Bioactive Peptides in Milk: From Encrypted Sequences to Nutraceutical Aspects
Abstract
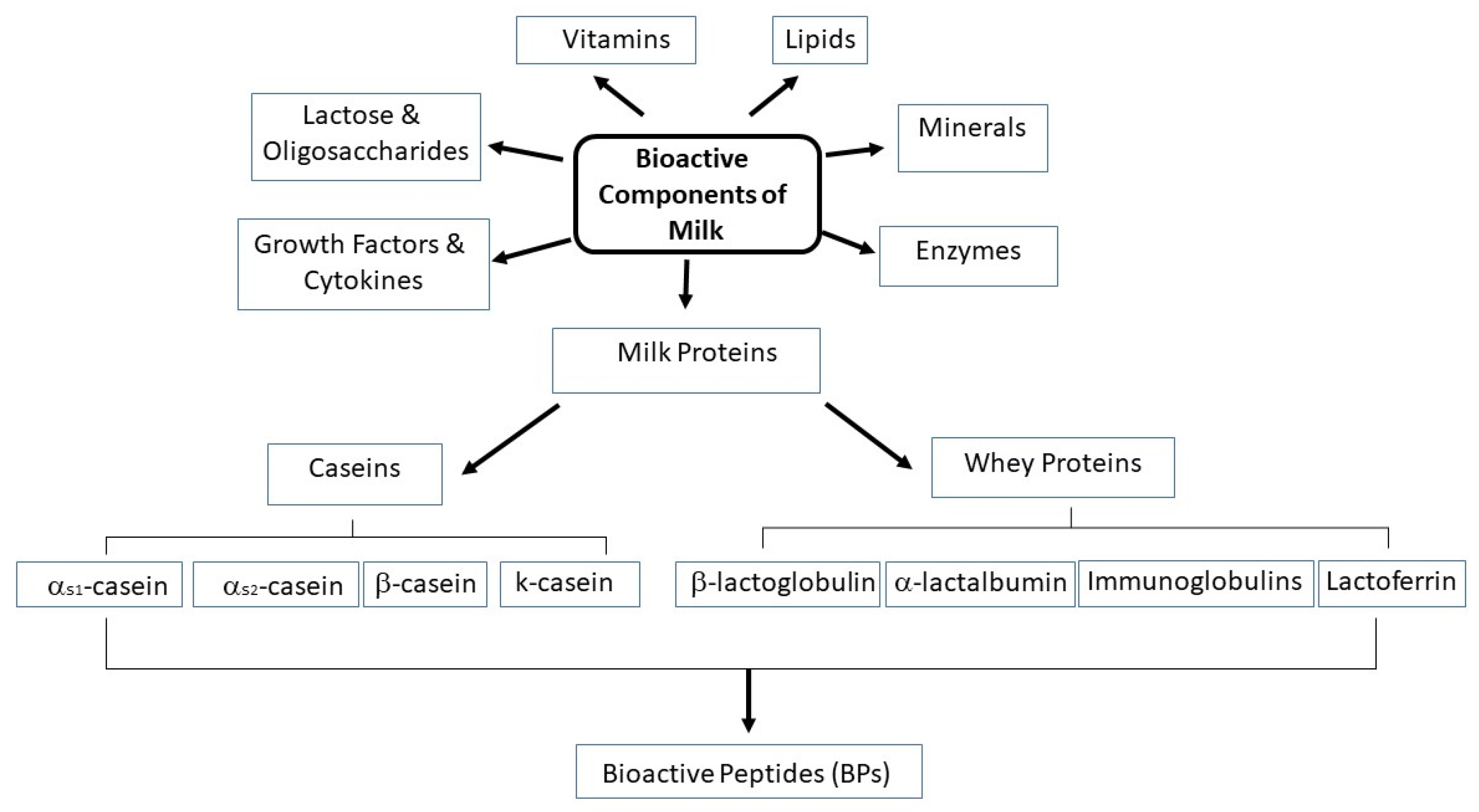
:1. Bioactive Components of Milk: Focus on BPs
2. Biochemical Properties
- −
- the transport of minerals (caseinophosphopeptides), such as calcium, and intestinal transport of amino acids, such as leucine, through the beta-casomorphin receptors;
- −
- the transport of intestinal fluid (beta-casomorphine);
- −
- the motility of the gastrointestinal tract (beta-casomorphine);
- −
- the stimulation of the postprandial hormone secretion (insulin, somatostatin) (beta-casomorphine);
- −
- the regulation of insulin secretion based on glucose concentration;
- −
- immunostimulant peptides (alpha and beta casein fragments);
- −
- anti-hypertensive peptides enzyme inhibitors converting angiotensin I (ACE) (casokinine);
- −
- antithrombotic peptides such as ADP-activated platelet aggregation inhibitors, as well as fibrinogen binding (γ-chain) to ADP-treated platelets (casoplateline);
- −
- opioid activities;
- −
- antioxidative functions;
- −
- hypocholesterolemic activities;
- −
- antitumor activities.
3. Bioavailability
4. Nutraceutical Aspects
4.1. Methodology toward Innovative Aspects
4.2. Applications
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Park, Y.W. Overview of bioactive components in milk and dairy products. In Bioactive Components in Milk and Dairy Products; Park, Y.W., Ed.; Wiley-Blackwell Publishers: Oxford, UK, 2009; pp. 3–14. [Google Scholar]
- Baum, F.; Fedorova, M.; Ebner, J.; Hoffmann, R.; Pischetsrieder, M. Analysis of the endogenous peptide profile of milk: Identification of 248 mainly casein-derived peptides. J. Proteome Res. 2013, 12, 5447–5462. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P.; Jenness, R. Dairy Chemistry and Physics; John Wiley: New York, NY, USA, 1984. [Google Scholar]
- Teschemacher, H.; Csontos, K.; Westenthanner, A.; Brantl, V.; Kromer, W. Endogenous opioids: Cold-induced release from pituitary tissue in vitro; extraction from pituitary and milk. In Endorphins in Mental Health Research; Usdin, E., Bunney, W.E., Kline, N.S., Eds.; Palgrave Macmillan: Basingstoke, UK, 1979; pp. 203–208. [Google Scholar]
- Phelan, M.; Aherne, A.; FitzGerald, R.J.; O’Brien, N.M. Casein-derived peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009, 19, 643–654. [Google Scholar] [CrossRef]
- Kamiński, S.; Cieslińska, A.; Kostyra, E. Polymorphism of bovine beta-casein and its potential effect on human health. J. Appl. Genet. 2007, 48, 189–198. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Review of the potential health impact of β-casomorphins and related peptides. Eur. Food Saf. Auth. 2009, 7. [Google Scholar] [CrossRef]
- Lopez-Fandino, R.; Otte, J.; van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Kamau, S.M.; Lu, R.-R.; Chen, W.; Liu, X.-M.; Tian, F.-W.; Shen, Y.; Gao, T. Functional significance of bioactive peptides derived from milk proteins. Food Rev. Int. 2010, 26, 386–401. [Google Scholar] [CrossRef]
- Nagpal, R.; Behare, P.; Rana, R.; Kumar, A.; Kumar, M.; Arora, S.; Morotta, F.; Jain, S.; Yadav, H. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food Funct. 2011, 2, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Chemical characterization and opioid activity of an exorphin isolated from in vivo digests of casein. FEBS Lett. 1986, 196, 223–227. [Google Scholar] [CrossRef]
- Cieślińska, A.; Kostyra, E.; Kostyra, H.; Oleński, K.; Fiedorowicz, E.; Kamiński, S. Milk from cows of different β-casein genotypes as a source of β-casomorphin-7. Int. J. Food Sci. Nutr. 2012, 63, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Nakagomi, K.; Tomizuka, N.; Suzuki, H. Angiotensin I-converting enzyme inhibitor derived from an enzymatic hydrolysate of casein. II. Isolation and bradykinin-potentiating activity on the uterus and the ileum of rats. Agric. Biol. Chem. 1985, 49, 1405–1409. [Google Scholar]
- Maruyama, S.; Suzuki, H. A peptide inhibitor of angiotensin I-converting enzyme in the tryptic hydrolysate of casein. Agric. Biol. Chem. 1982, 46, 1393–1394. [Google Scholar]
- Maeno, M.; Yamamoto, N.; Takano, T. Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1996, 79, 1316–1321. [Google Scholar] [CrossRef]
- Migliore-Samour, D.; Floch, F.; Jollès, P. Biologically active casein peptides implicated in immunomodulation. J. Dairy Res. 1989, 56, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, H.; Yonezawa, K.; Tohno, M.; Shimosato, T.; Kawai, Y.; Saito, T.; Wang, J.M. Enzymatic digestion of the milk protein beta-casein releases potent chemotactic peptide(s) for monocytes and macrophages. Int. Immunopharmacol. 2007, 7, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Stanton, C.; Slattery, H.; O’Sullivan, O.; Hill, C.; Fitzgerald, G.F.; Ross, R.P. Casein fermentate of Lactobacillus animalis DPC6134, contains a range of novel propeptide angiotensin-converting enzyme inhibitors. Appl. Environ. Microbiol. 2007, 73, 4658–4667. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Shindo, M.; Gunshin, H.; Noguchi, T.; Naito, H. Characterization of phosphopeptide derived from bovine beta-casein: An inhibitor to intra-intestinal precipitation of calcium phosphate. Biochim. Biophys. Acta 1991, 1077, 413–415. [Google Scholar] [CrossRef]
- Loukas, S.; Varoucha, D.; Zioudrou, C.; Streaty, R.A.; Klee, W.A. Opioid activities and structures of .alpha.-casein-derived exorphins. Biochemistry 1983, 22, 4567–4573. [Google Scholar] [CrossRef] [PubMed]
- Karaki, H.; Doi, K.; Sugano, S.; Uchiwa, H.; Sugai, R.; Murakami, U.; Takemoto, S. Antihypertensive effect of tryptic hydrolysate of milk casein in spontaneously hypertensive rats. Comp. Biochem. Physiol. C 1990, 96, 367–371. [Google Scholar] [PubMed]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Prakash, V. Bioactive peptides from bovine milk alpha-casein: Isolation, characterization and multifunctional properties. Int. J. Pept. Res. Ther. 2010, 16, 7–15. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tani, F.; Ashikaga, T.; Yoshimura, T.; Chiba, H. Purification and Characterization of an Opioid Antagonist from a Peptic Digest of Bovine κ-Casein. Agric. Biol. Chem. 1986, 50, 2951–2954. [Google Scholar] [CrossRef]
- Chiba, H.; Tani, F.; Yoshikawa, M. Opioid antagonist peptides derived from kappa-casein. J. Dairy Res 1989, 56, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Jollès, P.; Lévy-Toledano, S.; Fiat, A.M.; Soria, C.; Gillessen, D.; Thomaidis, A.; Dunn, F.W.; Caen, J.P. Analogy between fibrinogen and casein. Effect of an undecapeptide isolated from kappa-casein on platelet function. Eur. J. Biochem. 1986, 158, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Patten, G.S.; Head, R.J.; Abeywardena, M.Y. Effects of casoxin 4 on morphine inhibition of small animal intestinal contractility and gut transit in the mouse. Clin. Exp. Gastroenterol. 2011, 4, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, M.L.; Sipola, M.; Kaarto, H.; Pihlanto-Leppälä, A.; Piilola, K.; Korpela, R.; Tossavainen, O.; Korhonen, H.; Vapaatalo, H. Alpha-lactorphin lowers blood pressure measured by radiotelemetry in normotensive and spontaneously hypertensive rats. Life Sci. 2000, 66, 1535–1543. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Synthetic peptides corresponding to alpha-lactalbumin and beta-lactoglobulin sequences with angiotensin-I-converting enzyme inhibitory activity. Biol. Chem. Hoppe Seyler 1996, 377, 259–260. [Google Scholar] [PubMed]
- FitzGerald, R.J.; Meisel, H. Lactokinins: Whey protein-derived ACE inhibitory peptides. Nahrung 1999, 43, 165–167. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Identification of a novel angiotensin-I-converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine beta-lactoglobulin. FEBS Lett. 1997, 402, 99–101. [Google Scholar] [CrossRef]
- Yamauchi, R.; Ohinata, K.; Yoshikawa, M. Beta-lactotensin and neurotensin rapidly reduce serum cholesterol via NT2 receptor. Peptides 2003, 24, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Tani, F.; Shiota, A.; Chiba, H.; Yoshikawa, M. Serophin, an opioid peptide derived from serum albumin. In β-Casomorphins and Related Peptides: Recent Developments; Brantl, V., Teschemacher, H., Eds.; Wiley: Weinheim, Germany, 1994; pp. 49–53. [Google Scholar]
- Meisel, H.; Bockelmann, W. Bioactive peptides encrypted in milk proteins: Proteolytic activation and thropho-functional properties. Antonie Van Leeuwenhoek 1999, 76, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Multifunctional peptides encrypted in milk proteins. Biofactors 2004, 21, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Fiat, A.M.; Jolles, P. Caseins of various origins and biologically active casein peptides and oligosaccharides: Structural and physiological aspects. Mol. Cell. Biochem. 1989, 87, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Overview on milk protein-derived peptides. Int. Dairy J. 1998, 8, 363–373. [Google Scholar] [CrossRef]
- Meisel, H.; FitzGerald, R.J. Opioid peptides encrypted in milk proteins. Br. J. Nutr. 2000, 84, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Vermeirssen, V.; van Camp, J.; Verstraete, W. Bioavailability of angiotensin I-converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Brandsch, M.; Leibach, F.H. Intestinal transport of amino acids and peptides. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Ed.; Raven Press Ltd.: New York, NY, USA, 1994; pp. 1773–1794. [Google Scholar]
- Pauletti, G.M.; Gangwar, S.; Knipp, G.T.; Nerurkar, M.M.; Okumu, F.W.; Tamura, K.; Siahaan, T.J.; Borchardt, R.T. Structural requirements for intestinal absorption of peptide drugs. J. Control. Release 1996, 41, 3–17. [Google Scholar] [CrossRef]
- Pauletti, G.M.; Okumu, F.W.; Borchardt, R.T. Effect of size and charge on the passive diffusion of peptides across Caco-2 cell monolayers via the paracellular pathway. Pharm. Res. 1997, 14, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Gentilucci, L.; Tolomelli, A.; Calienni, M.; Qasem, A.R.; Spampinato, S. Stability against enzymatic hydrolysis of endomorphin-1 analogues containing beta-proline. Org. Biomol. Chem. 2003, 1, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Nishimura, S.; Matsuura, K.; Gotou, T.; Yamamoto, N. Release of short and proline-rich antihypertensive peptides from casein hydrolysate with an Aspergillus oryzae protease. J. Dairy Sci. 2004, 87, 3183–3188. [Google Scholar] [CrossRef]
- Savoie, L.; Agudelo, R.A.; Gauthier, S.F.; Marin, J.; Pouliot, Y. In vitro determination of the release kinetics of peptides and free amino acids during the digestion of food proteins. J. AOAC Int. 2005, 88, 935–948. [Google Scholar] [PubMed]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Svedberg, J.; de Hass, J.; Leimenstoll, G.; Paul, F.; Teschemacher, H. Demonstration ofmbeta-casomorphin immunoreactive materials in in vitro digests of bovine milk and in small intestine contents after bovine milk ingestion in adult humans. Peptides 1985, 6, 825–830. [Google Scholar] [CrossRef]
- Matar, C.; Amiot, J.; Savoie, L.; Goulet, J. The effect of milk fermentation by Lactobacillus helveticus on the release of peptides during in vitro digestion. J. Dairy Sci. 1996, 79, 971–979. [Google Scholar] [CrossRef]
- Miquel, E.; Gomez, J.A.; Alegria, A.; Barbera, R.; Farre, R.; Recio, I. Identification of casein phosphopeptides released after simulated digestion of milk-based infant formulas. J. Agric. Food Chem. 2005, 53, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. The scientific evidence for the role of milk protein-derived bioactive peptides in humans: A review. J. Funct. Foods 2015, 17, 640–656. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derivedpeptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Laganà, A. Recent Trends in the analysis of bioactive peptides in milk and dairy products. Anal. Bioanal. Chem. 2016, 408, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Muro Urista, C.; Álvarez Fernández, R.; Riera Rodriguez, F.; Arana Cuenca, A.; Téllez Jurado, A. Review: Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Lee, H.; Parc, A.L.; de Moura Bell, J.M.L.N.; Barile, D. Coupling Mass Spectrometry-Based “Omic” Sciences with Bioguided Processing to Unravel Milk’s Hidden Bioactivities. Adv. Dairy Res. 2013, 1, 104. [Google Scholar] [CrossRef]
- Sánchez-Rivera, L.; Martínez-Maqueda, D.; Cruz-Huerta, E.; Miralles, B.; Recio, I. Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides. Food Res. Int. 2014, 63, 170–181. [Google Scholar] [CrossRef]
- Kareb, O.; Gomaa, A.; Champagne, C.P.; Jean, J.; Aïder, M. Electro-activation of sweet defatted whey: Impact on the induced Maillard reaction products and bioactive peptides. Food Chem. 2017, 221, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Bitri, L. Optimization Study for the Production of an Opioid-like Preparation from Bovine Casein by Mild Acidic Hydrolysis. Int. Dairy J. 2004, 14, 535–539. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Krissansen, G.W. Emerging health properties of whey proteins and their clinical implications. J. Am. Coll. Nutr. 2007, 26, 713S–723S. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides—Opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Marnila, P. Bovine milk antibodies for protection against microbial human diseases. In Nutraceutical Proteins and Peptides in Health and Disease; Mine, Y., Shahidi, S., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 137–159. [Google Scholar]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980–988. [Google Scholar]
- Dziuba, B.; Dziuba, M. Milk proteins-derived bioactive peptides in dairy products: Molecular, biological and methodological aspects. Acta Sci. Pol. Technol. Aliment. 2014, 13, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Folmer Corrêa, A.P. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Yadav, J.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Manfra, M.; Tenore, G.; Russo, M.; Novellino, E.; et al. Detailed peptide profiling of “Scotta”: From a dairy waste to a source of potential health-promoting compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar] [CrossRef]
- Patel, S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J. Food Sci. Technol. 2015, 52, 6847–6858. [Google Scholar] [CrossRef] [PubMed]
- Athira, S.; Mann, B.; Saini, P.; Sharma, R.; Kumar, R.; Singh, A.K. Production and characterisation of whey protein hydrolysate having antioxidant activity from cheese whey. J. Sci. Food Agric. 2015, 95, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, M.H.; El-Shibiny, S. Preparation, properties, and uses of enzymatic milk protein hydrolysates. Crit. Rev. Food Sci. Nutr. 2017, 57, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
| Precursor Protein | Fragment | Peptide Sequence | Name | Biological Activity | Preparation | References |
|---|---|---|---|---|---|---|
| Casein Protein | ||||||
| β-casein | 60–70 | YPFPGPIPNSL | β-casomorphin-11 | Opioid | Hydrolysis with digestive enzymes Trypsin | [13] |
| 60–66 | YPFPGPI | β-casomorphin-7 | Opioid ACE Inhibitory Immunomodulatory | Mixture of gastro-intestinal enzymes Trypsin | [14] | |
| 60–64 | YPFPG | β-casomorphin-5 | Opioid ACE Inhibitory | Hydrolysis with trypsin | [15] | |
| 177–183 | AVPYPQR | β-casokinin-7 | ACE Inhibitory | Hydrolysis with trypsin | [15] | |
| 193–202 | YQQPVLGPVR | β-casokinin-10 | ACE Inhibitory Immunomodulatory | Hydrolysis with trypsin | [16] | |
| 169–175 | KVLPVPQ | ACE inhibition | Hydrolysis with proteinase | [17] | ||
| 63–68 | PGPIPN | Immunopeptide | Immunomodulatory | Trypsin or chymosin | [18] | |
| 191–193 | LLY | Immunopeptide | Immunomodulatory | Trypsin or chymosin | [18] | |
| 114–118 | YPVEP | βcasochemotide-1 | Immunomodulatory | Hydrolysis with proteinase | [19] | |
| 210–221 | EPVLGPVRGPFP | ACE-inhibition | Fermentation | [20] | ||
| (1–25)4P | RELEELNVPGEIVESLSSSEESITR | Caseinophosphopeptide | Ca++ binding | Trypsin or chymosin | [21] | |
| αs1-casein | 90–96 | RYLGYLE | α-casein exorphin | Opioid | Hydrolysis with pepsin | [22] |
| 90–95 | RYLGYL | α-casein exorphin | Opioid | Hydrolysis with pepsin | [22] | |
| 91–96 | YLGYLE | α-casein exorphin | Opioid | Hydrolysis with pepsin | [22] | |
| 23–27 | FFWAP | αs1-Casokinin-5 | ACE inhibition | Hydrolysis with trypsin | [16] | |
| 28–34 | FPEWFGK | αs1-Casokinin-7 | ACE inhibition | Hydrolysis with trypsin | [16] | |
| 194–199 | TTMPLW | αs1-Casokinin-6 | ACE inhibition, Immunomodulatory | Hydrolysis with trypsin | [23] | |
| 169–193 | LGTQYTDAPSFSDIPNPIGSENSEK | ACE-inhibition | Trypsin or chymosin | [24] | ||
| αs2-casein | 94–103 | QKALNEINQF | Antimicrobial ACE inhibition | Hydrolysis with chymotrypsin | [25] | |
| 163–176 | TKKTKLTEEEKNRL | ACE inhibition | Hydrolysis with chymotrypsin | [25] | ||
| k-Casein | 33–38 | SRYPSY | Casoxin 6 | Anti-Opioid | Hydrolysis with pepsin | [26] |
| 25–34 | YIPIQYVLSR | Casoxin C | Anti-Opioid | Hydrolysis with trypsin | [27] | |
| 106–116 | MAIPPKKNQDK | Casoplatelin | Antithrombotic:inhibition of platelet aggragation | Hydrolysis with trypsin | [28] | |
| YPSY | Casoxin 4 | Opioid agonist | Synthetic | [29] | ||
| Whey Proteins | ||||||
| α-lactalbumin | 50–53 | YGLF | α-lactorphin | Opioid agonist ACE inhibition | Hydrolysis with gastric and pancreatic enzymes | [30] |
| β-lactoglobulin | 102–105 | TLLF | β-lactorphin | Non-opioid ACE-inhibition | Tryptic digest | [31] |
| β-lactoglobulin | 142–148 | ALPMHIR | ACE-inhibition | Proteolytic digestion | [32,33] | |
| 146–149 | HIRL | β-lactotensin | Ileum contraction, hypocholesterolemic activity | Synthetic | [31,34] | |
| Bovine Serum Albumin | 208–216 | ALKAWSVAR | Albutensin A | Ileum contraction, ACE inhibition | Hydrolysis with proteinase | [32] |
| 399–404 | YGFQDA | Serorphin | Opioid | Hydrolysis with pepsin | [35] | |
| Lactoferrin | 17–41/42 | FKCRRWQWRMKKLGAPSICURRAF/A | Lactoferricin | Antimicrobial | Hydrolysis with pepsin | [36] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, M. Bioactive Peptides in Milk: From Encrypted Sequences to Nutraceutical Aspects. Beverages 2017, 3, 41. https://doi.org/10.3390/beverages3030041
Lucarini M. Bioactive Peptides in Milk: From Encrypted Sequences to Nutraceutical Aspects. Beverages. 2017; 3(3):41. https://doi.org/10.3390/beverages3030041
Chicago/Turabian StyleLucarini, Massimo. 2017. "Bioactive Peptides in Milk: From Encrypted Sequences to Nutraceutical Aspects" Beverages 3, no. 3: 41. https://doi.org/10.3390/beverages3030041
APA StyleLucarini, M. (2017). Bioactive Peptides in Milk: From Encrypted Sequences to Nutraceutical Aspects. Beverages, 3(3), 41. https://doi.org/10.3390/beverages3030041





