Quality Green Tea (Camellia sinensis L.) Clones Marked through Novel Traits
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
2.3. Statistical Analysis
3. Results and Discussions
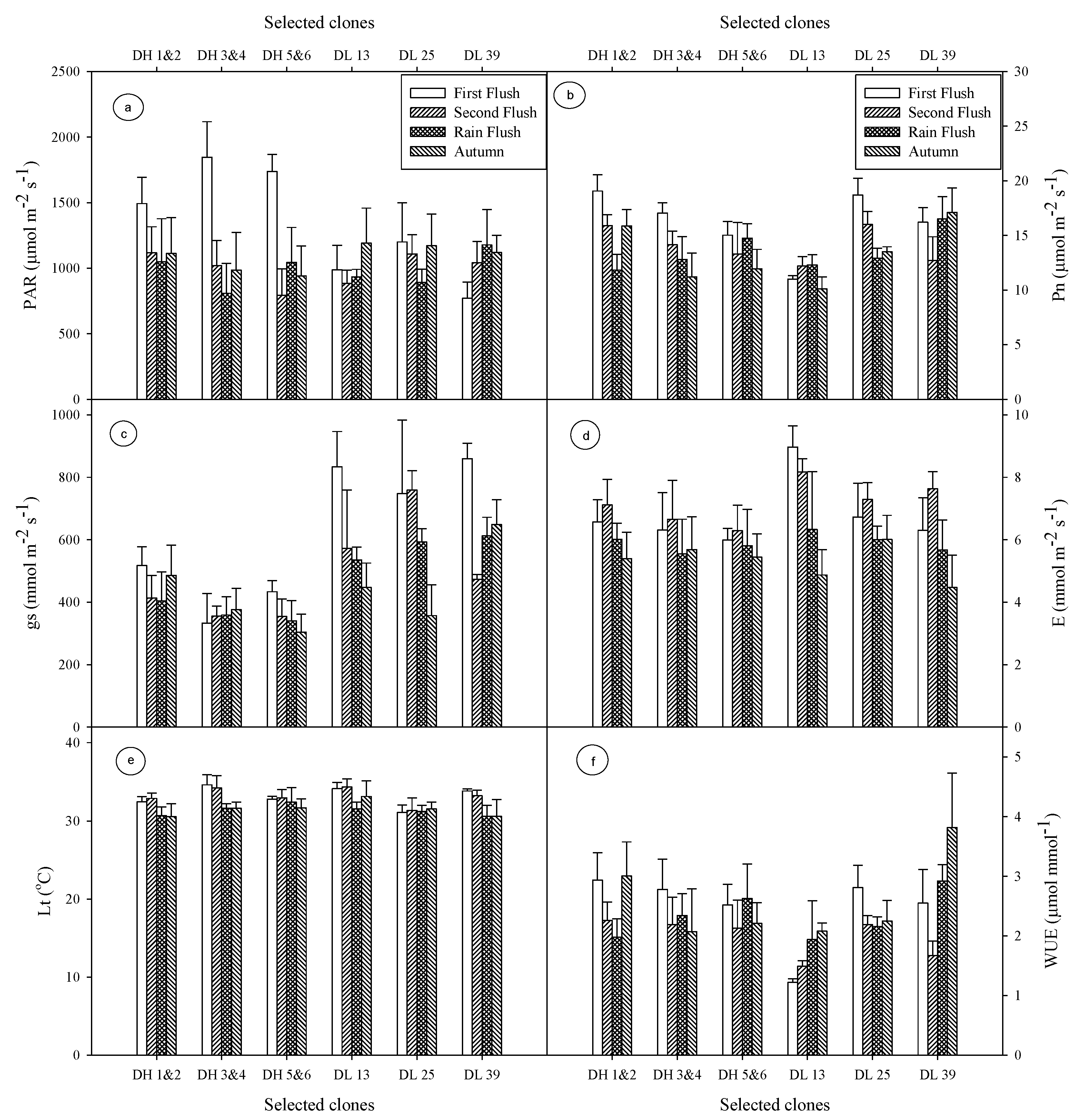
3.1. Photosynthetically Active Radiation (PAR)
3.2. Net Photosyntheis (Pn)
3.3. Leaf Temperature (Lt)
3.4. Stomatal Conductance (gs) and Transpiration (E)
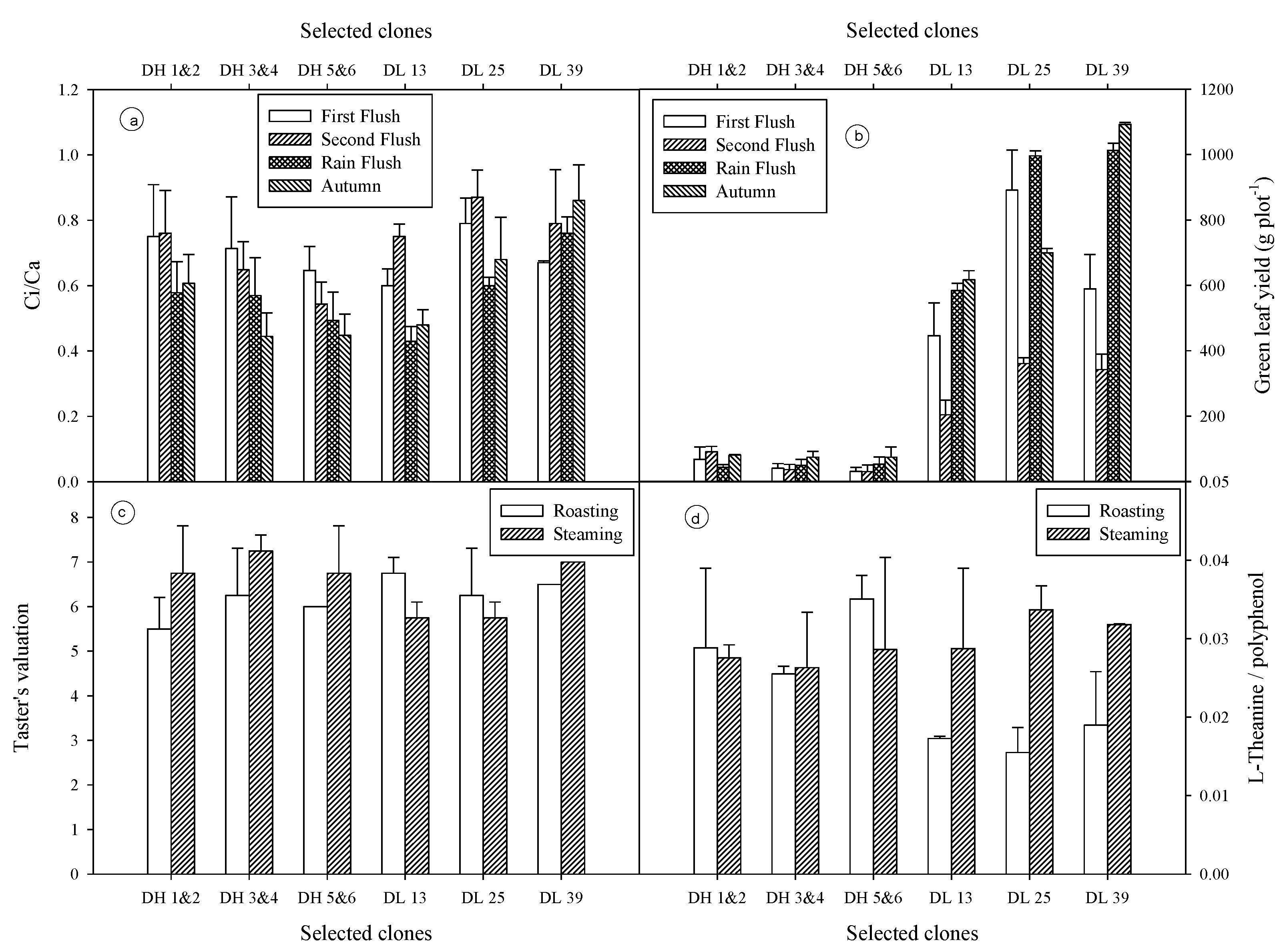
3.5. Water-Use Efficiency (WUE) and Carboxylation Efficiency (Ci/Ca)
3.6. Green Leaf Yield
3.7. Taster’s Score and L-Theanine to Ployphenol Ratio
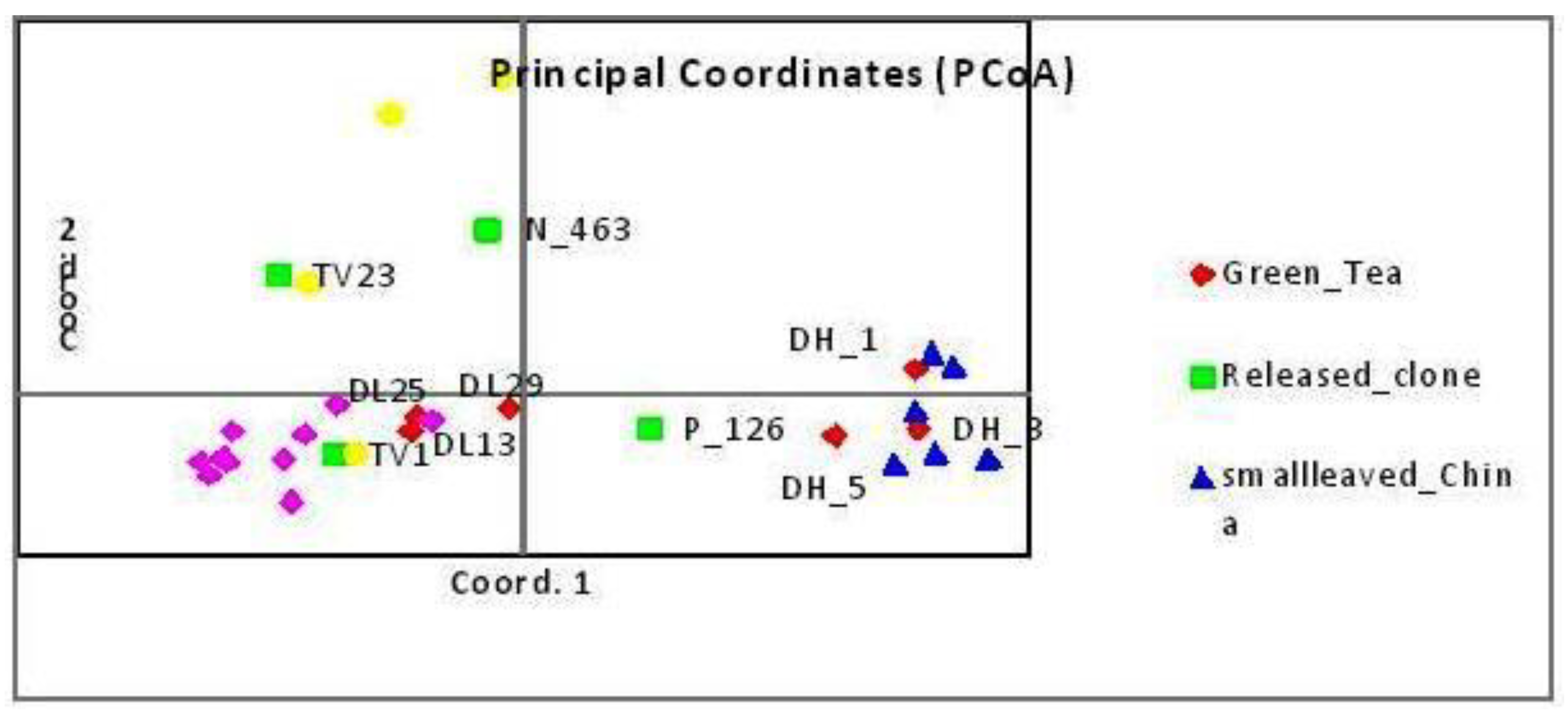
3.8. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhen, Y.S. Tea: Bioactivity and Therapeutic Potential; Taylor & Francis: New York, NY, USA, 2002. [Google Scholar]
- Ho, C.T.; Lin, J.K.; Shahidi, F. Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Konwar, B.K. Biodiversity of tea in North East India and their conservation at Tocklai. Two Bud 1999, 46, 7–12. [Google Scholar]
- Singh, I.D. Indian tea germplasm and its contribution to the world tea industry. Two Bud 1979, 26, 23–26. [Google Scholar]
- Das, S.C.; Barua, D.N. Mechanism of tea dormancy: Effect of temperature on growth and dormancy of tea plant in North East India. Two Bud 1987, 34, 36–41. [Google Scholar]
- Barman, T.S.; Baruah, U.; Saikia, J.K.; Borah, D.; Deka, D.K.; Lahon, T. Seasonal varation of physiological activiteis and its effect on yield of tea in North East India. Two Bud 2006, 53, 6–12. [Google Scholar]
- McAlpine, M.; Ward, W.E. Influence of Steep Time on Polyphenol Content and Antioxidant Capacity of Black, Green, Rooibos, and Herbal Teas. Beverages 2006, 2, 17. [Google Scholar] [CrossRef]
- ISO. 14502-1 Determination of Substances Characteristic of Green and Black Tea Part 1: Content of Total Polyphenols in Tea-Colorimetric Method Using Folin-Ciocalteu Reagent; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- ISO. DIS 19563: Determination of Theanine in Tea and Instant Tea in Solid Form Using High-Performance Liquid Chromatography; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- ISO. 3103 Tea—Preparation of Liquor for Use in Sensory Tests; International Organization for Standardization: Geneva, Switzerland, 1980. [Google Scholar]
- Gogoi, R.C.; Sanyal, S. Tea Testing Manual; Tocklai Tea Research Institute Tea Research Association: Jorhat, India, 2016; p. 25. [Google Scholar]
- Fang, W.P.; Meinhardt, L.W.; Tan, H.W.; Zhou, L.; Mischke, S.; Zhang, D. Varietal identification of tea (Camellia sinensis) usingnanofluidic array of single nucleotide polymorphism (SNP) markers. Hort. Res. 2014, 1, 14035. [Google Scholar] [CrossRef] [PubMed]
- Fluidigm. Fluidigm SNP Genotyping User Guide Rev H1; PN 68000098; Fluidigm Corporation: South San Francisco, CA, USA, 2011. [Google Scholar]
- Peakall, R.O.D.; Smouse, P.E. Genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. Gen AlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; A. Willy-Interscience Publication: New York, NY, USA, 1984. [Google Scholar]
- Barman, T.S.; Baruah, U.; Saikia, J.K. Irradiance influence tea leaf (Camellia sinensis L.) photosynthesis and transpiration. Photosynthetica 2008, 46, 618–621. [Google Scholar] [CrossRef]
- Ghosh Hajara, N.; Kumar, R. Seasonal variation in photosynthesis and productivity of young tea. Exp. Agric. 1999, 35, 71–85. [Google Scholar] [CrossRef]
- Ghosh Hajara, N.; Kumar, R. Diurnal and seasonal variation in gas exchange property of tea leaves. J. Plant Biol. 2002, 29, 169–173. [Google Scholar]
- Barua, D.N. Photosynthesis and respiration. In Science and Pratice in Tea Culture; Pub. TRA, Tea Research Association: Tocklai Experimental Station, Jorhat, India, 1989; pp. 374–377. [Google Scholar]
- Hadfield, W. Leaf temperature, leaf pose and productivity of tea bush. Nature 1968, 219, 282–284. [Google Scholar] [CrossRef]
- Whitehead, D.; Okali, D.V.W.; Fasehun, F.E. Stomatal response to environmental variables in two tropical rainforest species during the dry season in Nigeria. J. Appl. Ecol. 1981, 18, 581–587. [Google Scholar] [CrossRef]
- Cawan, I.R. Stomatal behaviour and environment. Adv. Bot. Res. 1977, 4, 117–228. [Google Scholar]
- Frank, A.B.; Baker, R.C.; Berdahl, J.D. Water use efficiency of grasses grown under controlled and field condition. Agron. J. 1987, 79, 541–544. [Google Scholar] [CrossRef]
- Weber, J.A.; Jurik, T.W.; Tenhunen, J.D.; Gates, D.M. Analysis of gas exchange in seedlings of Acer saccharum: Integration of field and laboratory studies. Oceologia 1985, 65, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Squire, G.R.; Callander, B.A. Tea plantations. In Water Deficits and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1981; Volume VI, pp. 471–510. [Google Scholar]
- Wickremasinghe, R.L. Monographs on Tea Production in Sri Lanka; Tea Research Institute of Sri Lanka, St. Coombs: Talawakelle, Sri lanka, 1978; Volume 7, p. 65. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.K.; Zhang, D.; Borthakur, D.; Hazarika, M.; Boruah, P.; Barooah, R.; Sabhapondit, S.; Neog, N.J.; Gogoi, R.C. Quality Green Tea (Camellia sinensis L.) Clones Marked through Novel Traits. Beverages 2019, 5, 63. https://doi.org/10.3390/beverages5040063
Patel PK, Zhang D, Borthakur D, Hazarika M, Boruah P, Barooah R, Sabhapondit S, Neog NJ, Gogoi RC. Quality Green Tea (Camellia sinensis L.) Clones Marked through Novel Traits. Beverages. 2019; 5(4):63. https://doi.org/10.3390/beverages5040063
Chicago/Turabian StylePatel, Pradeep Kumar, Dapeng Zhang, Devajit Borthakur, Monpi Hazarika, Pulakesh Boruah, Raj Barooah, Santanu Sabhapondit, Naba Jyoti Neog, and Romen Chandra Gogoi. 2019. "Quality Green Tea (Camellia sinensis L.) Clones Marked through Novel Traits" Beverages 5, no. 4: 63. https://doi.org/10.3390/beverages5040063
APA StylePatel, P. K., Zhang, D., Borthakur, D., Hazarika, M., Boruah, P., Barooah, R., Sabhapondit, S., Neog, N. J., & Gogoi, R. C. (2019). Quality Green Tea (Camellia sinensis L.) Clones Marked through Novel Traits. Beverages, 5(4), 63. https://doi.org/10.3390/beverages5040063




