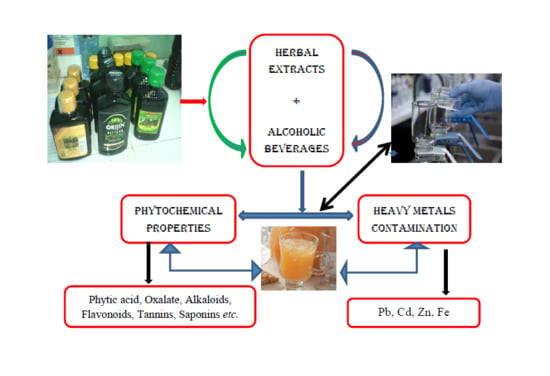
Phytochemical Properties and Heavy Metal Contents of Commonly Consumed Alcoholic Beverages Flavouredwith Herbal Extract in Nigeria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples for Laboratory Analysis
2.2. Quantitative Phytochemical Analysis
2.3. Determination of Heavy Metals
2.4. Statistical Analysis
3. Results
3.1. Physical Properties of the Alcoholic Beverages
3.2. Phytochemical Quantitative Analysis of Alcoholic Beverages Flavoured with Herbal Extracts
3.3. Heavy Metals Content of the Alcoholic Beverages Flavoured with Herbal Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chikere, E.I.; Mayowa, O. Prevalence and Perceived health effect of Alcohol use among male undergraduate students in Owerri, South-East, Nigeria: A descriptive cross-sectional study. BMC Public Health 2011, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Falodun, A. Herbal medicine in Africa—Distribution, standardization and prospects. Res. J. Phytochem. 2010, 4, 156–161. [Google Scholar] [CrossRef]
- Ritch, R. Potential role for Ginkgo biloba extract in the treatment of glaucoma. Med. Hypotheses 2000, 54, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, L. Challenges and opportunities in the Chinese herbal drug industry. In Natural Products; Humana Press: New York, NY, USA, 2005; pp. 229–250. [Google Scholar]
- World Health Organization. Traditional Medicine, Fact Sheet, No. 134. Available online: http://www.who.int/mediacentre/factsheets/2003/fs134/en/ (accessed on 3 November 2017).
- Aruoma, O.L. Methodological consideration for characterizing potential antioxidants actions of bioactive components in plant foods. Mutat. Res. 2003, 523, 9–20. [Google Scholar] [CrossRef]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Maduabuchi, J.M.U.; Nzegwu, C.N.; Adigba, E.O.; Oragwu, C.I.; Agbo, F.N.; Agbata, C.A.; Ani, G.C.; Orisakwe, O.E. Iron, manganese and nickel exposure from beverages in Nigeria: A public health concern? J. Heath Sci. 2008, 54, 335–338. [Google Scholar] [CrossRef]
- Magomya, A.M.; Yebpella, G.G.; Okpaegbe, U.C. An Assessment of metal contaminant levels in selected soft drinks sold in Nigeria. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 517–522. [Google Scholar]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, W.L.; Jung, G.B.; Yun, S.G. Safety assessment of heavy metals in agricultural products of Korea. Korean J. Environ. Agric. 2001, 20, 169–174. [Google Scholar]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Okwu, D.E. Phytochemicals and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain. Agric. Environ. 2004, 6, 30–34. [Google Scholar]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Springer: Dordrecht, The Netherland, 2005; p. 320. ISBN1 10 8181283104. ISBN2 13 9788181283108. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Ilarslan, H.; Palmer, G.; Imsande, J.; Horner, H.T. Quantitative determination of calcium oxalate and oxalate in developing seeds of soybean (leguminosae). Am. J. Bot. 1997, 84, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, E.E. Phytochemical screening. In Medicinal Plants and Traditional Medicine in Africa; John Wiley and Sons Inc.: New York, NY, USA, 1984; pp. 35–50. [Google Scholar]
- Maccance, R.A.; Widdowson, E.M. Phytin in Human nutrition. Biochem. J. 1953, 29, 2694–2699. [Google Scholar] [CrossRef]
- AOAC SMPR 2012.007: Standard Method Performance Requirements for Determination of Heavy Metals in a Variety of Foods and Beverages. J. AOAC Int. 2013, 96, 704. [CrossRef]
- Codex Alimentarius. General Requirements for Natural Flavourings (CAC/GL 29.1987). Available online: www.codexalimentarius.net (accessed on 12 November 2017).
- CODEX. Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF; Codex Alimentarius Commission: Rome, Italy, 2011; pp. 13–15. [Google Scholar]
- World Health Organization (WHO). Guideline for Drinking Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Standard Organization of Nigeria (SON). Nigerian Standard for Drinking Water Quality; Standards Organisation of Nigeria: Abuja, Nigeria, 2007. [Google Scholar]
- Riboli, E.; Norat, T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003, 78, 559S–569S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanner, S.A.; Hughes, J.; Kelly, C.N.; Buttriss, J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Pub. Health Nutr. 2004, 7, 407–422. [Google Scholar] [CrossRef]
- Olayemi, F.O. A review on some causes of male infertility. A recent comprehensive review summarizing marijuana’s negative health effects highlights particular risks for adolescents and young adults. AJBT 2010, 9, 2834–3842. [Google Scholar]
- Glahn, R.P.; Wortley, G.M.; South, P.K.; Miller, D.D. Inhibition of iron uptake by Phytic Acid, Tannic Acid and Z-C 12 Studies Using in vitro Digestion CaCo-2 Cell Model. J. Agric. Food Chem. 2002, 50, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Amzal, H.; Alaoui, K.; Tok, S.; Errachidi, A.; Charof, R.; Cherrah, Y.; Benjouad, A. Protective effect of saponins from Argania spinosa against free radical-induced oxidative haemolysis. Fitoterapia 2008, 79, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Bakovic, M. Biologically Active Triterpenoids and their Cardio-protective and Anti-Inflammatory Effects. J. Bioanal. Biomed. 2015, 12. [Google Scholar] [CrossRef]
- Topcu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Holst, B. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Br. J. Nutr. 2008, 99, S55–S58. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.B.; Hels, O.; Morberg, C.M.; Marving, J.; Büge, S.; Tetens, I. Total zinc absorption in young women, but not fractional zinc absorption, differs between vegetarian and meat-based diets with equal phytic acid content. Br. J. Nutr. 2006, 95, 963–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurrell, R.F.; Juillerat, M.A.; Reddy, M.B.; Lynch, S.R.; Dassenko, S.A.; Cook, J.D. Soy protein, phytate and iron absorption in man. Am. J. Clin. Nutr. 1992, 56, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Adepoju-Bello, A.A.; Oguntibeju, O.O.; Onuegbu, M.T.; Ayoola, G.A.A.; Coker, H.A.B. Analysis of selected metallic impurities in soft drinks marketed in Lagos, Nigeria. Afr. J. Biotechnol. 2012, 11, 4676–4680. [Google Scholar]
- Adepoju-Bello, A.A.; Issa, O.A.; Oguntibeju, O.O.; Ayoola, G.A.; Adejumo, O.O. Analysis of some selected toxic metals in registered herbal products manufactured in Nigeria. Afr. J. Biotechnol. 2012, 11, 6918–6922. [Google Scholar] [CrossRef]
- Onianwa, P.C.; Adetola, I.G.; Iwegbue, C.M.A.; Ojo, M.F.; Tella, O.O. Trace heavy metals composition of some Nigerian beverages and food drinks. Food Chem. 1999, 66, 275–279. [Google Scholar] [CrossRef]
- Obi, E.; Akunyili, D.N.; Ekpo, B.; Orisakwe, O.E. Heavy metal hazards of Nigerian herbal remedies. Sci. Total Environ. 2006, 369, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Orisakwe, O.E.; Oragwu, C.I.; Maduabuchi, J.M.U.; Nzegwu, C.N.; Nduka, J.K.C. Copper, selenium and zinc content of canned and non-canned beverages in Nigeria. Afr. J. Environ. Sci. Technol. 2009, 3, 042–049. [Google Scholar]
- Godwill, E.A.; Jane, I.C.; Scholastica, I.U.; Marcellus, U.; Eugene, A.L.; Gloria, O.A. Determination of some soft drink constituents and contamination by some heavy metals in Nigeria. Toxic. Rep. 2015, 2, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Ogunlana, O.O.; Ogunlana, O.E.; Akinsanya, A.E.; Ologbenla, O.O. Heavy metal analysis of selected soft drinks in Nigeria. J. Glob. Biosci. 2015, 4, 1335–1338. [Google Scholar]
| Samples | NAFDAC Reg. No. | pH | Alcohol Label Claim (%) | a Alcohol (%) Determined in the Laboratory | t-Test Value (p Value) |
|---|---|---|---|---|---|
| Sample 1 | B1-4103L | 3.28 ± 0.04 | 42 | 41.1 ± 0.02 | −7.794 (0.01) |
| Sample 2 * | - | 4.57 ± 0.3 | NA | 34.0 ± 5 | Nil |
| Sample 3 | B1-7529 | 6.57 ± 0.07 | 40 | 42.0 ± 0.55 | 6.709 (0.003) |
| Sample 4 | 08-0630 | 4.34 ± 0.2 | 30 | 40.6 ± 0.35 | 52.114 (0.000) |
| Sample 5 | A1-8029 | 5.77 ± 0.1 | 42 | 51.5 ± 0.03 | 53.116 (0.000) |
| No. | Parameters | Sample 1 | Sample 2 * | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|---|---|
| 1 | Phytic acid (mg/g) | 2.37 ± 0.30 | 2.43 ± 0.1 | 0.00 | 0.72 ± 0.1 | 0.00 |
| 2 | Oxalate (mg/g) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | Alkaloids (mg/g) | 4.11 ± 0.2 | 1.93 ± 0.3 | 0.27 ± 0.1 | 0.37 ± 0.2 | 0.42 ± 0.1 |
| 4 | Flavonoids (mg rutin equivalents/g) | 3.64 ± 0.05 | 0.77 ± 0.1 | 0.68 ± 0.1 | 0.22 ± 1 | 0.52 ± 0.2 |
| 5 | Tannins (mg/g) | 0.00 | 0.00 | 0.00 | 0.12 ± 0.4 | 1.43 ± 0.4 |
| 6 | Saponins (mg diosgenin equivalents/g) | 0.17 ± 0.02 | 0.00 | 0.00 | 0.00 | 0.22 ± 0.1 |
| 7 | Total Phenolic (mg gallic acid equivalents/g) | 3.66 ± 0.05 | 1.13 ± 0.1 | 1.58 ± 0.2 | 0.00 | 1.55 ± 0.3 |
| 8 | Anthraquinones (mg/g) | 1.93 ± 0.30 | 0.00 | 0.74 ± 0.1 | 0.00 | 0.00 |
| 9 | Triterpenoids | 0.11 ± 0.01 | 0.24 ± 0.1 | 0.93 ± 0.5 | 0.00 | 0.00 |
| Samples | Pb | Cd | Cr | Co | Zn | Fe | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 2.13 ± 0.01 a | 0.07 ± 0.02 | 0.00 | 0.13 ± 0.1 | 0.40 ± 0.02 a | 4.22 ± 0.02 a | |
| 2 * | 2.39 ± 0.01 | 0.06 ± 0.03 | 0.15 ± 0.02 | 0.12 ± 0.03 | 0.19 ± 0.04 | 0.83 ± 0.01 | |
| 3 | 3.91 ± 0.01 a | 0.06 ± 0.03 | 0.35 ± 0.02 a | 0.23 ± 0.01 | 0.14 ± 0.03 | 1.01 ± 0.01a | |
| 4 | 2.51 ± 0.04 a | 0.06 ± 0.01 | 0.22 ± 0.04 a | 0.13 ± 0.02 | 0.17 ± 0.01 | 0.72 ± 0.05 | |
| 5 | 4.70 ± 0.50 a | 0.07 ± 0.01 | 0.31 ± 0.01 a | 0.20 ± 0.50 a | 0.22 ± 0.01 | 1.10 ± 0.10 a | |
| WHO | 0.01 | 0.03 | 0.05 | - | 60 | 100 | [23] |
| SON | 0.01 | 0.03 | 0.05 | - | 3.0 | 0.3 | [24] |
| RDA | 0.3 mg/week | 10–60 | - | 100 | 10–60 | ||
| F(p value) | 6345.3 (0.000) | 0.56 (0.695) | 223.038 (0.000) | 33.545 (0.000) | 128.78 (0.000) | 2044 (0.000) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okareh, O.T.; Oyelakin, T.M.; Ariyo, O. Phytochemical Properties and Heavy Metal Contents of Commonly Consumed Alcoholic Beverages Flavouredwith Herbal Extract in Nigeria. Beverages 2018, 4, 60. https://doi.org/10.3390/beverages4030060
Okareh OT, Oyelakin TM, Ariyo O. Phytochemical Properties and Heavy Metal Contents of Commonly Consumed Alcoholic Beverages Flavouredwith Herbal Extract in Nigeria. Beverages. 2018; 4(3):60. https://doi.org/10.3390/beverages4030060
Chicago/Turabian StyleOkareh, Oladapo T., Tosin M. Oyelakin, and Oluwaseun Ariyo. 2018. "Phytochemical Properties and Heavy Metal Contents of Commonly Consumed Alcoholic Beverages Flavouredwith Herbal Extract in Nigeria" Beverages 4, no. 3: 60. https://doi.org/10.3390/beverages4030060
APA StyleOkareh, O. T., Oyelakin, T. M., & Ariyo, O. (2018). Phytochemical Properties and Heavy Metal Contents of Commonly Consumed Alcoholic Beverages Flavouredwith Herbal Extract in Nigeria. Beverages, 4(3), 60. https://doi.org/10.3390/beverages4030060