Mini-Review: The Role of Saccharomyces cerevisiae in the Production of Gin and Vodka
Abstract
:1. Introduction
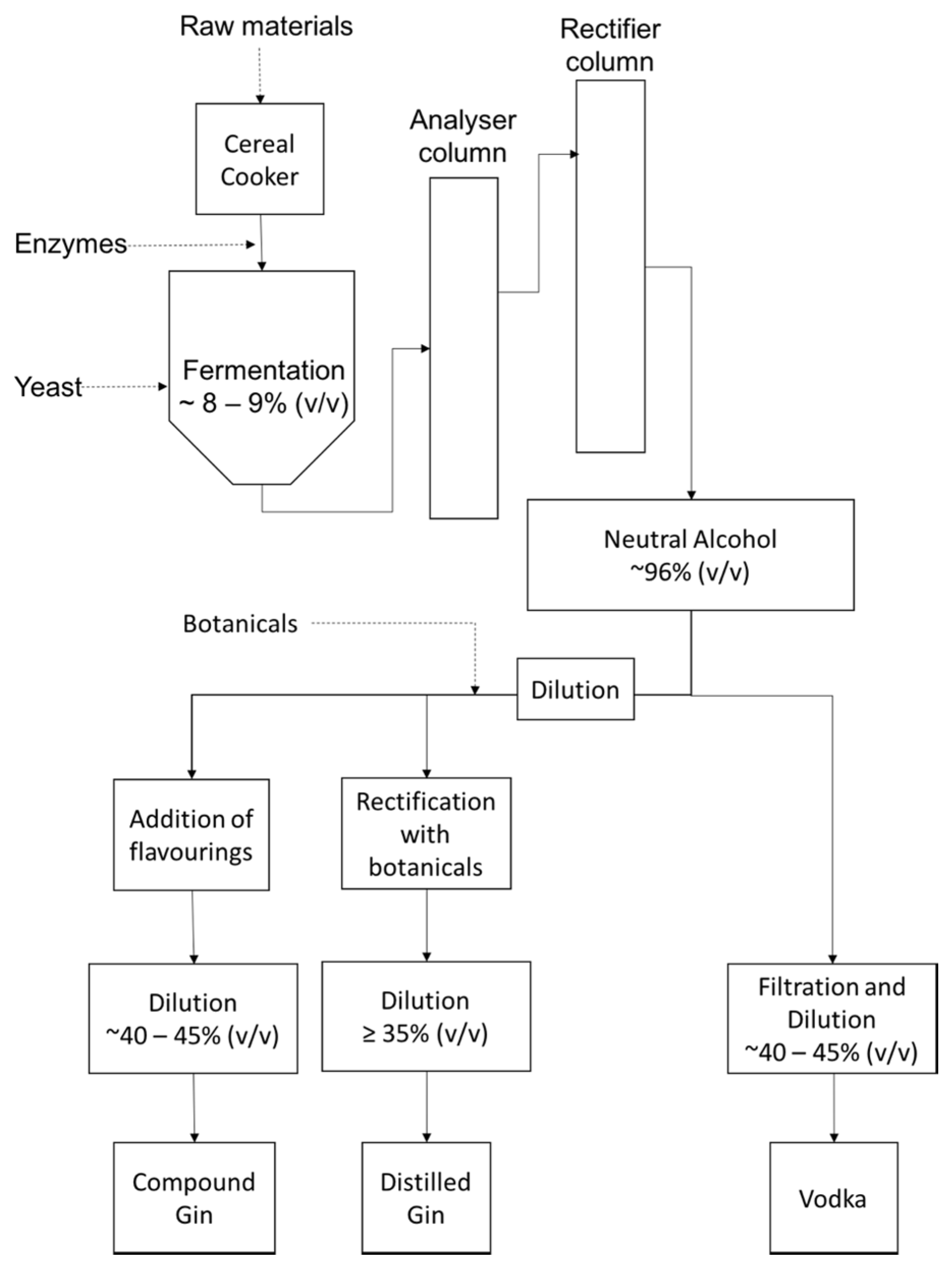
2. Process Overview
3. Microbiological Aspects
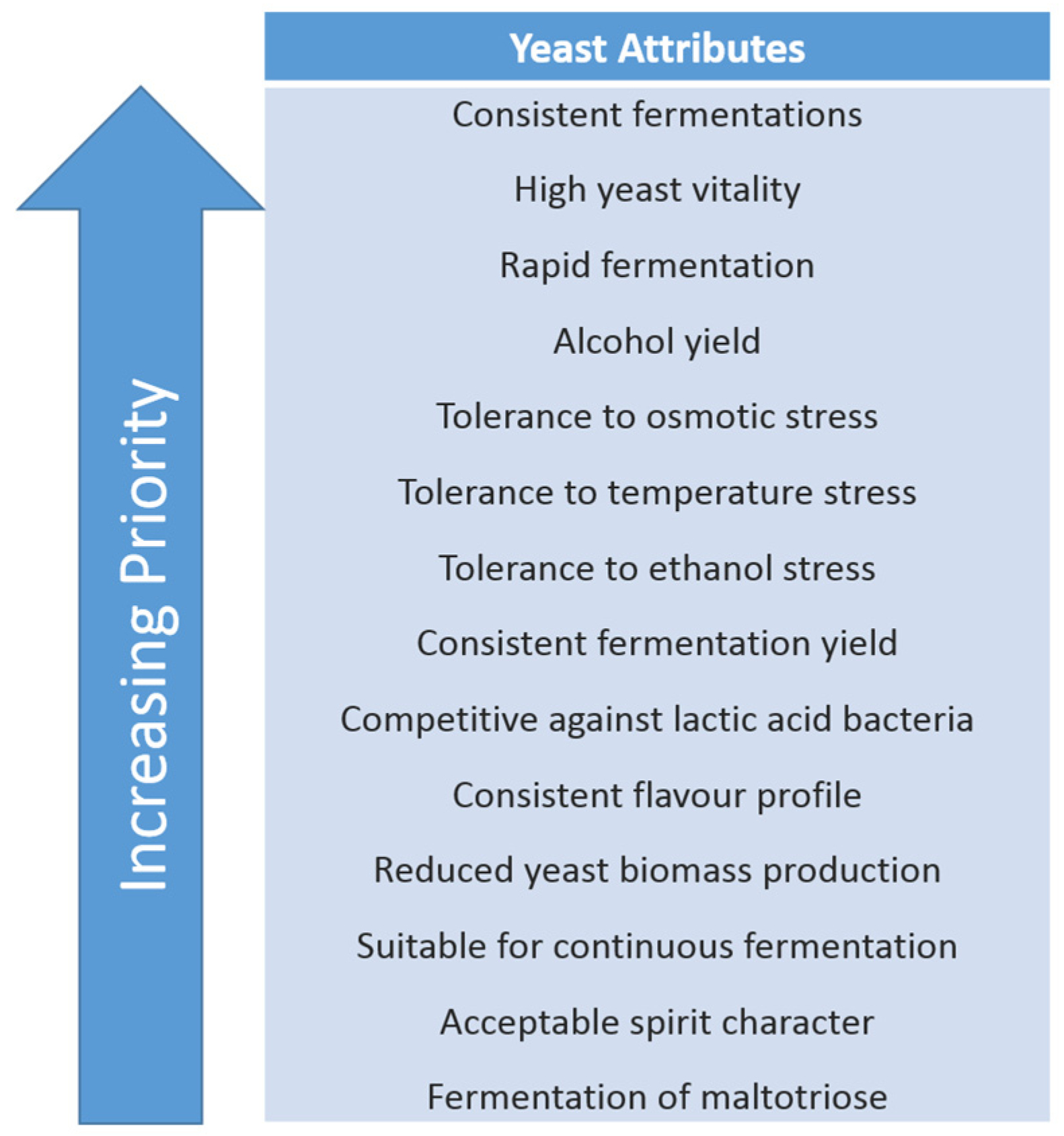
4. Role of Yeast
5. Yeast Strain Selection and Improvement
6. Conclusions
Conflicts of Interest
References
- Aylott, R.I. Gin and Vodka. In Production, Technology and Innovation, Proceedings of the Worldwide Distilled Spirits Conference, Edinburgh, UK, 18–22 September 2005; Bryce, J.H., Piggott, J.R., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2008; pp. 299–303. [Google Scholar]
- Buglass, A.J.; McKay, M.; Lee, C.G. Chapter 3.4: Other cereal based spirits. In Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects, 1st ed.; Buglass, A.J., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 535–553. [Google Scholar]
- Christian, D. ‘Living Water’: Vodka and Russian Society on the Eve of Emancipation, 1st ed.; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Benjamins, A.N. Vodka. Russia’s Gigantic Monopoly in Drink. How it is made; How it is sold and what is done with the money. The Idler; An Illustrated Monthly Magazine 1902, 159–165. [Google Scholar]
- Murghagh, J.E. Chapter 13: Production of neutral spirits and preparation of gin and vodka. In The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries, 3rd ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University Press: Nottingham, UK, 1999; pp. 195–210. [Google Scholar]
- European Union (EU). Regulation (EC) No. 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, Description, Presentation, Labelling and the Protection of Spirit Drinks, 2008. Available online: http://www.wipo.int/wipolex/en/details.jsp?id=5422 (accessed 4 February 2017).
- Aylott, R.I. Flavoured Spirits. In Fermented Beverage Production, 1st ed.; Lea, A.G.H., Piggott, J., Eds.; Blackie Academic and Professional: Glasgow, UK, 1995; pp. 275–300. [Google Scholar]
- Korhola, M. Developments in vodka production. In New Horizons: Energy, Environment and Enlightenment, Proceedings of the Worldwide Distilled Spirits Conference, Edinburgh, 7–10 September 2008; Walker, G.M., Hughes, P.S., Eds.; EnergyNottingham University Press: Nottingham, UK, 2010; pp. 53–61. [Google Scholar]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Differentiation between spirits according to their botanical origin. Food Anal. Methods 2016, 9, 1029–1035. [Google Scholar] [CrossRef]
- Alcohol and Tobacco Tax and Trade Bureau. Electronic Code of Federal Regulations: Title 27, Chapter 1, Subchapter A, Part 5. US Government Publishing Office. 2017–01–04. Available online: http://www.ecfr.gov/cgi-bin/text-idx?SID=eddaa2648775eb9b2423247641bf5758&mc=true&node=pt27.1.5&rgn=div5#se27.1.5_121 (accessed on 4 January 2017).
- MarketLine. Industry Profile: Global Spirits; Datamonitor: London, UK, 2015. [Google Scholar]
- Hughes, N. Gin. Nutr. Food Sci. 1992, 92, 14–146. Available online: http://dx.doi.org/10.1108/EUM0000000000962 (accessed on 1 September 2016). [Google Scholar]
- McCabe, M.; Gohdes, D.; Morgan, F.; Eakin, J.; Sanders, M.; Schmitt, C. Herbal Therapies and Diabetes Among Navajo Indians. Diabetes Care 2005, 28, 1534–1535. [Google Scholar] [CrossRef] [PubMed]
- Pennington, W.; Tutin, T.G. Vegetation History in the North West of England: A Regional Synthesis. In Studies in the Vegetation History of the British Isles: Essays in Honour of Harry Godwin; Walker, D., West, R.G., Eds.; Cambridge University Press: London, UK, 1970; p. 43. [Google Scholar]
- Van Acker–Beittel, V. Genever: 500 Years of History in A Bottle, 1st ed.; Flemish Lion LLC: Richmond, VA, USA, 2014. [Google Scholar]
- Abel, E.L. The gin epidemic: much ado about what? Alcohol Alcohol. 2001, 36, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Coates, G. Classic Gin, 1st ed.; Prion Press: London, UK, 2000; pp. 77–84. [Google Scholar]
- Simpson, A.C. Gin Manufacture. Process Biochem. 1966, 1, 355–358, 365. [Google Scholar]
- Simpson, A.C. Gin and Vodka. In Alcoholic Beverages; Rose, A.H., Ed.; Academic Press: London, UK, 1977; pp. 537–594. [Google Scholar]
- Clutton, D.W. The production of Gin and Vodka. Brew. Guard. 1979, 108, 25–30. [Google Scholar]
- Barnett, R. The Book of Gin; Grove Press: New York, NY, USA, 2011. [Google Scholar]
- Knoll, A.J.; Smith, D.T. The Craft of Gin; White Mule Press: Hayward, CA, USA, 2013. [Google Scholar]
- Phelan, A.D.; Jack, F.R.; Conner, J.M.; Reid, K.J.G.; Priest, F.G. Sensory assessment of gin flavour. In Tradition and Innovation, Proceedings of the Worldwide Distilled Spirits Conference, Edinburgh, UK, 8–11 September, 2002; Bryce, J.H., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2004; pp. 53–58. [Google Scholar]
- Vichi, S.; Riu-Aumatell, M.; Mora-Pons, M.; Buxaderas, S.; López-Tamames, E. Characterization of volatiles in different dry gins. J. Agric. Food Chem. 2005, 53, 10154–10160. [Google Scholar] [CrossRef] [PubMed]
- MacNamara, K.; Howell, J.; Huang, Y.; Albert, R. Analysis of gin essential oil mixtures by multidimensional and one-dimensional gas chromatography/mass spectrometry with spectral deconvolution. J. Chromat. A 2007, 1164, 281–290. [Google Scholar]
- El-Ghorab, A.; Shaaban, H.A.; El-Massry, K.F.; Shibamoto, T. Chemical composition of volatile extract and biological activities of volatile and less-volatile extracts of juniper berry (Juniperus drupacea L.) fruit. J. Agric. Food Chem. 2008, 56, 5021–5025. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Riu-Aumatell, M.; Buxaderas, S.; López-Tamames, E. Assessment of some diterpenoids in commercial distilled gin. Anal. Chim. Acta 2008, 628, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.; Kowalsick, A.; Howell, J. Tracking juniper berry content in oils and distillates by spectral deconvolution of gas chromatography/mass spectrometry data. J. Chromatog. A 2011, 1218, 5531–5541. [Google Scholar]
- Dussort, P.; Deprêtre, N.; Bou-Maroun, E.; Fant, C.; Guichard, E.; Bruinerie, P.; Le Fur, Y.; Le Quéré, J.-L. An original approach for gas chromatography-olfactometry detection frequency analysis: Applications to gin. Food Res. Int. 2012, 49, 253–262. [Google Scholar] [CrossRef]
- Sádecká, J.; Uríčková, V.; Hrobońová, K.; Májek, P. Classification of juniper-flavoured spirit drinks by multivariate analysis of spectroscopic and chromatographic data. Food Anal. Methods 2015, 8, 58–69. [Google Scholar] [CrossRef]
- Greer, D.; Pfahl, L.; Rieck, J.; Daniels, T.; Garza, O. Comparison of a novel distillation method in a model gin system using liquid/liquid extraction. J. Agric. Food Chem. 2008, 56, 9030–9036. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.R. Contamination: Bacteria and wild yeasts in a whisky fermentation. In Whisky: Technology, Production and Marketing, 2nd ed.; Russell, I., Stewart, G., Eds.; Academic Press: Oxford, UK, 2014; pp. 147–154. [Google Scholar]
- Walker, G.M.; Brosnan, J.; Bringhurst, T.; Jack, F. Selecting new distilling yeast for improved fermentation and for sustainability. In Science and Sustainability, Proceedings of the Worldwide Distilled Spirits Conference, Glasgow, UK, 12–15 September 2011; Walker, G.M., Goodall, I., Fotheringham, R., Murray, D., Eds.; Nottingham University Press: Nottingham, UK, 2012; pp. 127–136. [Google Scholar]
- Richards, C. Diversity of yeast supply for distilled spirit fermentation. In Future Challenges, New Solutions, Proceedings of the Worldwide Distilled Spirits Conference, Glasgow, UK, 8–11 September 2014; Goodall, I., Fotheringham, R., Murray, D., Speers, R.A., Walker, G.M., Eds.; Context Products Ltd.: Packington, UK, 2015; pp. 37–41. [Google Scholar]
- Simpkins, W.A. Congener profiles in the detection of illicit spirits. J. Sci. Food Agric. 1985, 36, 367–376. [Google Scholar] [CrossRef]
- Gregori, M.; Motta, P.; Caldarelli, G. Chemistry supports marketing: Characterisation of vodkas. In Future Challenges, New Solutions, Proceedings of the Worldwide Distilled Spirits Conference, Glasgow, UK, 8–11 September 2014; Goodall, I., Fotheringham, R., Murray, D., Speers, R.A., Walker, G.M., Eds.; Context Products Ltd.: Packington, UK, 2015; pp. 221–223. [Google Scholar]
- Datamonitor. Skyy Vodka Case Study: Creating Differentiation in the Premium Vodka Market; Informa PLC: London, UK, 2009. [Google Scholar]
- Kotarska, K.; Klosowski, G.; Czupryński, B. Characterization of technological features of dry yeast (strain I-7–43) preparation, production of electrofusion between Saccharomyces cerevisiae and Saccharomyces diastaticus, in industrial application. Enzym. Microb. Technol. 2011, 49, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Janser, E.; Andersen, E.A. Advanced enzymatic pre-treatment for High Gravity fermentation. In New Horizons: Energy, Environment and Enlightenment, Proceedings of the Worldwide Distilled Spirits Conference, Edinburgh, 7–10 September 2008; Walker, G.M., Hughes, P.S., Eds.; Nottingham University Press: Nottingham, UK, 2010; pp. 20–26. [Google Scholar]
- Butcher, S.; Koistinen, T.; Väisänen, E. The growth of contaminating Lactobacilli and alcohol or baker’s yeast production organisms under inhibitory conditions. In Proceedings of the COMETT Course on Microbial Contaminants, Helsinki, Finland, 9–10 June 1992; Korhola, M., Backström, V., Eds.; Foundation for Biotechnological and Industrial Fermentation Research: Helsinki, Finland, 1992; Volume 7, pp. 99–130. [Google Scholar]
- Lim, Y.; Jang, Y.; Kim, K. Production of a high concentration of ethanol from potato tuber by high gravity fermentation. Food Sci. Biotechnol. 2013, 22, 441–448. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Menezes, E.G.T.; Alves, J.G.L.F.; Rodrigues, L.F.; das Graças Cardoso, M. Vodka production from potato (Solanum tuberosum L.) using three Saccharomyces cerevisiae isolates. J. Inst. Brew. 2016, 122, 76–83. [Google Scholar]
- Tomaszewska, M.; Białończyk, L. Ethanol production from whey in a bioreactor coupled with direct contact membrane distillation. Catal. Today 2016, 268, 156–163. [Google Scholar] [CrossRef]
- Perederii, M.; Tsodikov, M.; Uvarov, V. Purification of aqueous alcohol solutions in two-bed adsorber filters. Solid Fuel Chem. 2011, 45, 34–38. [Google Scholar] [CrossRef]
- Cai, L.; Rice, S.M.; Koziel, J.A.; Jenks, W.S.; van Leeuwen, J.H. Further purification of food-grade alcohol to make a congener-free product. J. Inst. Brew. 2016, 122, 84–92. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Qualitative characteristics and comparison of volatile fraction of vodkas made from different botanical materials by comprehensive two-dimensional gas chromatography and the electronic nose based on the technology of ultra-fast gas chromatography. J. Sci. Food Agric. 2016. Available online: http//dx.doi.org/10.1002/jsfa.7868 (accessed on 10 August 2016). [Google Scholar]
- Siříšťová, L.; Přinosilová, S.; Riddellová, K.; Hajšlová, J.; Melzoch, K. Changes in quality parameters of vodka filtered through activated charcoal. Czech J. Food Sci. 2012, 30, 474–482. [Google Scholar]
- Cheung, A.W.Y.; Brosnan, J.M.; Smart, K.A. The tolerance of Saccharomyces cerevisiae distilling and bioethanol strains to ethanol. In Science and Sustainability, Proceedings of the Worldwide Distilled Spirits Conference, Glasgow, UK, 12–15 September 2011; Walker, G.M., Goodall, I., Fotheringham, R., Murray, D., Eds.; Nottingham University Press: Nottingham, UK, 2012; pp. 117–125. [Google Scholar]
- Walker, G.; Bringhurst, T.; Brosnan, J. The ideal distillers yeast. Brew. Distill. Int. 2011, 7, 30–32. [Google Scholar]
- Cheung, A.W.Y.; Brosnan, J.M.; Phister, T.; Smart, K.A. Impact of dried, creamed and cake supply formats on the genetic variation and ethanol tolerance of three Saccharomyces cerevisiae distilling strains. J. Inst. Brew. 2012, 118, 152–162. [Google Scholar]
- Lallemand Biofuels and Distilled Spirits. Stabilized Liquid Yeast. 2017–01–04. Available online: http://www.lallemandbds.com/wp-content/uploads/2012/12/2013_LBDS_Data-Sheet_SLY_10090_Data_Rev.00.01.01.2013.pdf (accessed on 4 January 2017).
- Plutowska, B.; Biernacka, P.; Wardencki, W. Identification of volatile compounds in raw spirits of different organoleptic quality. J. Inst. Brew. 2010, 116, 433–439. [Google Scholar] [CrossRef]
- Harrison, J.S.; Graham, J.C.J. Yeasts in distillery practice. In The Yeasts, Volume 3; Rose, A.H., Harrison, J.S., Eds.; Academic Press: London, UK, 1970; pp. 283–348. [Google Scholar]
- Biernacka, P.; Wardencki, W. Volatile composition of raw spirits of different botanical origin. J. Inst. Brew. 2012, 118, 393–400. [Google Scholar] [CrossRef]
- Klosowski, G.; Czupryński, B. Kinetics of acetals and esters formation during alcoholic fermentation of various starchy raw materials with application of yeasts Saccharomyces cerevisiae. J. Food. Eng. 2006, 72, 242–246. [Google Scholar] [CrossRef]
- Ng, L.-K.; Hupé, J.; Harnois, J.; Moccia, D. Characterisation of commercial vodkas by solid-phase microextraction and gas chromatography/mass spectrometry analysis. J. Sci. Food Agric. 1996, 71, 380–388. [Google Scholar] [CrossRef]
- Klosowski, G.; Czupryński, B.; Wolska, M. Characteristics of alcoholic fermentation with the application of Saccharomyces cerevisiae yeasts: As-4 strain and I-7–43 fusant with amylolytic properties. J. Food Eng. 2006, 76, 500–505. [Google Scholar] [CrossRef]
- Pietruszka, M.; St. Szopa, J. Agricultural distillates from Polish varieties of rye. Czech. J. Food Sci. 2014, 32, 406–411. [Google Scholar]
- De Nicola, R.; Hall, N.; Melville, S.G.; Walker, G.M. Influence of zinc on distiller’s yeast: Cellular accumulation of zinc and impact on spirit congeners. J. Inst. Brew. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. 2007, 31, 535–569. [Google Scholar] [CrossRef] [PubMed]
- James, T.C.; Campbell, S.; Donnelly, D.; Bond, U. Transcription profile of brewery yeast under fermentation conditions. J. Appl. Microbiol. 2003, 94, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.M.; Huang, X.W.; Zhang, L.M.; Zhao, N.; Yang, D.M.; Zhang, K.Q. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009, 85, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Moffat, F.; Jamieson, D.J. Analysis of a distiller’s yeast during industrial grain fermentation. In Science and Sustainability, Proceedings of the Worldwide Distilled Spirits Conference, Glasgow, UK, 12–15 September 2011; Walker, G.M., Goodall, I., Fotheringham, R., Murray, D., Eds.; Nottingham University Press: Nottingham, UK, 2012; pp. 109–116. [Google Scholar]
- Hansen, R.; Ferguson, A.M.; Pearson, S.Y.; Brosnan, J.M.; Meaden, J.M.; Jamieson, D.J. Proteomic analysis of distillers’ yeast. In Production, Technology and Innovation. Proceedings of the Worldwide Distilled Spirits Conference, Edinburgh, UK, 18–22 September 2005; Bryce, J.H., Piggott, J.R., Stewart, G.G., Eds.; Nottingham University Press: Nottingham, UK, 2008; pp. 93–107. [Google Scholar]
- Watson, D.C. Distilling Yeasts. Dev. Ind. Microbiol. 1984, 25, 213–220. [Google Scholar]
- Walker, G.M. Current challenges and future opportunities for distillers yeast: Discussion forum. In Science and Sustainability, Proceedings of the Worldwide Distilled Spirits Conference, Glasgow, UK, 12–15 September 2011; Walker, G.M., Goodall, I., Fotheringham, R., Murray, D., Eds.; Nottingham University Press: Nottingham, UK, 2012. [Google Scholar]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvabhowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 2013, 505, 239. [Google Scholar] [CrossRef] [PubMed]
- Grunert, K.G.; Bredahl, L.; Scholderer, J. Four questions on European consumers’ attitudes toward the use of genetic modification in food production. Inn. Food Sci. Emerg. Technol. 2003, 4, 435–445. [Google Scholar] [CrossRef]
- Frewer, L.J.; van der Lans, I.A.; Fischer, A.R.H.; Reinders, M.J.; Menozzi, D.; Zhang, X.; van den Berg, I.; Zimmerman, K.L. Public perception of agri-food applications of genetic modification―A systematic review and meta-analysis. Trends Food Sci. Technol. 2013, 30, 142–152. [Google Scholar] [CrossRef]
| Common Name | Botanical Name | Principle Flavour Contribution | Principal Origins |
|---|---|---|---|
| Traditional Gin Botanicals | |||
| Juniper berries | Juniperus communis | Dry pine, resin | Italy, Central Europe |
| Coriander seed | Coriandrum sativum | Citreous, grapefruit | Morocco, Eastern Europe |
| Angelica root | Archangelica officinalis | Savoury, dry and incense | Germany |
| Orris Root | Iris germanica, Iris pallida | Floral, fixative of flavours | Italy |
| Sweet orange peel | Citrus sinensis | Warming citrus | Italy |
| Bitter orange peel | Citrus aurantiun | Marmalade, dry citrus | Spain |
| Lemon peel | Citrus limon | Clean citrus, zesty | Mediterranean |
| Contemporary Gin Botanicals | |||
| Szechuan Pepper (Sichuan pepper) | Zanthoxyum simulans Zanthoxylum bungeanum | Tingly spice, numbing, warmth | China |
| Cucumber | Cucumis sativus | Palate cleansing and slight melon | South Asia /Europe |
| Rose | Various family Rosa | Floral rose | Various |
| Cumin | Cuminum cyminum | Rich spicy, musky | Pakistan India |
| Cubeb Berries | Piper cubeba | Woody, camphor | Java/ Sumatra |
| Grains of Paradise | Aframomum melegueta | Citrus and pepper | Ethiopia |
| Company (Location) | Product | Strain | Notes |
|---|---|---|---|
| ABMauri (Hull, UK) | Pinnacle Distillers Yeast | S. cerevisiae | Optimised conversion of sugar to alcohol, consistent flavours. |
| Kerry Foods (Menstrie, UK) | M | Hybrid of S. cerevisiae and S. diastaticus | Mainstay of many Scotch whisky distilleries. Fast fermenting, super attenuator. Recommended for gin and vodka. |
| Lallemand (Felixstowe, UK) Or Lallemand BDS (Milwaukee, WI, USA) | Distillamax DS | S. cerevisiae | For vodka and neutral grain alcohol (also base for light whiskies). |
| Distillamax HT | S. cerevisiae | Tolerant of high fermentation temperatures and high gravity fermentations. Aimed at a wide range of beverage alcohol fermentations. | |
| Distillamax LS | S. cerevisiae bayanus | Suitable for batch and semi-continuous fermentation systems. Recommended for tequila, fruit brandies, and neutral grain spirit. | |
| Distillamax SR | S. cerevisiae | Recommended for the fermentation of sugar cane and sugar beet products to produce neutral and light spirits. | |
| Lallemand (Duluth, GA, USA) | Superstart | S. cerevisiae | An active dried yeast designed for biofuel production, but it is also said to be suitable for neutral spirits. |
| Lallemand (Australia) | Lalvin 71B | S. cerevisiae | An active dried yeast for neutral spirits, vodka, and gin. Also used in nouveau wines. |
| Fermentis (France) | DADY | S. cerevisiae | For grain mash fermentations for light spirits and whiskeys. |
| SafSpirit GR-2 | S. cerevisiae | For very neutral alcohol production, especially vodka. | |
| SafSpirit HG-1 | S. cerevisiae | Produces a spirit with a neutral flavour profile |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauley, M.; Maskell, D. Mini-Review: The Role of Saccharomyces cerevisiae in the Production of Gin and Vodka. Beverages 2017, 3, 13. https://doi.org/10.3390/beverages3010013
Pauley M, Maskell D. Mini-Review: The Role of Saccharomyces cerevisiae in the Production of Gin and Vodka. Beverages. 2017; 3(1):13. https://doi.org/10.3390/beverages3010013
Chicago/Turabian StylePauley, Matthew, and Dawn Maskell. 2017. "Mini-Review: The Role of Saccharomyces cerevisiae in the Production of Gin and Vodka" Beverages 3, no. 1: 13. https://doi.org/10.3390/beverages3010013
APA StylePauley, M., & Maskell, D. (2017). Mini-Review: The Role of Saccharomyces cerevisiae in the Production of Gin and Vodka. Beverages, 3(1), 13. https://doi.org/10.3390/beverages3010013






