Abstract
Soursop is an unexploited fruit with numerous health benefits. Its underutilization is attributable to its rapid softening and ripening, which leads to great postharvest loss. The current study was carried out to formulate a functional drink from blends of soursop, milk and honey and also to assess the phytoconstituents of the beverages. The proximate composition, physicochemical, antioxidant, sensory and microbial properties of the formulated drink were evaluated. The formulated milkshake exhibited a significant ascorbic acid content which was above 50% of the recommended daily requirement for both adult and children. The soursop fresh extract (SFE) showed high antioxidant activity which qualifies it as a functional drink. The incorporation of milk to the soursop drink also produced a high flavonoid content and antioxidant properties (DPPH• scavenging activity). The soursop fresh extract and drink with 30% milk inclusion had high sensory acceptability and showed no significant difference to the market sample (control). The richness in ascorbic acid, high phenolic content and antioxidant activity of the formulated drink suggests it may be an important source of antioxidants and could be used as a functional drink for mediation in health-related issues.
1. Introduction
Tropical fruits have been used to produce several products which have gained global importance over time owing to their characteristic exotic aroma and colour [1]. There are a number of these fruits readily available for utilization, including orange, grape, pineapple, banana, guava and watermelon. The use of these fruits depends on the intended product (juice, drink, jam etc.). Juices produced over the years had been done using a fruit or combination of fruits depending on the choice of the producer or even with regard to consumer demand. Fruits possess antioxidant capacities as they often serve as scavengers of free radicals, thus preventing oxidative damage. Studies have also shown that fruits possess anticancer and antioxidant capabilities [2]. The consumption of fresh fruit extracts or drinks is increasing all over the world and this is attributable to the perception that they contribute to a healthy diet and are good sources of vitamins and minerals as well as having the ability to reduce the risk of many diseases.
Soursop (Annona muricata L.) is a tropical fruit belonging to the Annonaceae family with a characteristic unique aroma and flavour [3]. It is an exotic fruit that is recognized for its very pleasant, sub-acid, aromatic and juicy flesh [4]. The fruit is an important source of vitamins, minerals and dietary fibre [5]. Soursop fruit is often eaten raw or occasionally extracted and consumed as fresh soursop juice; consequently, the fruit is grossly underutilized and unexploited. Similar to many other tropical fruits with short shelf lives, soursop fruit softens very rapidly during ripening and becomes mushy and difficult to consume fresh. Extreme injury resulting from postharvest handling or uneven shape and size is responsible for rejection by buyers [4]. Processing into juice, drinks, beverages or other related products capable of preserving the nutritional and health benefits is of great importance to minimize postharvest loss and maximize its potential in the food processing industry. The processing and consumption of soursop is of increasing interest owing to the functional and beneficial claims from several quarters. However, commercial utilization of soursop products is uncommon. Sweeteners are often incorporated into fruit drinks to improve sensory appeal. Honey is a complex nutritional sweetener, composed primarily of carbohydrates (60–85%) and small amounts of other compounds [6]. Honey also possesses antioxidant capacity, phenolics and organic acid compounds which makes it valuable in the treatment of many diseases [7]. Previous studies have successfully evaluated the nutritional composition of a formulated drink from several other fruits, such as African star apple [8], pineapple [9], and custard apple [10]. So far, there is limited information on the antioxidant properties of soursop drink or milkshake. Hence, the current study seeks to evaluate the nutritional composition and antioxidant properties of a formulated soursop drink.
2. Materials and Methods
Mature soursop (Annona muricata L.) fruits, sugar and filled milk powder were purchased from Oba market in Akure, Ondo State, Nigeria. Honey was purchased from Ondo State Sunshine Honey and Agro Industries Limited, Akure, Ondo State. The fruits were sorted from a selection of several matured fruits, which were determined by their dark green skin with smooth numerous fleshy spines. All reagents used were of analytical grade.
2.1. Preparation of Soursop Drink
Soursop drink was produced according to the modified method of Ndife et al. [11]. The pulp was obtained by allowing the matured fruit to ripen for five days at 25 °C. The drink was produced from different blends of soursop extract, milk, honey and sugar syrup, as outlined in Table 1. The formulated milkshake was bottled, pasteurized at 95 °C for 5 min, cooled and stored at 4 °C prior to analyses.

Table 1.
Raw materials and quantities used in the formulation of the soursop drink.
2.2. Determination of Physicochemical Properties
pH was measured using a standardized pH meter (Hanna instruments, HI 8314 membrane pH meter). The amount of total soluble solids (TSS) was determined using a bench type Abbé refractometer (0–32°) ATAGO N1 (model 2313 MASTER-M) and expressed as oBrix.
The ascorbic acid content was determined using the oxidation–reduction titration method, as described by Mau et al. [12]. Briefly, 10 mL of 3% metaphosphoric acid was added to 5 mL of sample and filtered to remove possible protein interference. The filtrate was then titrated against freshly standardized 2, 6-dichloroindophenol (DCP). The standardization was with 10 mL of standard ascorbic acid from Natural Health Direct (OxyMin®, Krpan Enterprises, Queensland, Australia).
The total titratable acidity (TTA) was determined, as described in AOAC [13]. An aliquot (10 mL) of the sample was pipette into a test tube and two drops of phenolphthalein indicator was added and thoroughly shaken. The mixture was titrated against 0.1 M NaOH until a change in colour was observed, and then, acidity was calculated.
2.3. Determination of Proximate Composition
Determination of the proximate composition of the fresh extract and formulated drink was carried out. Protein, ash, crude fibre, fat and moisture contents were determined as described in AOAC [13], carbohydrate was calculated by difference.
2.4. Evaluation of Antioxidant Properties
The free radical scavenging ability of the soursop drink against DPPH (1, 1-diphenyl-2-picryhydrazyl) was evaluated using the method described by Gyamfi et al. [14]. Briefly, the appropriate dilution of sample (1 mL) was mixed with 1 mL of 0.4 mM methanolic DPPH solution. The mixture was left in the dark for 30 min, and absorbance was measured at 516 nm.
The total flavonoid content of the soursop drink was determined using a colorimeter assay developed by Bao et al. [15]. An aliquot (0.2 mL) of the drink was added to 0.3 mL of 5% NaNO3 at zero time. After 5 min, 0.6 mL of 10% AlCl3 was added and after 6 min, 2 mL of 1 M NaOH was added to the mixture, followed by the addition of 2.1 mL of distilled water. Absorbance was read at 510 nm against the reagent blank.
The total phenolic content (TPC) was determined according to the modified Folin–Ciocalteau method [16]. Briefly 0.2 mL of diluted soursop drink was added to 1 mL of Folin–Ciocalteau reagent (prediluted 10-fold with distilled water) and shaken well. The mixture was allowed to stand at room temperature for 8 min. Then, 0.8 mL of sodium carbonate (7.5%) was added to the mixture, shaken and left at room temperature for 30 min. Absorbance was measured at 765 nm in a spectrophotometer (Ultrospec 4300 Pro UV/Vis, Amersham Biosciences, NJ, USA). The TPC was assessed by plotting the gallic acid calibration curve and expressed as milligrams of gallic acid equivalents per mL of sample.
The reducing property of the soursop drink was determined as described by Pulido et al. [17] Aliquot (0.25 mL) of the soursop drink sample was mixed with 0.25 mL of 200 mM sodium phosphate buffer (pH 6.6) and 0.25 mL of 1% KFC. The mixture was incubated at 50 °C for 20 min; thereafter, 0.25 mL of 10% TCA was also added and centrifuged at 2000 rpm for 10 min; then, 1 mL of the supernatant was mixed with 1 mL of distilled water and 0.1% of FeCl3, and the absorbance was measured at 700 nm.
2.5. Microbiological Analysis
The total plate count was evaluated using the standard plate method [18]. An aliquot (25 mL) of soursop drink was mixed with 225 mL of sterile buffered peptone water. A serial dilution for each sample was made using sterilized, buffered peptone water up to 10−2 dilution. Dilutions of 10−1 and 10−2 were surface spread onto duplicate sterile plate count agar (PCA) (70152 Sigma-Aldrich, Germany) and incubated at 37 °C for 24 h, after which colony-forming units were counted. Yeast and mould counts were enumerated according to Harrigan and MacCance [19] using potato dextrose agar. The plates were incubated at 25 °C for 72 h, and the spore-forming units were counted.
2.6. Sensory Evaluation
Sensory evaluation of the formulated drink was carried out as follows. Twenty (20) panellists experienced in the evaluation of fruit drink and milkshakes were chosen for the assessment of the sensory attributes of soursop drink. A 30 min training session was conducted to evaluate the use of the attributes by the panellists during the sensory analysis. The sensory attributes allowed the differentiation of samples in terms of appearance (colour), texture (viscosity, consistency/flow), flavour (flavour and aroma) and taste (palatability). Samples were coded and served to the panellists for independent evaluation; all sensory attributes assessed by the panellists were rated using a 9-point hedonic scale (1 = dislike extremely, 9 = like extremely).
2.7. Statistical Analysis
Data was generated in triplicate and subjected to Analysis of Variance (ANOVA) using Statistical Package for Social Sciences (SPSS) V. 17.0. The means were separated using Duncan Multiple Range Test (DMRT) at a 95% confidence level.
3. Results and Discussion
3.1. Physicochemical Properties of Formulated Soursop Drink
Table 2 presents the physicochemical properties of the different soursop drink formulations. Microorganisms have optimal pH requirements for growth and proliferation. More so, the storage quality or shelf life of a fruit juice or drink is dependent on its pH. The pH values of the drinks ranged from 3.7 to 5.6. Soursop drink incorporated with 30% milk (SSM) had the highest pH value (5.6). The addition of milk to soursop extract caused an increase in pH. This could be due to the alkalinity of milk which caused the observed reduction in the acidity of the formulated drink. The obtained values were within the range of 3.0–5.0 reported by Akusu et al. [9] for fruit juices, pineapple juice (3.97), and orange juice (3.50). Yapo et al. [20] and Islam et al. [21] also reported similar values for pineapple juice and mango juice, respectively. An increase in pH was reported for sugar-apple pulp substituted with milk [22]. The pH values observed for the milkshakes were higher than those reported by de Moura et al. [23] for a milk drink flavoured with mangaba pulp (4.03–4.12). A low pH is favourable for microbial stability in juices; moreover, low pH values have been reported to inhibit bacterial growth in fresh, unpasteurized fruit juices [24].

Table 2.
Physicochemical compositions of soursop extract and drinks.
The total titratable acidity (TTA) ranged from 0.25 to 0.52%. The fresh soursop extract (SFE) showed an average titratable acidity of 0.52% which is significantly higher than the value of 0.19% reported earlier by Othman et al. [5] for fresh soursop fruit. Obasi et al. [25] also reported a low TTA (0.10%) for laboratory-prepared orange juice. Soursop sweetened with honey (SSH) and soursop sweetened with sugar (SSS) showed significantly higher TTA values. This may be attributable to the low pH values observed in the drinks (SSH and SSS) which may have caused increased acidity levels. The result showed a correlation between pH (low) and total titratable acidity (high). SSM had the lowest titratable acidity, which correlates with the fact that the drink had the highest pH; hence, the low amount of acid in the food sample was neutralized by a strong alkali in the formulated drink.
The viscosity of the soursop drink formulations ranged from 0.40 to 0.92 dPa. Higher viscosities were observed in SSM (0.92 dPa) and SSMH (0.78 dPa) which could be attributed to the addition of milk. The incorporation of milk to the formulation may have caused a reduction in the free flow of the milkshake and consequently, increased viscosity. Chavan et al. [26] reported that the addition of whey improved the mouthfeel of drink as a result of increased beverage viscosity. This observation correlates with the findings of the present study, as the drinks containing milk solutions had higher viscosities.
The brix value ranged from 15 to 22° Brix. The highest brix was recorded in SSM (with 30% milk incorporation). The high brix level may be a result of the high total solid content of milk. The total soluble solids observed in fresh extract (SFE) was higher than the value of 12.83° reported for soursop pulp by Orsi et al. [27], and this may be attributable to the level of fruit ripeness and geographical variation. The present result is similar to the finding of Duarte et al. [22], who reported a value of 23.67° Brix for a blend of 75% Annona pulp and 25% milk. However, Bajwa and Mittal [28] reported a lower brix value (13.9°) for mango flavoured milk drink.
SFE had the highest ascorbic acid content of 44.00 mg/100 mL, which was higher than the average contents of 29.6 mg/100 g and 22.6 mg/100 g for soursop pulp previously reported by Waston and Preedy [29] and Badrie and Schauss [30], respectively, but similar to the value of 42.38 mg/100 g reported for custard apple juice [31]. The ascorbic acid levels of the drink ranged from 28.67 to 32.92 mg/100 mL with SSMH having the highest ascorbic acid content. The ascorbic acid levels of the drinks were lower than the content observed for the fresh extract, which may be due to the introduction of other components to the formulated drink as well as the dilution ratios of these materials. The increase in the amount of ascorbic acid in the honey sweetened soursop drink corroborates the report of Chua et al. [32] that honey is rich in ascorbic acid; thus, honey may have contributed to the ascorbic acid content of the drink. The ascorbic acid content of the drinks is similar to the content reported for pineapple juice [9,33]. Ascorbic acid prevents scurvy and protects the body against oxidative stress [34]. The high amount of ascorbic acid showed that the fruit is a good source of ascorbic acid as it could meet over 50% of the recommended daily intake of ascorbic acid for both adults (65 mg/day) and children (25 mg/day) [35].
3.2. Proximate Composition of Formulated Drink
The proximate compositions of the soursop drink formulations are presented in Table 3. The moisture contents of the soursop drinks ranged from 74.3 to 81.3%. Soursop fresh extract (SFE) had a lower moisture content (70.26%) while soursop sweetened with honey (SSH) had the highest. The higher moisture content observed in this drink may be attributed to the fact that the components are majorly water-based (water, honey, sugar solution). Othman et al. [5] reported a similar moisture content for soursop pulp, whereas other authors [29,36,37] reported higher values. The result for the moisture content of soursop drinks is similar to the amount reported for juice produced from the pulp of soursop [1].

Table 3.
Proximate compositions of formulated soursop drinks.
The fat content ranged from 1.00 to 7.40%. SSM had the highest fat content which could be as a result of the addition of 30% of milk, a known high fat dairy product. SSMH also had a considerably high fat content (5.40%), equally connected to the incorporation of milk (10%). SSH and SSS showed low fat contents (1.00 and 1.40%, respectively) which could be due to the fact that soursop fruit is generally low in fat. This result corroborates the report of Onimawo [36]. The fat content of the soursop drinks without the addition of milk is low and almost negligible, as previously reported by Abbo et al. [1] and Onyechi et al. [38]. The low level of fat content of the drink suggests that it is a poor source of calories; however, it may be useful as a functional drink for weight management therapy.
The protein content followed a similar trend as the fat content of milkshakes. SSM (drink containing 30% milk) had the highest protein content (5.13%), closely followed by SSMH (drink containing 10% milk) (3.51%). The other drinks without milk, SSH and SSS, had lower protein contents of 2.0 and 2.6%, respectively. This implies that the nutrient composition of the drink was significantly improved by the incorporation of milk as fruit juice is generally a poor source of protein [39].
The ash content ranged from 0.32 to 0.73% among the drink samples. The carbohydrate content on the other hand ranged from 11.36 to 17.54%, suggesting that the drink formulations may be a potential low-calorie milk-shake; likewise, they are a potential functional drink for blood pressure management therapy [40]. The energy value observed in this study showed that soursop drink sweetened with honey (SSH) had the least value; hence, it may be useful for weight management. The higher energy values obtained for the milkshakes (SSM, SSMH) may be attributed to the high fat content of the milk incorporated into the drinks.
3.3. Antioxidant Properties of Soursop Drink
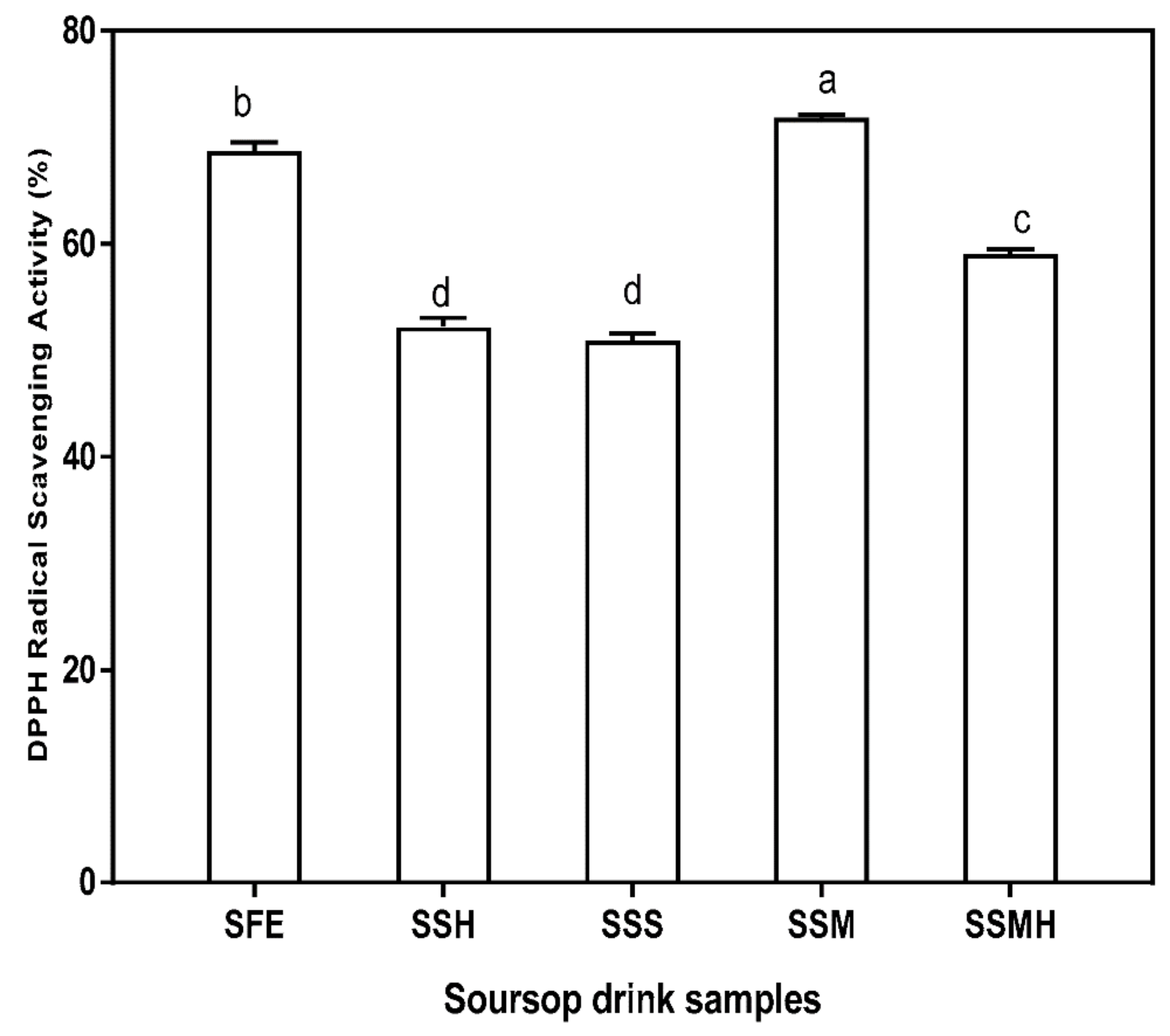
Antioxidants acts as free radical scavengers and inhibit lipid peroxidation and other free radical mediated processes, thereby helping to protect the human body from several diseases attributed to the reactions of radicals [41]. Annona spp. has been reported as one of the tropical fruits that demonstrates significant antioxidant properties [42]. The DPPH radical scavenging activity of the drink formulation is presented in Figure 1. The presence of antioxidants in the soursop drink was significant thus, the drink may possess possible beneficial potential as free radical scavenger. There was no significant difference (p < 0.05) in the DPPH radical scavenging activities of SSH (52.21%) and SSS (50.97%) which suggests that sweetener variation have not contributed to the radical scavenging activity of the drink. SSM had the highest DPPH radical scavenging activity (71.88%), attributable to the addition of milk to soursop extract which significantly (p < 0.05) increased the radical scavenging activity of the formulated drink. Similarly, the fresh extract (SFE) also had high scavenging activity (68.78%). Relative to the results of this study, Madurangi and Gunathilake [43] also reported a high DPPH radical scavenging activity for soursop pulp and beverage. The high DPPH radical scavenging activity observed in this study showed that despite variation, the formulated drinks were active in scavenging DPPH radicals. The high DPPH radical scavenging activity of SSM corresponds to its total phenolic content. Akomolafe and Ajayi [44] correlated the strong inhibitory effect of soursop on DPPH• to the presence of polyphenolic compounds which donate electrons to neutralize free radicals. Also, Bonoli-Carbognin et al. [45] reported a synergistic effect resulting from milk proteins binding with polyphenols, translating into increased antioxidant activity.
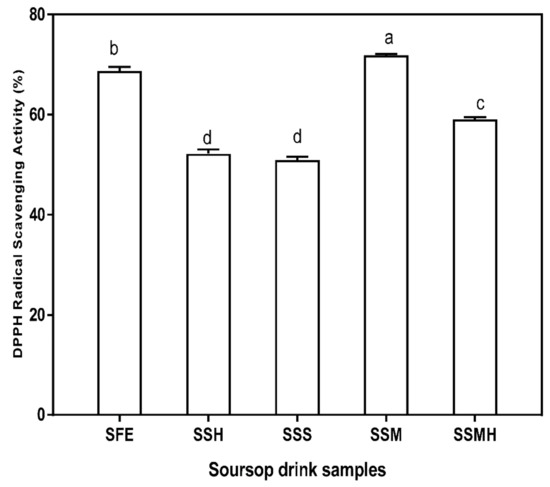
Figure 1.
DPPH (1, 1-diphenyl-2-picryhydrazyl) radical scavenging activity of formulated soursop drink. Values are means ± SDs; bars with different letters (a–d) are significantly different (p < 0.05); SFE: soursop fresh extract; SSH: soursop + honey; SSS: soursop + sugar solution; SSM: soursop + milk; SSMH: soursop + milk + honey.
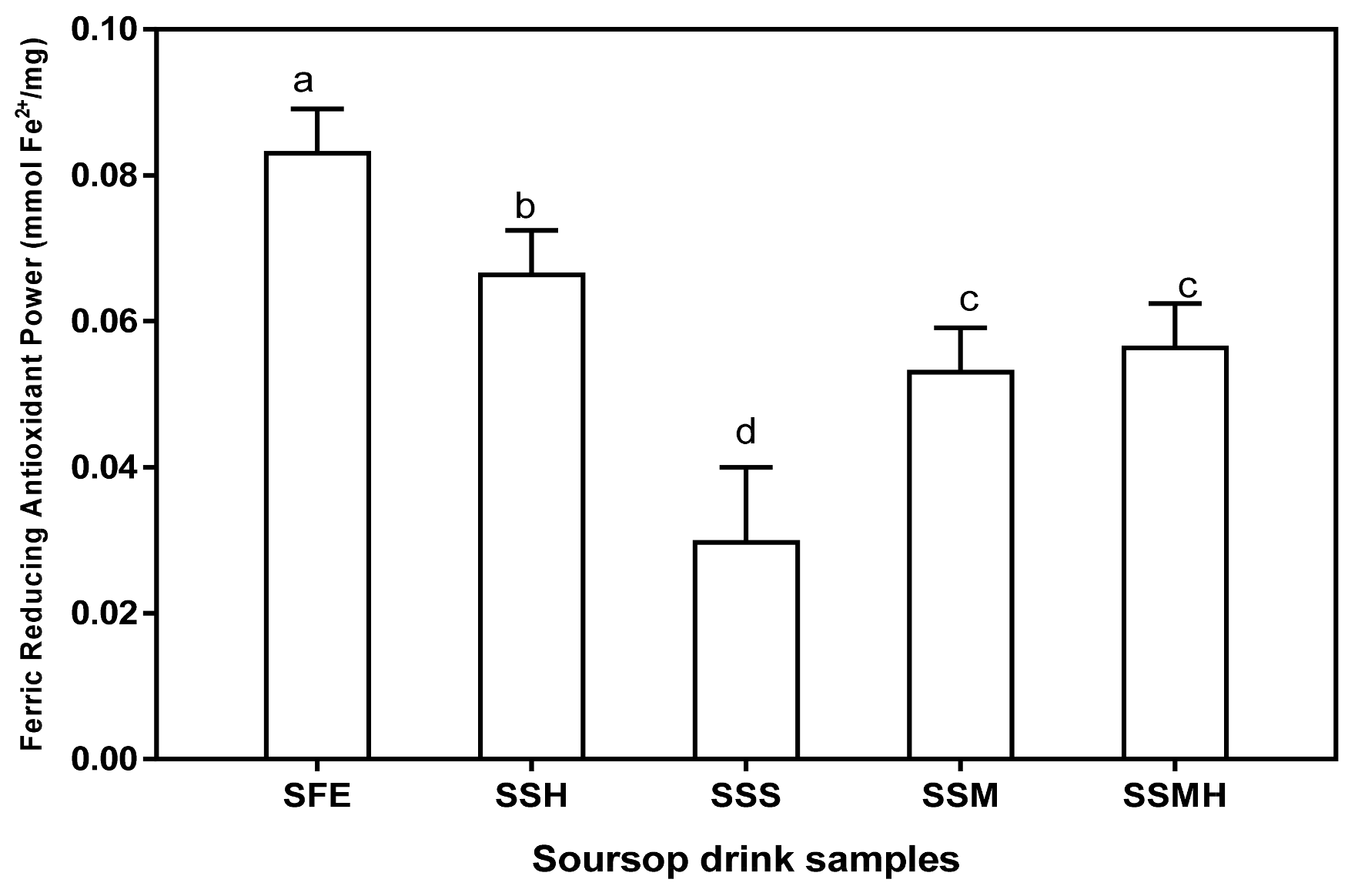
The antioxidant function of the drinks was also proven by their ferric reducing antioxidant power (Figure 2). Fresh extract showed the highest ability to reduce Fe3+ to Fe2+ (0.08 mmol Fe2+/mg). Amongst the formulated drink, SSH had the highest ferric reducing antioxidant power (0.07 mmol Fe2+/mg) showing a double-fold reducing effect compared to the ferric reducing ability of SSS (0.03 mmol Fe2+/mg). This result suggests that the sweetener type significantly correlates with ability of the drink to reduce Fe3+ to Fe2+. Thus, honey may be a better sweetener that is more capable of maintaining or boosting antioxidant activity of the formulated drinks. The results of antioxidant study revealed that the formulated drinks/milkshake can easily donate electrons capable of reacting with free radicals, thus terminating the chain reaction as well as creating a stable product and consequently, preventing oxidative stress-related diseases.
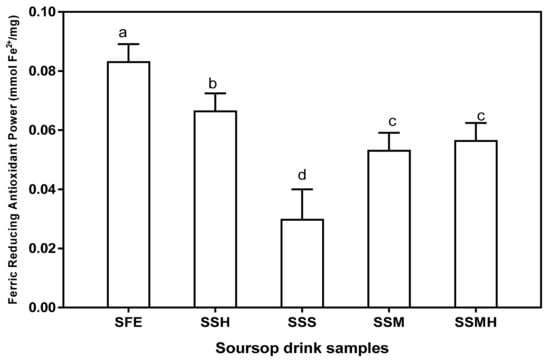
Figure 2.
Ferric reducing antioxidant power of formulated soursop drink. Values are means ± SDs; bars with different letters (a–d) are significantly different (p < 0.05); SFE: soursop fresh extract; SSH: soursop + honey; SSS: soursop + sugar solution; SSM: soursop + milk; SSMH: soursop +milk + honey.
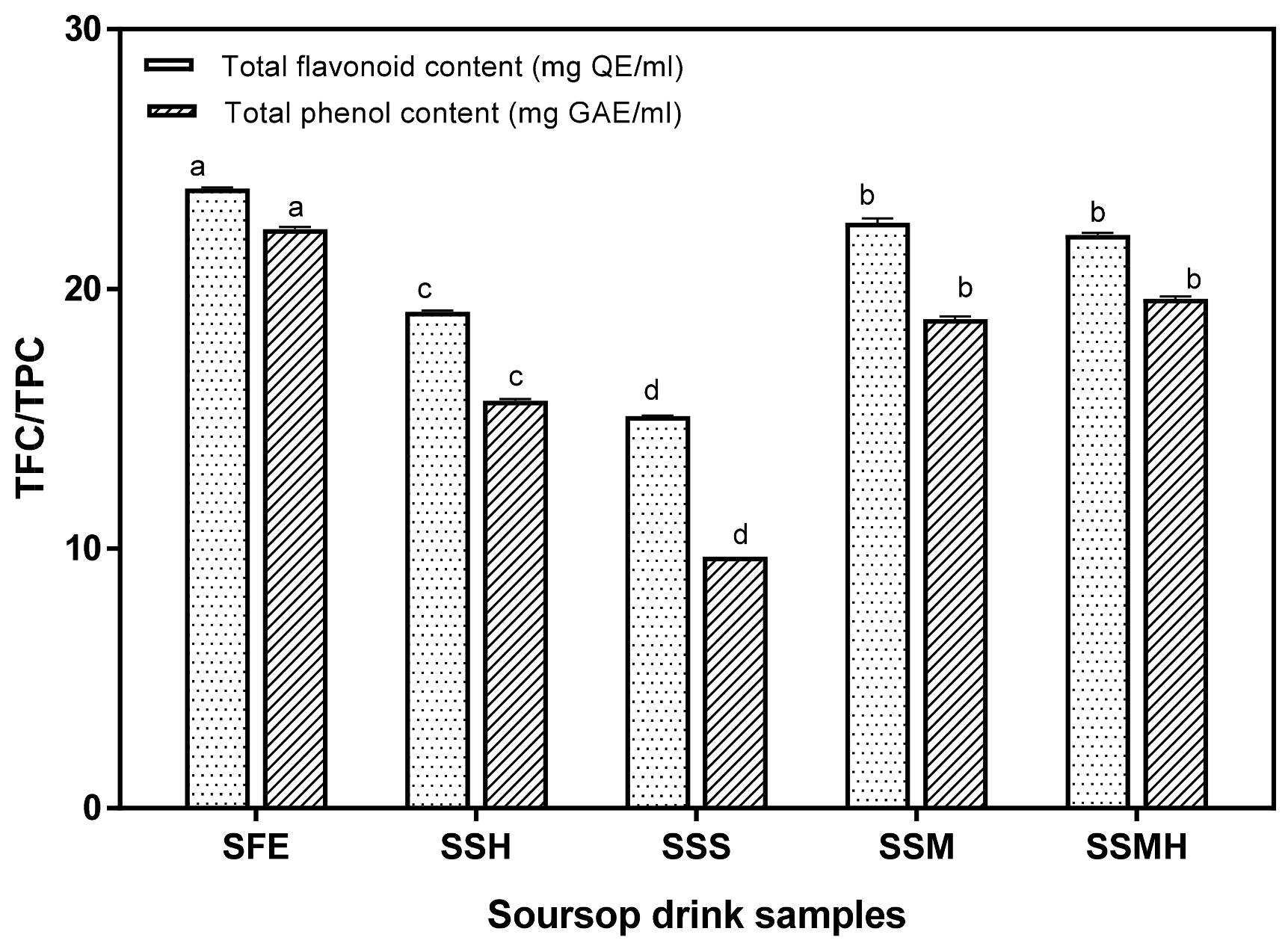
Flavonoids are a large group of compounds that are widely distributed in plant foods. They have antioxidant properties which help to protect the body against cardiovascular diseases and some forms of cancer [38]. The antioxidant potential of the drink was also evaluated by total flavonoid and total phenol contents. Similar to other antioxidant properties, the fresh extract exhibited the highest flavonoid content (23.86 mg/g), and the soursop formulations with milk (SSM and SSMH) also had significant flavonoid contents of 22.54 and 22.08 mg QE/mL, respectively (Figure 3). The presence of flavonoids in soursop drink is desirable as flavonoids display antioxidative properties via several mechanisms, including the chelation of metal ions, scavenging of free radicals and inhibition of enzymes that propagate the generation of free radicals [46]. Similarly, the formulated drink possessed a significant (p < 0.05) quantity of phenolics ranging from 9.68 to 22.30 mg GAE/mL. There was a similar trend for the total flavonoid content with SSS having the lowest total phenol content. A previous study [47] reported that phenolic antioxidants induce greater biological health-promoting effects than vitamins. Phenolic compounds exert scavenging effect on free radicals, thus displaying an antioxidative capability. Overall, the soursop drink sweetened with sugar syrup exhibited the lowest antioxidant activity while the soursop milkshake containing 30% milk (SSM) exhibited the highest antioxidant activity.
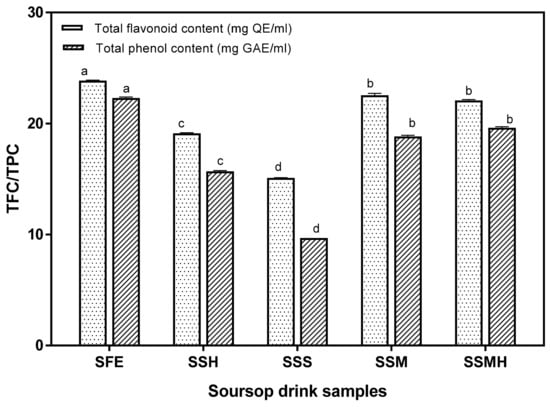
Figure 3.
Total flavonoid and total phenolic contents of formulated soursop drinks. Values are means ± SDs; bars with different letters (a–d) are significantly different (p < 0.05); SFE: soursop fresh extract; SSH: soursop + honey; SSS: soursop + sugar solution; SSM: soursop + milk; SSMH: soursop + milk + honey.
3.4. Microbiological Assessment of the Soursop Drink Formulations
The microbial quality of the soursop formulated drink was evaluated to ascertain its safety by comparing the microbial load with bacteriological standards. The result revealed a total viable count (TVC) of 2.3 × 103 cfu/mL for sugar sweetened soursop drinks (SSS) while milk incorporated soursop drinks (SSM and SSMH) had TVCs of 4.5 × 103 and 2.5 × 103 cfu/mL, respectively (Table 4). However, no visible growth was observed in the other samples. The total viable counts (TVC) of the drink samples were less than 1.0 × 105 cfu/mL which is the total plate count level permitted in food according to the Codex Alimentarius Commission (CAC) of the Food and Agricultural Organization (2003). This low microbial population may be attributed to the positive effect of pasteurization in destroying microorganisms in liquid food products. A low pH is also known to favour microbial stability, which explains the low microbial populations observed in the drinks. However, a high pH favours yeast growth and this may be responsible for the highest microbial count (3.20 × 104 sfu/mL) observed in SSM (milkshake with highest pH of 5.6). The results from the microbial assessment of the formulated drinks suggests that they are safe for human consumption and not likely to cause any microbiological hazard.

Table 4.
Microbial counts of soursop drinks.
3.5. Sensory Attributes of the Soursop Drink Formulations
The sensory attributes of the soursop drinks from different blends of pulp, sugar syrup, honey and milk were evaluated using a nine-point hedonic scale (Table 5). The drinks were evaluated for appearance, mouthfeel, aroma, consistency, and taste, as well as overall acceptability. For all the parameters evaluated, drink formulations, SSH, SSS and SSMH showed lower sensory attributes. However, SFE and SSM showed higher attributes while there was no significant difference (p < 0.05) in their overall acceptability when compared with VMD (control) which is a milk-based drink. The acceptability of SSM may be attributed to the addition of a higher percentage of milk (30%).

Table 5.
Sensory attributes of formulated soursop drinks.
4. Conclusions
Soursop as a tropical fruit showed good potential for use in the formulation of a functional drink. It also displayed potential for incorporating unique raw materials such as honey (a highly medicinal and nutritious food) and milk (to improve its mouthfeel, taste and to impart some creaminess) for value addition purposes. The increased protein content and significant antioxidant properties may increase the willingness of consumers to buy and consume the drink, thereby promoting its utilization and industrial processing as well as improved cardiovascular health of the consumers.
Author Contributions
Conceptualization, A.I.O.; Formal Analysis: A.I.O. and O.E.S.; Writing-Original Draft Preparation: O.E.S.; Writing-Review & Editing: A.I.O., Supervision: A.I.O.
Acknowledgments
Authors wish to thank J.B. Adeloye for her valuable contribution and A.A. Badejo for his technical assistance and grammar editing. Both in the Department of Food Science and Technology, Federal University of Technology, Akure, Ondo State, Nigeria.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbo, E.S.; Olurin, T.O.; Odeyemi, G. Studies on the storage stability of soursop (Annona muricate L.) juice. Afr. J. Biotechnol. 2006, 5, 1808–1812. [Google Scholar]
- Luzia, D.M.M.; Jorge, N. Soursop (Annona muricata L.) and sugar apple (Annona squamosa L.): Antioxidant activity, fatty acids profile and determination of tocopherols. Nutr. Food Sci. 2012, 42, 434–441. [Google Scholar] [CrossRef]
- Singh, D.R.; Medhi, R.P. Soursop Cultivation in Andaman; Director CIARI: Port Blair, India, 2003. [Google Scholar]
- Minh, N.P. Application of alginate film for soursop fruit preservation. Int. J. Appl. Eng. Res. 2017, 12, 15287–15291. [Google Scholar]
- Othman, O.C.; Fabian, C.; Lugwisha, E. Postharvest physicochemical properties of soursop (Annona muricate L.) fruits of Coast region, Tanzania. J. Food Nutr. Sci. 2014, 2, 220–226. [Google Scholar]
- Almeida-Muradian, L.B.; Stramm, K.M.; Horita, A.; Barth, O.M.; Freitas, A.S.; Estevinho, L.M. Comparative study of the physicochemical and palynological characteristics of honey from Melipona subnitida and Apis mellifera. Int. J. Food Sci. Technol. 2013, 48, 1698–1706. [Google Scholar] [CrossRef]
- Hussein, A.M.S.; Hegazy, N.A.; Kamil, M.M.; Ola, S.S.M. Formulation and evaluation of some healthy natural juice blends. Asian J. Sci. Res. 2016, 10, 160–168. [Google Scholar] [CrossRef]
- Awolu, O.O.; Aderinola, T.A.; Adebayo, I.A. Physicochemical and rheological behaviour of African star apple (Chrysophyllum albidium) juice as affected by concentration and temperature variation. J. Food Process. Technol. 2013, 4, 229–235. [Google Scholar]
- Akusu, O.M.; Kiin-Kabari, D.B.; Ebere, C.O. Quality characteristics of orange/pineapple fruit juice blends. Am. J. Food Sci. Technol. 2016, 4, 43–47. [Google Scholar]
- Karthikeyan, K.; Abitha, S.; Kumar, V.G. Identification of bioactive constituents in peel, pulp of prickly custard apple (Annona muricate) and its antimicrobial activity. Int. J. Pharm. Phytochem. Res. 2016, 8, 1833–1838. [Google Scholar]
- Ndife, J.; Kwaya, P.J.; Bello, S. Production and evaluation of storage changes in soursop-juice. Asian J. Agric. Food Sci. 2014, 2, 425–433. [Google Scholar]
- Mau, J.L.; Tsai, S.Y.; Tseng, Y.H.; Huang, S.J. Antioxidant properties of methanolic extracts from Ganoderma tsugae. Food Chem. 2005, 93, 641–649. [Google Scholar] [CrossRef]
- Association of official Analytical Chemists (AOAC). Official Methods of Analysis, Association of Official Analytical Chemists, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free radical scavenging activity of medicinal herb of Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Bao, J.Y.; Cai, M.; Sun, G.; Wang, G.; Corke, H. Anthocyanins, flavonoid and free radical scavenging activity of myrialrubia extracts and their colour properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Pulido, R.; Bravo, L.; Sauro-Calixo, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Morton, D. Aerobic plate count. In Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; Downes, F.P., Ito, K., Eds.; American Public Health Association: Washington, DC, USA, 2001; pp. 63–67. [Google Scholar]
- Harrigan, W.F.; MacCance, M.E. Laboratory Methods in Food and Dairy Microbiology, 1st ed.; Academic Press: London, UK, 1976; pp. 25–29. [Google Scholar]
- Yapo, E.S.; Kouakou, K.L.; Bognonkpe, T.P.; Kouame, P.; Kouakou, T.H. Comparison of pineapple fruit characteristics of plants propagated in three different ways: by suckers, micropropagation and somatic embryogenesis. J. Nutr. Food Sci. 2011, 1, 110–118. [Google Scholar]
- Islam, M.K.; Khan, M.Z.; Sarkar, M.A.; Absar, N.; Sarkar, S.K. Changes in acidity, TSS, and sugar content at different storage periods of the postharvest mango (Mangifera indica L.) influenced by Bavistin DF. Int. J. Food Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.M.; Gouveia, D.S.; Mata, M.E.; Queiroz, A.J. Rheological behaviour of mixed drink of Annona and milk. Eng. Agric. Jaboticabal 2012, 32, 333–341. [Google Scholar]
- De Moura, L.C.; da Silva, M.A.; Plácido, G.R.; Caliari, M.; Souza, D.G.; e Souza, J.L.F.; Célia, J.A.; de Oliveira, K.B.; Leão, K.M.; do Nascimento, L.E.C. Functional properties of milk drinks flavoured with mangaba pulp and enriched with passion fruit bark flour. Afr. J. Biotechnol. 2016, 15, 1846–1854. [Google Scholar]
- Nwachukwu, E.; Ezeigbo, C.G. Changes in microbial population of pasteurized soursop juice treated with benzoate and lime during storage. Afr. J. Microbiol. Res. 2013, 7, 3992–3995. [Google Scholar]
- Obasi, B.C.; Whong, C.M.; Ameh, J.B. Nutritional and sensory qualities of commercially and laboratory prepared orange juice. Afr. J. Food Sci. 2017, 11, 189–199. [Google Scholar] [CrossRef]
- Chavan, R.S.; Shraddha, R.C.; Kumar, A.; Nalawade, T. Whey based beverage: Its functionality, formulations, health benefits and applications. J. Food Process. Technol. 2015, 6, 495–503. [Google Scholar]
- Orsi, D.C.; Carvalho, V.S.; Nishr, A.C.; Damiani, C.; Asquieri, E.R. Use of sugar apple, atemoya, and soursop for technological development of jams-chemical and sensorial composition. Ciênc. Agrotecnol. Lavras 2012, 36, 560–566. [Google Scholar] [CrossRef]
- Bajwa, U.; Mittal, S. Quality characteristics of no added sugar ready to drink milk supplemented with mango pulp. J. Food Sci. Technol. 2015, 52, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Waston, R.R.; Preedy, V. Bioactive Foods in Promoting Health: Fruits and Vegetables; Academic Press: London, UK, 2009; pp. 628–629. [Google Scholar]
- Badrie, N.; Schauss, A.G. Composition, nutritional value, medicinal uses, and toxicology. In Bioactive Foods in Promoting Health; Waston, R.R., Preedy, V.R., Eds.; Academic Press: London, UK, 2009; pp. 621–641. [Google Scholar]
- Amoo, I.A.; Emenike, A.E.; Akpambang, V.O.E. Compositional evaluation of Annona cherimoya (custard apple) fruit. Trends Appl. Sci. Res. 2008, 3, 216–220. [Google Scholar]
- Chua, L.S.; Rahaman, N.L.; Adnan, N.A.; Tan, T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013. [Google Scholar] [CrossRef] [PubMed]
- Awsi, J.; Er-Dorcus, M. Development and quality evaluation of pineapple juice blend with carrot and orange juice. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–25. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health, Office of Dietary Supplements. Vitamin C Fact Sheet for Consumers. pp. 1–3. Available online: https://ods.od.nih.gov/pdf/factsheets/VitaminC-Consumer.pdf (accessed on 3 February 2018).
- Onimawo, I.A. Proximate composition and selected physicochemical properties of the seed, pulp and oil of soursop (Annona muricata). Plant Foods Hum. Nutr. 2002, 57, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cancer.Vg. Cancer Information & Resources. Available online: http://cancer.vg/en/annona-muricata-soursop (accessed on 26 February 2018).
- Onyechi, A.C.; Ibeanu, V.N.; Eme, P.E.; Kelechi, M. Nutrient, phytochemical composition and sensory evaluation of soursop (Annona muricate) pulp and drink in south eastern Nigeria. Int. J. Basic Appl. Sci. 2012, 12, 53–57. [Google Scholar]
- Emelike, N.J.T.; Hart, A.D.; Ebere, C.O. Influence of drying techniques on the properties, physicochemical and mineral composition of beetroot juice. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 20–26. [Google Scholar]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical Evaluation of Blood Pressure Lowering, Endothelial Function Improving, Hypolipidemic and Anti-Inflammatory Effects of Pomegranate Juice in Hypertensive Subjects. Phytopathol. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and herbal infusions; their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Syahida, M.; Maskat, M.Y.; Suri, R.; Mamot, S.; Hadijah, H. Soursop (Anona muricate L.): Blood hematology and serum biochemistry of Sprague-dawley rats. Int. Food Res. J. 2012, 19, 955–959. [Google Scholar]
- Madurangi, G.W.; Gunathilake, D. Development of an antioxidant rich beverage using soursop fruit and ginger extract. Ann. Food Sci. Technol. 2016, 17, 179–185. [Google Scholar]
- Akomolafe, S.F.; Ajayi, O.B. A comparative study on antioxidant properties, proximate and mineral composition of peel and pulp of ripe Annona muricata (L.) fruit. Int. Food Res. J. 2015, 22, 2381–2388. [Google Scholar]
- Bonoli-Carbognin, M.; Cerretani, L.; Bendini, A.; Almajano, M.P.; Gordon, M.H. Bovine serum albumin produces a synergistic increase in the antioxidant activity of virgin olive oil phenolic compounds in oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 7076–7081. [Google Scholar] [CrossRef] [PubMed]
- Benavente, G.O.; Castillo, J.; Marin, F.R. Uses and properties of Citrus flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515. [Google Scholar] [CrossRef]
- Vinson, J.A.; Zubik, L.; Bose, P.; Samman, N.; Proch, J. Dried fruits: Excellent in vitro and in vivo antioxidants. J. Am. Coll. Nutr. 2005, 24, 44–50. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).