. Chem. 1993, 51, 79–88. [Google Scholar] [CrossRef]
- Ahrens, H.; Schröpfer, J.; Stumpf, L.; Pahl, R.; Brauer, J.; Schildbach, S. Enhancing flavour stability in beer using biological scavengers part 2: Screening of yeasts. Mon. Brauwiss. 2018, 71, 24. [Google Scholar]
- Saison, D.; Vanbeneden, N.; De Schutter, D.; Daenen, L.; Mertens, T.; Delvaux, F.; Delvaux, F. Characterisation of the flavour and the chemical composition of lager beer after ageing in varying conditions. Brew. Sci. 2010, 63, 41–53. [Google Scholar]
- Wietstock, P.C.; Kunz, T.; Methner, F.J. Relevance of oxygen for the formation of Strecker aldehydes during beer production and storage. J. Agric. Food Chem. 2016, 64, 8035–8044. [Google Scholar] [CrossRef]
- Bamforth, C.W. 125th Anniversary Review: The non-biological instability of beer. J. Inst. Brew. 2011, 117, 488–497. [Google Scholar] [CrossRef]
- Baert, J.J.; De Clippeleer, J.; Hughes, P.S.; De Cooman, L.; Aerts, G. On the origin of free and bound staling aldehydes in beer. J. Agric. Food Chem. 2012, 60, 11449–11472. [Google Scholar] [CrossRef]
- Derdelinckx, G.; Vanderhasselt, B.; Maudoux, M.; Dufour, J. Refermentation in bottles and kegs. A rigorous approach. Brauwelt Int. 1992, 2, 156–164. [Google Scholar]
- Verachtert, H.; Iserentant, D. Properties of Belgian acid beers and their microflora. The production of Gueuze and related refreshing acid beers. Cerevisia 1995, 20, 37–41. [Google Scholar]
- Van Landschoot, A.; Vanbeneden, N.; Vanderputten, D.; Derdelinckx, G. Effect of pitching yeast preparation on the refermentation of beer in bottles. Cerevisia 2004, 29, 140–146. [Google Scholar]
- Fels, S.; Reckelbus, B.; Gosselin, Y. Dried yeasts-A truly multifunctional product [for brewing]. Cerevisia 1999, 24, 17–20. [Google Scholar]
- Reckelbus, B.; Fels, S.; Gosselin, Y.; Debourg, A. Optimising the use of dried yeasts in breweries. TQ MBAA 2000, 37, 21–25. [Google Scholar]
- Muller, R.; Fels, S.; Gosselin, Y. Brewery fermentations with dried lager yeast. In Proceedings of the European Brewery Convention—the 26th Congress, Maastricht, The Netherlands, 1 January 1997; Van Wijngaarden, M., Ed.; Oxford University Press: Oxford, UK, 1998; pp. 431–438. [Google Scholar]
- Guldfeldt, L.; Piper, J. Yeast typing and propagation of dry brewing yeast cultures. TQ MBAA 1999, 36, 1–6. [Google Scholar]
- Spearot, J. Microscale and macroscale effect of the early pitching method on beer composition during the brewing process. TQ MBAA 2014, 51, 97–105. [Google Scholar] [CrossRef]
- Bayrock, D.; Ingledew, W. Mechanism of viability loss during fluidized bed drying of baker’s yeast. Food Res. Int. 1997, 30, 417–425. [Google Scholar] [CrossRef]
- Yüzgeç, U.; Türker, M. Comparison of different modeling concepts for drying process of baker’s yeast. IFAC Proc. Vol. 2009, 42, 816–821. [Google Scholar] [CrossRef]
- Hieronymus, S. Brew Like a Monk: Trappist, Abbey and Strong Belgian Ales and How to Brew Them; Brewers Publications: Boulder, CO, USA, 2005. [Google Scholar]
- Vanbeneden, N.; Vanderputten, D.; Vanderhaegen, B.; Derdelinckx, G.; Van Landschoot, A. Influence of the sugar composition of the added extract on the refermentation of beer in bottles. J. Am. Soc. Brew. Chem. 2006, 64, 206–213. [Google Scholar] [CrossRef]
- Liger-Belair, G. Uncorked: The Science of Champagne; Princeton University Press: Princeton, NJ, USA, 2013; pp. 37–58. [Google Scholar]
- Liger-Belair, G. Effervescence in Champagne and sparkling wines: From grape harvest to bubble rise. Eur. Phys. J. Spec. Top. 2017, 226, 3–116. [Google Scholar] [CrossRef]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef]
- Dysvik, A.; La Rosa, S.L.; De Rouck, G.; Rukke, E.-O.; Westereng, B.; Wicklund, T. Microbial dynamics in traditional and modern sour beer production. Appl. Environ. Microbiol. 2020, 86, e00566-20. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [PubMed]
- De Roos, J.; Van der Veken, D.; De Vuyst, L. The interior surfaces of wooden barrels are an additional microbial inoculation source for lambic beer production. Appl. Environ. Microbiol. 2019, 85, e02226-18. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Suzuki, N.; Washizu, Y. Microbial oxidation of alcohols by Candida boidinii: Selective oxidation. In ACS Symposium Series. Biotechnology for Improved Foods and Flavors; Takeoka, G.R., Teranishi, R., Williams, P., Kobayashi, A., Eds.; American Chemical Society: Washington, DC, USA, 1996; Volume 637, pp. 188–195. [Google Scholar]
- Cartledge, T.G. Substrate utilization, non-carbohydrate substrates. In Yeast Biotechnology; Berry, D., Russel, I., Stewart, G., Eds.; Allen and Unwin: London, UK, 1987; pp. 311–342. [Google Scholar]
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Spitaels, F.; Wieme, A.D.; Snauwaert, I.; De Vuyst, L.; Vandamme, P. Microbial ecology of traditional beer fermentations. In Brewing Microbiology: Current Research, Omics and Microbial Ecology; Bokulich, N., Bamforth, C., Eds.; Caister Academic Press: Poole, UK, 2017; pp. 179–196. [Google Scholar]
- Walker, G.M. Yeast metabolism. In Yeast Physiology and Biotechnology; John Wiley & Sons: Chicheter, UK, 1998; p. 229. [Google Scholar]
- Tataridis, P.; Kanelis, A.; Logotetis, S.; Nerancis, E. Use of non-Saccharomyces Torulaspora delbrueckii yeast strains in winemaking and brewing. Zb. Matice Srp. Prir. Nauk. 2013, 415–426. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii contribution in mixed brewing fermentations with different Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 259, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L. Occurrence and taxonomic characteristics of strains of Saccharomyces cerevisiae predominant in African indigenous fermented foods and beverages. FEMS Yeast Res. 2003, 3, 191–200. [Google Scholar] [CrossRef]
- Tonsmeire, M. American Sour Beer: Innovative Techniques for Mixed Fermentations; Brewers Publications: Boulder, CO, USA, 2014. [Google Scholar]
- Peyer, L.C.; Zarnkow, M.; Jacob, F.; De Schutter, D.P.; Arendt, E.K. Sour brewing: Impact of Lactobacillus Amylovorus Fst2. 11 on technological and quality attributes of acid beers. J. Am. Soc. Brew. Chem. 2017, 75, 207–216. [Google Scholar] [CrossRef]
- Bossaert, S.; Crauwels, S.; De Rouck, G.; Lievens, B. The power of sour—A review: Old traditions, new opportunities. Brew. Sci. 2019, 72, 78–88. [Google Scholar]
- Domizio, P.; House, J.; Joseph, C.; Bisson, L.; Bamforth, C. Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Callejo, M.; Navas, J.G.; Alba, R.; Escott, C.; Loira, I.; González, M.; Morata, A. Wort fermentation and beer conditioning with selected non-Saccharomyces yeasts in craft beers. Eur. Food Res. Technol. 2019, 245, 1229–1238. [Google Scholar] [CrossRef]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The microbial diversity of an industrially produced lambic beer shares members of a traditionally produced one and reveals a core microbiota for lambic beer fermentation. Food Microbiol. 2015, 49, 23–32. [Google Scholar] [CrossRef]
- De Roos, J.; Vandamme, P.; De Vuyst, L. Wort substrate consumption and metabolite production during lambic beer fermentation and maturation explain the successive growth of specific bacterial and yeast species. Front. Microbiol. 2018, 9, 2763. [Google Scholar] [CrossRef] [PubMed]
- Spitaels, F.; Van Kerrebroeck, S.; Wieme, A.D.; Snauwaert, I.; Aerts, M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. Microbiota and metabolites of aged bottled gueuze beers converge to the same composition. Food Microbiol. 2015, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.; Iserentant, D.; Verachtert, H. Microbiological aspects of a mixed yeast—Bacterial fermentation in the production of a special Belgian acidic ale. J. Inst. Brew. 1997, 103, 85–91. [Google Scholar] [CrossRef]
- Snauwaert, I.; Roels, S.P.; Van Nieuwerburgh, F.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. Microbial diversity and metabolite composition of Belgian red-brown acidic ales. Int. J. Food Microbiol. 2016, 221, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Beer | Yeast Cells (Million/mL) |
|---|---|
| Duvel | 1 |
| Orval | 3 |
| Rochefort | 1 |
| Westmale | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štulíková, K.; Novák, J.; Vlček, J.; Šavel, J.; Košin, P.; Dostálek, P. Bottle Conditioning: Technology and Mechanisms Applied in Refermented Beers. Beverages 2020, 6, 56. https://doi.org/10.3390/beverages6030056
Štulíková K, Novák J, Vlček J, Šavel J, Košin P, Dostálek P. Bottle Conditioning: Technology and Mechanisms Applied in Refermented Beers. Beverages. 2020; 6(3):56. https://doi.org/10.3390/beverages6030056
Chicago/Turabian StyleŠtulíková, Kateřina, Jan Novák, Jakub Vlček, Jan Šavel, Petr Košin, and Pavel Dostálek. 2020. "Bottle Conditioning: Technology and Mechanisms Applied in Refermented Beers" Beverages 6, no. 3: 56. https://doi.org/10.3390/beverages6030056
APA StyleŠtulíková, K., Novák, J., Vlček, J., Šavel, J., Košin, P., & Dostálek, P. (2020). Bottle Conditioning: Technology and Mechanisms Applied in Refermented Beers. Beverages, 6(3), 56. https://doi.org/10.3390/beverages6030056






