Abstract
The partial substitution of barley malt has been one of the main strategies of breweries to reduce production costs. In Brazil, as in other countries, maize is a raw material that is used mostly for this purpose. Aiming for strategic cost management, some large breweries have adopted a reduction in the time and energy costs of the process. As an example, changes have been made to the traditional mashing curves by reducing the times or omitting the proteolytic step. The objective of this work was to compare the physical and chemical aspects of sweet and bitter worts prepared through experimental design with and without the addition of maize as adjunct, as well as the execution or not of the proteolytic step in mashing. Color, total acidity, extract, total reducing sugars, total phenolic compounds, and proteins content were evaluated. In addition, the antioxidant activity was determined. The wort obtained with maize and not submitted to the proteolytic step presented a bright color as well as reduced phenolic compounds, protein, and antioxidant activity. Comparatively, the action of proteases in the wort resulted in a greater release of total reducing sugars, a more intense color, and a higher content of total nitrogen and phenolic compounds. The results indicate that despite being an alternative to reduce costs in the brewing process, the use of the adjunct and the exclusion of the proteolytic step of mashing will imply a poor wort in regards to nutrition, which can compromise the activity of the yeast during the process and therefore affect the quality of the final product.
1. Introduction
Beer is an alcoholic beverage obtained from the fermentation of wort prepared with water, barley malt, and hops [1]. Water is the raw material used in the greatest quantity and its quality is independent of the locality of origin, since it can be treated in the brewery itself to adapt to the needs of the brewing stages and fermentation, which is considered the most important function for the final quality of the drink [2,3]. On the other hand, barley and hops are dependent on the soil type and climate, which restricts cultivation to some regions of the planet. Therefore, many countries are dependent on the importation of these raw materials. In Brazil, the third largest beer producer in the world, all hops and a large portion of barley malt are imported, since domestic production is inadequate to meet the brewing market’s demands [4,5]. As a result, the cost of beer is greatly affected by variations in exchange taxes as well as import taxes.
The need for large quantities of barley for brewing makes breweries, whether small, medium, or large producers, adopt cost reduction strategies, such as the partial substitution of barley malt with adjuncts, defined as complementary sources of carbohydrates [6,7]. For this purpose, domestic raw materials, available in abundance and at a lower cost, may be used, respecting current legislation. In general, large national breweries use maize, rice, or high-maltose syrup as a partial substitute for barley malt [8].
Another characteristic of the brewing process is the mashing step, in which the cereals (barley malt and possible adjuncts) are added with water and the mixture is submitted to successive heating with rest periods at predetermined temperatures. Therefore, adequate enzymatic action occurs for the hydrolysis of the main macromolecules present in the cereal, such as glucans, proteins, and starch [9]. This stage demands time as well as high energy costs. Due to this, another strategy to minimize productions cost is the reduction of the mashing time [10]. The suppression of the proteolytic step is the most common practice, so that the mixture does not rest at temperatures between 45 and 55 °C, thus reducing the processing time and energy expenditure [11,12].
It is known that the proteolytic enzymes of malt act on proteins and polypeptides and in turn the nitrogen content of wort and beer is an important factor for several characteristics of the beverage, such as foaming, texture, CO2 retention, and colloidal stability, rather than microbial nutrition during fermentation [13]. In previous studies, the influence of the proteolytic step and/or the addition of exogenous proteolytic enzymes for supplementation during mashing on the parameters of the wort have been evaluated [14,15].
The objective of this work was to evaluate the influence of the use of maize as a partial substitute for barley malt as adjunct and the influence of the proteolytic step in the mashing stage on the quality and composition of the brewer’s wort.
2. Materials and Methods
2.1. Materials
For preparation of the brewer’s wort, the following raw materials were used: (i) Potable water from a public supply service (CEDAE/Rio de Janeiro/Brazil), previously filtered in active charcoal for dechlorination; (ii) commercial Pilsner malt (Agrária/Paraná/Brazil); (iii) Kimilho (Yoki®) maize grains from a local supermarket, used as adjunct; and (iv) hops in pellets (Hallertau Perle, HGV®, Germany, 9.7% α-acids).
2.2. Experimental Design and Wort Production
The worts were produced in order to obtain typical Pilsner wort in beer production, with a original extract equal to or greater than 10.5 °Plato and less than 12.5 °Plato; an alcohol content between 2.8% and 4.2% ABV (alcohol by volume); and bitterness of 12 IBU (International Bitterness Units).
The use of maize as adjunct and the proteolytic step in the mashing were the variables adopted and varied according to the factorial experimental design (2k) shown in Table 1. The addition of maize was evaluated according to Brazilian legislation [16], between 0% and 45% relative to the total barley malt used. The proteolytic step on mashing was conducted at optimal malt protease performance temperatures, 45 and 55 °C, for 15 min each.

Table 1.
Experimental planning for the production of brewer’s worts.
The malt was milled in a disc mill (Guzzo®, Rio Grande do Sul, Brazil) adjusted to 1 mm. The mashing was conducted in beckers with a capacity of 2.0 L, in a thermostatic bath (NEW ethics) to control the heating ramps, and agitated by a propeller stirrer (Fisatom, 713D). The barley malt was mixed with water at a ratio of 1:4 (200 g and 800 mL) as described by Dragone and Silva [17]. For the wort prepared with adjunct, the same ratio as malt/adjunct:water (1:4) was used, with a mass ratio of malted barley and maize at 55% and 45%, respectively. However, the starch of the maize grains was gelatinized separated in a suspension with water [18,19], by heating to 60 °C [20] until gelatinization. Figure 1 and Figure 2 shows the mashing curves used. Heating ramps of at 1 °C/min were used and mashout was determined by the iodine test [21].
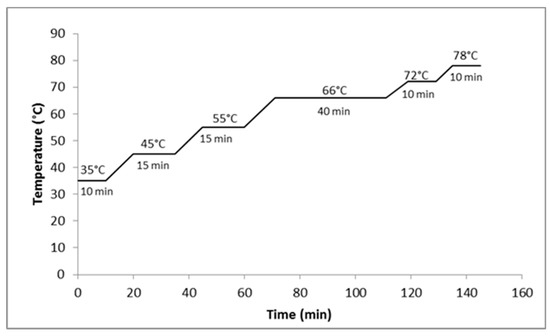
Figure 1.
Mashing profile with the proteolytic step.
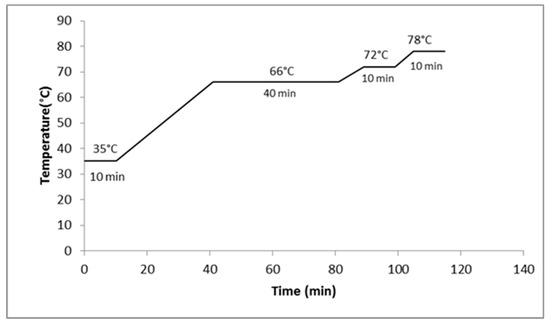
Figure 2.
Mashing profile without the proteolytic step.
After mashing, the wort was drained through a vessel with a false bottom (0.6 mm diameter) using the malt husk as a filtration medium. Then, the solid residue was washed twice with 800 mL of 78 °C water. This is the sweet wort, from which samples were taken and frozen for later analytical determinations. Once lautered, the wort was boiled for 60 min, with two steps of hop addition. Finally, the hopped wort was filtered for the removal of hot trub. This is the bitter wort, from which samples were taken and frozen for later analytical determinations.
Sweet and bitter wort were analyzed separately in order to evaluate the effects of the experimental design parameters on their composition. Also, how this composition influences the subsequent boiling step was also evaluated, since different sugar and protein compositions result in different parallel reactions during heating, such as non-enzymatic browning reactions.
2.3. Analytical Determinations
Both samples of sweet and hopped wort, previously centrifuged (10 g force/5 min), were analyzed for: Original extract (°P); color (Colorimeter Comparator 3000—AF607, EBC); total acidity by titration with 0.1 M NaOH [22]; pH by direct determination in a bench pHmeter (QUIMIS); total reducing sugar content (TRS) by the DNS method [22]; total nitrogen content by the Kjeldhal method [22]; content of total phenolic compounds expressed as mg of gallic acid/L by the Folin–Ciocalteau spectrophotometric technique [23]; antioxidant activity expressed in TEAC (trolox equivalent antioxidant capacity), in μmol TEAC/100 g of the sample; and inhibitory concentration, IC50, determined by the DPPH method [24,25].
All analyses were performed in triplicate and the results are presented as the mean and standard deviation (SD). An analysis of variance (ANOVA) was performed to evaluate the differences between them and the means values were evaluated by Tukey’s test for means comparison using Statistica 10 software. In addition, the effect sizes of each parameter on the response variables were calculated.
3. Results and Discussion
3.1. Physico-Chemical Parameters
Table 2 shows the mean values and deviations of the extract, acidity, color, total reducing sugar content (TRS), total phenolic content, and protein content for sweet and bitter worts for the four formulations carried out following the full factorial design (Table 1). Table 3 shows the effects of each independent variable (proteolytic step and adjunct addition) for each response parameter for sweet and bitter worts, indicating the magnitude of the influence and whether it is positive or negative.

Table 2.
Mean values and standard deviation of the parameters evaluated.

Table 3.
Parameter effects on response variables.
The 95% confidence level ANOVA indicated that for all parameters evaluated, there was a difference between treatments (p < 0.05) for both sweet and bitter worts. Thus, it is known that the chosen parameters (proteolytic step during mashing and the use of adjunct) interfere in the composition of the wort produced. In addition, the results of the Tukey’s test of comparison of means are presented, which allowed significant differences between treatments to be identified.
It is observed that only experiment C1 (without the addition of adjunct and without proteolytic step) resulted in a wort that is capable of producing a beer classified as extra beer, according to current legislation [16], as a function of the original extract (°P of the bitter wort) between 12.5 and 14 °P (14.88 ± 1.24). For the other worts, a content higher than 15 °P of the extract would lead to an extra-strong beer rather than a Pilsen style.
In the C2 (without adjunct) and C4 (with adjunct) worts, which included the proteolytic step during mashing, a higher extract content was observed compared to the C1 and C3 worts (without the proteolytic step) (Table 2). It is known that endosperm, the fraction of barley grain rich in starch, is enveloped by a matrix of insoluble structural proteins called aleurone, which must be degraded in order to facilitate amylases activity [26,27]. This proteolytic action promotes greater exposure of starch molecules, with a greater release of soluble material at the end of the mashing.
Regarding the use of adjunct, for the pairs of C1/C3 and C2/C4, which differ by adjunct addition, no significant increase was observed for the extract content for sweet and bitter wort for the different treatments (Table 2) when wort was added with maize for the substitution of barley malt. Although maize has a higher content of starch in its composition, its low diastase power compromises the efficiency of the hydrolysis, limiting the content of the extract obtained in the wort. Therefore, maize, which is a cheaper alternative for the partial substitution of barley, should have its use evaluated well because of the decrease of the product quality.
The statistical similarity between the extract contents of worts C2 and C3 and worts C3 and C4 indicates that, in terms of extract (and disregarding other must characteristics), the omission of the proteolytic step on mashing or the use of adjuncts may be parameters that can be used to reduce processing costs without a significant effect on mash efficiency. However, when the two parameters are used together (wort C1), the extract content decreases significantly.
It can be observed in Table 3 that for extract content, the effect is positive for both the proteolytic step and adjunct use. However, for the first case, the effect values are higher, corresponding to up to 20% of the average extract value for the four treatments, in both sweet must and bitter worts.
The presence of malt compounds, such as phenolic acids, only slightly affects pH, because they are weak organic acids; however, it promotes increased titratable acidity, as observed for barley worts C1 and C2 (Table 2) in relation to worts C3 and C4. The analysis of the effects corroborates this hypothesis (Table 3), where the negative effects of adjunct addition on wort acidity are indicated. Furthermore, the proteolytic step does not have a significant effect on the acidity of the wort, since the type of material used does not change, except for wort C4, for which the longest contact with malt husk during mashing may have compensated for the use of adjuncts. Bitter worts have a higher acidity in relation to the respective sweet worts due to the concentration by evaporation and due to the addition of hops (rich in acid compounds), as well as the occurrence of the Maillard reaction (between amino acids and reducing sugars) during boiling, which, according to Bobbio and Bobbio [28], promotes the release of acid compounds.
A significant increase in wort color was observed (Table 2) comparing the C1/C3 pair with the C2/C4 pair, as a function of the proteolytic step on mashing. This result may be due to different factors, such as the increase of the mashing time (in 30 min), which increases the extraction of malt compounds; the release of a higher amino acid content, which participates in the Maillard reaction when in the presence of reducing sugars at high temperatures with the formation of color compounds [29]; and the possible release of reducing sugars in a greater quantity (as will be discussed later), due to the improved activity of amylolytic enzymes, promoting caramelization of sugars and thte Maillard reaction.
However, when maize is introduced as an adjunct, there is a significant reduction in the wort color (Table 2), when comparing C1/C2 pairs with C3/C4 pairs. Although several factors are associated with this, it is important to highlight the reduction in the concentration of color compounds extracted from the malt. Steiner and collaborators [30] observed that the presence of the adjunct, even though it is rich in nitrogen but not malted (such as barley malt), promotes a decrease in color, confirming the hypothesis that the kilning stage of the malt grains influences the formation of the color compounds. Additionally, the introduction of maze minimizes the occurrence of browning reactions (Maillard) during heating because it is a poor cereal regarding protein content.
Table 3 shows the positive effect values of the proteolytic step and the negative effect values of the use of adjuncts on the color of the worts, the latter having a greater magnitude. In general, the color of the bitter worts is more intense in relation to the sweet worts (Table 2) because of the concentration and occurrence of parallel reactions during the boiling stage, such as caramelization and the Maillard reaction.
It was observed that the proteolytic step favored a significant increase of the TRS concentration in the worts (Table 2), noting C2 and C4 worts, which corroborates the previously established hypothesis for the extract results. The proteolytic step promotes the hydrolysis of proteins that involve the starch fraction, increasing its exposure for enzymatic action. This proposition is further confirmed by the results obtained by Hu and collaborators [31], who show that proteolysis has a great impact on the degradation of starch, including the sugar content of the resulting wort. Table 3 shows the positive effects for the proteolytic step on the TRS content.
The introduction of maize as an adjunct had small effects on the sugar content of sweet or bitter worts (Table 3), similar to the results obtained for the extract content. This can be seen by the similarity between the TRS contents of C1 and C3 musts and C2 and C4, in pairs (Table 2). This validates the hypothesis that although maize has a higher content of starch in its composition, there is a loss of total diastatic power in the mixture. This reduces the efficiency of the hydrolysis of the starch during mashing. The statistical equality of the TRS values for worts C2 and C4 indicates that performing the proteolytic step during mashing possibly compensates for the addition of adjunct devoid of enzymatic activity. Additionally, although maize has no significant relevance over an increase in the amount of TRS present in the wort, its use still provides a reduction in process costs, because it is cheaper than barley malt. Still, observing Table 3, it is noted that the proteolytic step has an up to four times greater effect on the TRS content than the use of adjunct in substitution for barley malt.
Worts produced exclusively with barley malt, C1 and C2, presented the highest levels of total phenolic compounds compared, respectively, with C3 and C4 worts (Table 2). Negative and expressive effects are presented in Table 3 for adjunct use on the phenolic compound content. This can be explained by the fact that the main phenolic compounds present come from barley husk (which supplies about 70% to 80% of the total wort), even though some of it comes from hops (accounting for 20 to 30% of the phenols) [32]. Maize, on the other hand, is composed of a low phenolic compound content. Studies of bioactive compounds performed by Paraginski and collaborators [33] demonstrate that the content of total phenolic compounds in maize grains was 1.77 mg GAE.g−1. According to Rocha and collaborators [34], barley malt grains contain significantly larger amounts of phenolic compounds, reaching up to 440.27 mg GAE.g−1.
On the other hand, a positive effect of the proteolytic step on the total phenolic compound content was observed (Table 3). The explanation is that a longer contact time (30 min or more) during the sampling increases the extraction of phenolic compounds from the husk of the grains [35]. Corroborating this hypothesis, comparing C1 (without adjunct and without the proteolytic step) and C4 (with adjunct and proteolytic step) worts, sweet worts have statistically similar values, indicating that the longer contact time with barley husk is important for phenolics extraction. For C4 bitter wort, the greatest drop in its content may be related to the complexation of phenolics compounds with high molar mass proteins (due to a lack of adequate hydrolysis), as will be proposed below.
It is worth mentioning that Gonzalez San José and collaborators [36] affirm that the release of phenols is directly related to the color of the wort and beer, which is consistent with the results of a more intense color for worts C1 and C2.
In addition to the phenolic compounds content, it was observed that the addition of adjunct also has a negative effect on the protein content in worts (Table 3). The worts C1 and C2, produced only with barley malt, have a higher protein content compared to their respective pairs, C3 and C4, which use maize as an adjunct (Table 2). The nitrogen content in the sweet wort is intrinsically related to the wort profile and the type of adjunct used. Kirsop [37] mentions that worts obtained only from barley malt have excess nitrogenous components, and that generally problems related to nitrogen deficiency are more frequent since the percentage of malt substitution by starchy adjuvants increases.
In general, a great part of the nitrogenous supply comes from malted cereals while maize, as used in this work, reduces this amount, due to the low protein content in its composition (9.8%) [5]. Although the protein content of maize is not so low in relation to barley’s protein content, the absence of an enzymatic content, including proteases, can compromise the hydrolysis of these molecules, which makes them more susceptible to precipitation during the boiling step, being removed from the wort. It should still be mentioned that this protein content refers to unprocessed whole maize grain and that it is common in the industry for gritz to be used as adjunct [38], which presents about 8% of proteins [39]. In this work, processed grains (flakes) were used, which, according to the producer, have an even lower protein content of 5.6%.
The execution of the proteolytic step promoted an increase of the total nitrogen content present in the sweet wort (Table 3). C2 wort had a higher nitrogen content than C1 wort and the same can be observed for the C4/C3 pair (Table 2). Protease action during wort production promotes a greater release of amino acids and low molar mass proteins and peptides, which are extracted for the soluble fraction, whereas unhydrolyzed proteins of high molar mass can be retained in malt bagasse during wort clarification.
Again, the similarity between the values of C1 and C4 bitter worts indicate the possibility that the execution of the proteolytic step compensates for the introduction of adjunct, although the C2 wort (without adjunct and with the proteolytic step) presented the highest protein content of all the experiments.
According to Kunze [40], wort has at least 0.5% of nitrogen sources, and similar values were obtained for C1 and C4 worts. For C2 wort, higher values were obtained, while for C3 wort, values were lower than the ideal. The amount and presentation form of nitrogen sources in the wort (amino acids; low, medium, and high molar masses) influences several sensory characteristics of the beverage. Specifically, the amino acid and peptide content have a positive effect on the metabolism of brewer’s yeast, as a low availability of these compounds promotes metabolic alterations, resulting in the generation of undesirable by-products during the fermentation stage [40]. On the other hand, high molar mass proteins influence foaming, texture, CO2 retention, and colloidal stability [13]; in addition, they also complex with each other, with the formation of the so-called hot trub, which is insoluble and precipitates, requiring removalfrom wort [41].
3.2. Evaluation of Antioxidant Activity
Table 4 shows the mean values of the IC50 results for the bitter worts samples calculated from the antioxidant activity (AA%), as determined by the DPPH method.

Table 4.
Antioxidant activity (%) and inhibitory concentration (IC50) of the bitter wort samples.
The percentage of antioxidant activity (% AA) corresponds to the amount of DPPH consumed by the antioxidant agent; and the concentration of antioxidant necessary to reduce the initial concentration of DPPH by 50% is called an “efficient concentration” or “inhibitory concentration” (IC50) [25]. The samples with the highest inhibitory concentration (IC50) are the ones with the lowest antioxidant activity (radical sequestering capacity) when compared to the other samples.
Table 4 shows that at low wort concentrations (10 and 25 µL/mL), no statistical differences were found between the antioxidant activity of the different treatments. However, as there is an increase in the concentration of wort used in the determination, there are significant differences. For the last concentration analyzed (100 µL/mL), it was observed that the proteolytic step promoted an increase in antioxidant activity (comparing C1 and C2 worts and C3 and C4 worts in pairs). Already, the use of maize as an adjunct promoted a reduction of this activity (comparing the worts C1 and C3 and the worts C2 and C4, in pairs). For all concentrations analyzed, wort C3 presented the lowest values of antioxidant activity, initiating the negative effect of both the omission of the proteolytic step and the use of adjunct. In contrast, the highest antioxidant activity values were obtained for the C2 wort, with the proteolytic step in the mash and adjunct introduction. Regarding the calculated IC50 values, the sample of bitter wort with the highest effective concentration required to sequester 50% of the free radicals in milligrams per liter of wort was C3 (64.66 mg/L), and the one with the lowest concentration was the C2 wort (37.16 mg/L).
A correlation between the antioxidant activity of the wort and the content of total phenolic compounds (Table 2) was also observed; the sample of the bitter wort C2, which presented the highest content of phenolic compounds (653.9 mg GAE/L), was the one with the highest antioxidant capacity. This is due to the large amount of phenolic acids present in the sample acting as antioxidant agents. Beer is a beverage that contains many components, including folic acid, B vitamins (B1, B2, B12), and polyphenols, which are known to be natural antioxidants, derived from malts and hops. It can be observed that the introduction of maize as an adjunct promotes its total phenolics content and antioxidant activity reduction, by comparing C3 and C4 worts with C1 and C2 worts, respectively. This is due to the reduction in the amount of barley malt (cereal containing a husk rich in phenolic compounds) added to the preparation of the wort, which aligns with the fact that maize is poor in antioxidant compounds [36].
Despite the adjunct use, the proteolytic step increased IC50 values, since these values were lower for C2 and C4 worts in relation to their respective pairs, C1 and C3. However, these values were not significantly different between C1 and C2 worts, but between C4 and C3 worts, indicating a possible interaction between the variables. That is, the proteolytic step compensates for the use of maize as an adjunct, increasing the antioxidant capacity of the wort. This is because of the longer contact time between the cereal husk and the wort, increasing the extraction of compounds favorable to an increase of antioxidant activity.
4. Conclusions
The results obtained allow us to conclude that the proteolytic step had a positive effect on the original extract and the content of TRS, color, and total nitrogen content, as well as on the content of phenolic compounds and antioxidant activity of sweet and bitter worts. Although the elimination of this step leads to a cost reduction for the process, the quality of the beverage may be impaired. In addition, the introduction of maize as an adjunct to starch, with a reduction in the barley malt amount, promoted a reduction of the color intensity, total nitrogen content, total phenolic compounds content, and antioxidant activity of the worts. So, although the use of this adjunct can reduce production costs, it negatively affects the characteristics of the final product.
Author Contributions
T.R.d.S.M. conceived and designed the experiments and wrote the paper; L.M.M. collaborated to perform the experiments; E.F.C.S. contributed reagents/materials the analysis of the results.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing Science and Practice; CRC Press LLC and Woodhead Publishing Limited: Boca Raton, FL, USA, 2004; 863p. [Google Scholar]
- Fillaudeau, L.; Bories, A.; Decloux, M. Brewing, winemaking and distilling: An overview of wastewater treatment and utilisation schemes. In Handbook of Water and Energy Management in Food Processing; Klemes, J., Smith, R., Kim, J.K., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Pires, E.; Brányik, T. Biochemistry of Beer Fermentation; Springer: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA, 2015. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Agribusiness Handbook: Barley Malt Beer; FAO: Roma, Italy, 2009. [Google Scholar]
- D’Avila, R.F.; Luvielmo, M.M.; Mendonça, C.R.B.; Jantzen, M.M. Adjuntos utilizados para produção de cerveja: Características e aplicações. Estudos Tecnol. Eng. 2012, 8, 60–68. [Google Scholar] [CrossRef][Green Version]
- Bamforth, C.W. Adjuncts. In The Oxford Companion to Beer; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Vasconcelos, Y. Innovations in brewing. Revista Pesquisa Fapesp 2017, 251, 19–25. [Google Scholar]
- Carvalho, L.G. Dossiê Técnico: Produção de Cerveja; REDETEC: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- Gomaa, A.M. Application of Enzymes in Brewing. J. Nutr. Food Sci. Forecast 2018, 1, 5. [Google Scholar] [CrossRef]
- Muster-Slawitsch, B.; Hubmann, M.; Murkovic, M.; Brunner, C. Process modelling and technology evaluation in brewing. Chem. Eng. Process. Process Intensif. 2014, 84, 98–108. [Google Scholar] [CrossRef]
- Denault, L.J.; Glenister, P.R.; Chau, S. Enzymology of the Mashing Step During Beer Production. J. Am. Soc. Brew. Chem. 1981, 39, 46–52. [Google Scholar] [CrossRef]
- Palmer, J.J. How to Brew, 4th ed.; Brewers Publications: Boulder, CO, USA, 2015. [Google Scholar]
- Mathias, T.R.S.; Mello, P.P.M.; Sérvulo, E.F.C. Nitrogen compounds in brewing wort and beer: A review. J. Brew. Distill. 2014, 5, 10–17. [Google Scholar] [CrossRef]
- Mathias, T.R.S.; Lopes, M.C.R.D.; Oliveira, C.A.; Carvalho, R.C.; Marques, F.F.C.; Sérvulo, E.F.C. Influence of mashing profile curve and addition of proteases on the composition of the wort and beer. MOJ Food Process. Technol. 2017, 5, 124. [Google Scholar] [CrossRef]
- Carvalho, R.C.; Mathias, T.R.S.; Netto, A.D.P.; Marques, F.F.C. Direct determination of amino acids in brewery worts produced by different processes by capillary zone electrophoresis. Electrophoresis 2018, 39, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Decreto n° 6.871. Ministério da Agricultura, Pecuária e Abastecimento 2009. Available online: http://www.agricultura.gov.br/assuntos/vigilancia-agropecuaria/ivegetal/bebidas-arquivos/decreto-no-6-871-de-04-de-junho-de-2009.pdf/view (accessed on 8 November 2019).
- Dragone, G.; Silva, J.B.A. Cerveja. In Bebidas. V1: Bebidas Alcoólicas Ciência e Tecnologia; Venturini-Filho, W.G., Ed.; Blucher: São Paulo, Brazil, 2010. [Google Scholar]
- Varnam, A.H.; Sutherland, J.P. Bebidas: Tecnología, Química e Microbiologia; Acribia: Zaragoza, Spain, 1997; 500p. [Google Scholar]
- Perpete, P.; Collin, S. How to improve the enzymatic wort flavor reduction in a cold contact fermentation. Food Chem. 2000, 70, 457–462. [Google Scholar] [CrossRef]
- Ciacco, C.F.; Cruz, R. Fabricação do amido e sua utilização; Secretaria da Indústria, Comércio, Ciência e Tecnologia: São Paulo, Brazil, 1982; Volume 7. [Google Scholar]
- Petkwics, C.L.O. Bioquímica: Aulas Práticas, 7th ed.; UFPR: Curitiba, Brazil, 2007. [Google Scholar]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1975. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybidic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sousa, M.P.; Matos, M.E.O.; Matos, F.J.A.; Machado, M.I.L.; Craveiro, A.A. Constituintes Químicos Ativos e Propriedades Biológicas de Plantas Medicinais Brasileiras; UFC: Fortaleza, Brazil, 2004. [Google Scholar]
- Lewis, M.J.; Young, T.W. Brewing, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Limberger, V.M.; Francisco, A.; Borges, M.R.; Oro, T.; Ogliari, P.J.; Scheuer, P.M.; Noronha, C.M. Extração de B-Glucanas de Cevada e Caracterização Parcial do Amido Residual. Ciência Rural. 2011, 41, 2217–2223. [Google Scholar] [CrossRef]
- Bobbio, F.O.; Bobbio, P.A. Introdução à Química de Alimentos, 2nd ed.; Livraria Varela: São Paulo, Brazil, 1995. [Google Scholar]
- Hough, J.S. Biotecnología de la Cerveza y de la Malta; Acribia: Zaragoza, Spain, 1990. [Google Scholar]
- Steiner, E.; Auer, A.; Becker, B.; Gastl, M. Comparison of beer quality attributes between beers brewed with 100% barley malt and 100% barley raw material. J. Sci. Food Agric. 2012, 92, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dong, J.; Fan, W.; Yu, J.; Yin, H.; Huang, S.; Liu, J.; Huang, S.; Zhang, X. The influence of proteolytic and cytolytic enzymes on starch degradation during mashing. J. Inst. Brew. 2014, 120, 379–384. [Google Scholar] [CrossRef]
- Freitas, G.L.; Kuskoski, E.M.; Gonzaga, L.; Fett, R. Avaliação da atividade antioxidante de diferentes cervejas aplicando os métodos ABTS e DPPH. Braz. J. Food Nut. 2006, 17, 303–307. [Google Scholar]
- Paraginski, R.T.; Talhamento, A.; Oliveira, M.; Elias, M.C. Efeitos da temperatura nas alterações do teor de compostos com potencial antioxidante em grãos de milho durante o armazenamento. Rev. Bras. Prod. Agroind. 2015, 17, 159–167. [Google Scholar] [CrossRef]
- Rocha, R.F.R.M. Monitorização de Parâmetros Físico-Químicos do Grão de Cevada/Malte ao Longo do Processo de Maltagem. Master’s Thesis, Faculdade de ciências, Universidade do Porto, Porto, Portugal, 2014. [Google Scholar]
- Curi, R.A. Produção de Cerveja Utilizando Cevada Como Adjunto de Malte. Ph.D. Thesis, USP, São Paulo, Brazil, 2009. [Google Scholar]
- Gonzalez San José, M.L.; Muniz Rodriguez, P.; Vall Bellés, V. Actividad antioxidante de la cerveza: Estúdios in vitro e in vivo. Cerveza Y Salud 2001, 154, 47–54. [Google Scholar]
- Kirsop, B.H. Developments in beer fermentation. Top. Enzyme Ferment Biotechnol. 1982, 6, 79–131. [Google Scholar]
- Reinold, M.R. Manual Prático de Cervejaria; Aden Editora: São Paulo, Brazil, 1997. [Google Scholar]
- Agraria. 2014. Available online: http://www.agraria.com.br/grits_flakes_produtos.php (accessed on 13 June 2018).
- Kunze, W. Technology Brewing and Malting, 2nd ed.; VLB: Berlin, Germany, 1999. [Google Scholar]
- Barchet, R. Hot Trub: Formation and removal. Brew. Tech. 1993, 1. Available online: http://www.morebeer.com/brewingtechniques/library/backissues/issue1.4/barchet.html (accessed on 8 November 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).