Saccharomyces cerevisiae in the Production of Fermented Beverages
Abstract
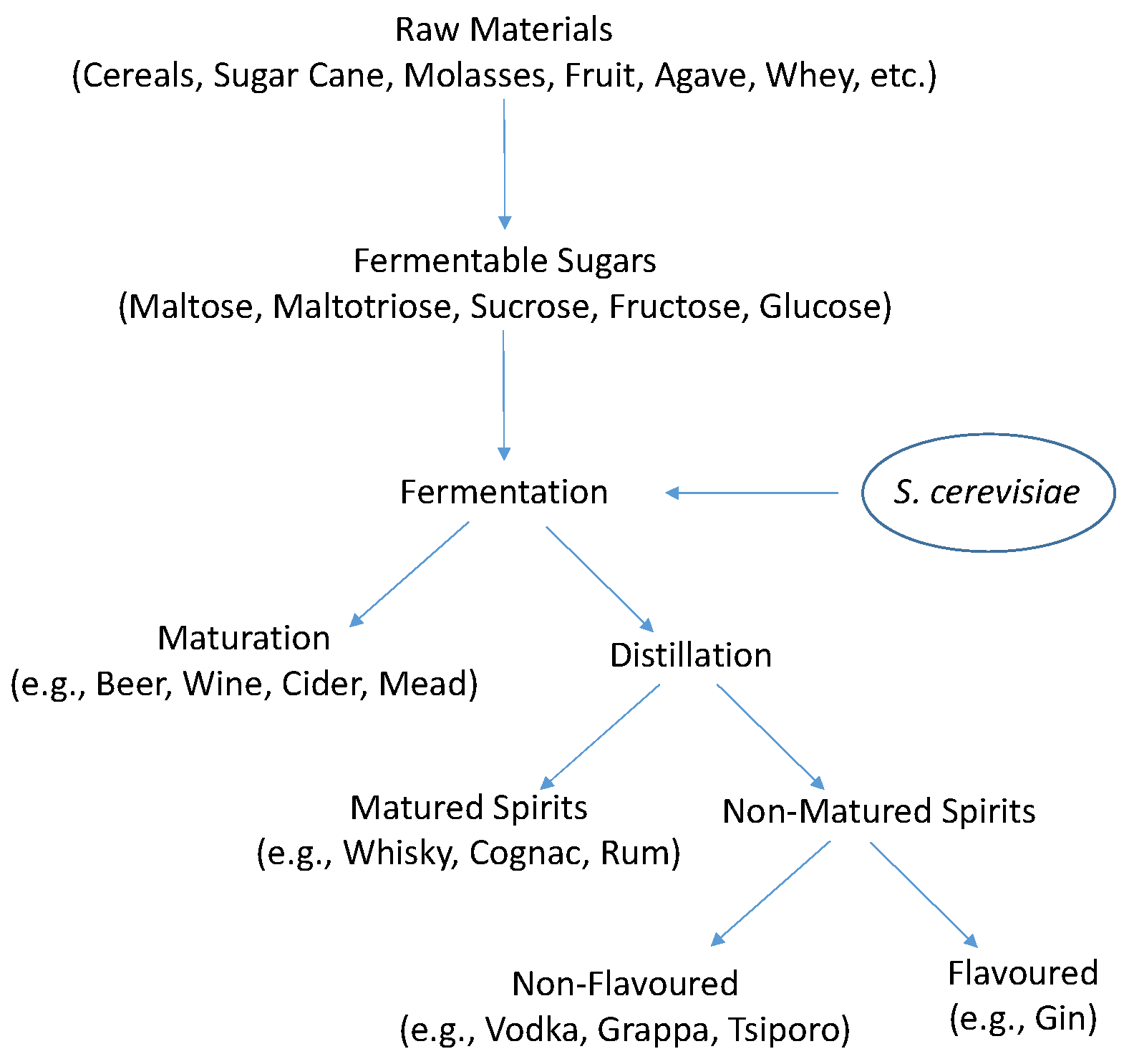
:1. Introduction
Yeasts in Alcoholic Beverage Fermentations
2. Physiology of Alcohol-Producing S. cerevisiae
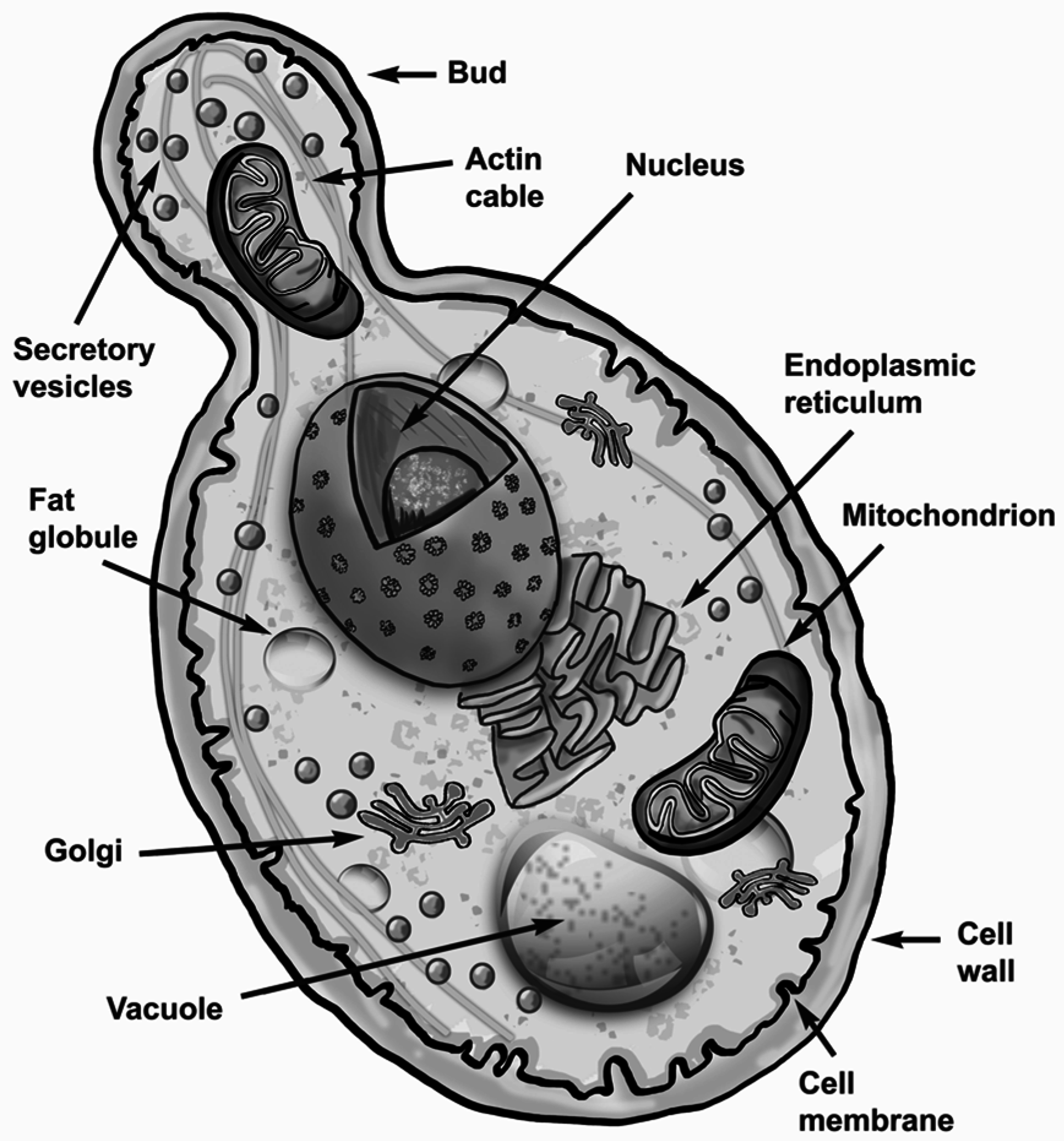
2.1. Form and Function of S. cerevisiae Cells
2.2. Physical Requirements of S. cerevisiae
2.3. Nutritional Requirements of S. cerevisiae
3. Yeast Nutrition
3.1. Nutrients Required by S. cerevisiae
3.1.1. Sources of Utilizable Carbon
3.1.2. Sources of Utilizable Nitrogen
3.1.3. Sources of Inorganic Nutrients
3.1.4. Sources of Growth Factors
3.2. Nutrient Composition of Fermentation Media
3.3. Nutrient Uptake by S. cerevisiae
3.4. S. cerevisiae Growth During Fermentation
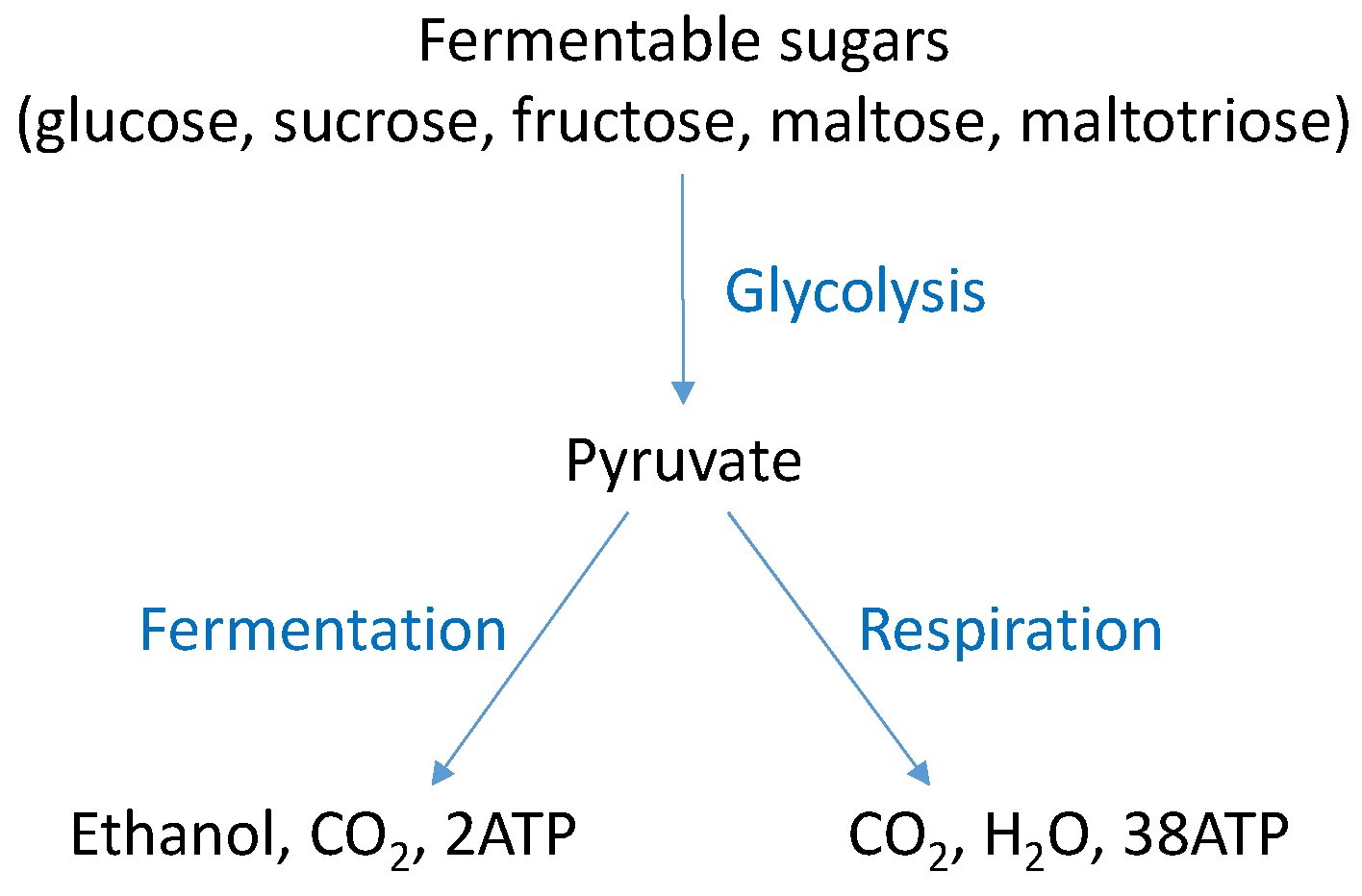
4. S. cerevisiae Fermentative Metabolism
4.1. Ethanol Fermentation by S. cerevisiae
4.2. Production of Secondary Fermentation Metabolites
5. Future Developments in Alcoholic Beverage Fermentations
Conflicts of Interest
References
- Walker, G.M. Microbiology of wine-making. In Encyclopedia of Food Microbiology; Batt, C., Tortorello, M.L., Eds.; Elsevier Science Publishers: Boston, MA, USA, 2014; pp. 787–792. [Google Scholar]
- Russell, I.; Stewart, G.G. Whisky: Technology, Production and Marketing, 2nd ed.; Academic Press/Elsevier: Boston, MA, USA, 2014. [Google Scholar]
- Walker, G.M. Yeast Physiology & Biotechnology; John Wiley & Sons: Chichester, UK; New York, NY, USA, 1998. [Google Scholar]
- Walker, G.M. Yeasts. In Eukaryotic Microbes; Schaechter, M., Ed.; Academic Press/Elsevier Science Publishers: Oxford, UK, 2011; pp. 3–17. [Google Scholar]
- Walker, G.M. Fermentation (Industrial). Media for Industrial Fermentations. In Encyclopedia of Food Microbiology; Batt, C., Tortorello, M.L., Eds.; Elsevier Science Publishers: Boston, MA, USA, 2014. [Google Scholar]
- Ingledew, W.M. Alcohol production by Saccharomyces cerevisiae: A yeast primer. In The Alcohol Textbook, 3rd ed.; Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University Press: Nottingham, UK, 1999; pp. 49–87. [Google Scholar]
- Walker, G.M. Metals in yeast fermentation processes. Adv. Appl. Microbiol. 2004, 54, 197–229. [Google Scholar] [PubMed]
- Chandrasena, G.; Walker, G.M.; Staines, H.J. Use of surface responses in predicting metal ion interactions in yeast fermentations. J. Am. Soc. Brew. Chem. 1997, 55, 24–29. [Google Scholar]
- Verstrepen, K.J.; Iserentant, D.; Malcorps, P.; Derdelinckx, G.; Van Dijck, P.; Winderickx, J.; Pretorius, I.S.; Thevelein, J.M.; Delvaux, F.R. Glucose and sucrose; hazardous fast-food for industrial yeast? Trends Biotechnol. 2004, 22, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Priest, F.G.; Stewart, G.G. Handbook of Brewing, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Berry, D.R.; Slaughter, J.C. Alcoholic beverage fermentations. In Fermented Beverage Production, 2nd ed.; Lea, A.G.H., Piggott, J.R., Eds.; Springer Science & Business Media: New York, NY, USA, 2003; pp. 25–39. [Google Scholar]
- Berry, D.R. Physiology and microbiology of Scotch whisky production. In Progress in Industrial Microbiology; Bushell, M.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 19, pp. 199–243. [Google Scholar]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; Blackwell Science Ltd.: Oxford, UK, 2006. [Google Scholar]
- Fleet, G.H. Wine Microbiology and Biotechnology; Harwood Academic Publishers: Chur, Switzerland, 1993. [Google Scholar]
- Stratford, M. Food and beverage spoilage yeasts. In The Yeast Handbook; Querol, A., Fleet, G.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 335–379. [Google Scholar]
- Deak, T. Handbook of Food Spoilage Yeasts, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Vriesekoop, F.; Krahl, M.; Hucker, B.; Menz, G. 125th Anniversary Review: Bacteria in brewing: The good, the bad and the ugly. J. Inst. Brew. 2012, 118, 335–345. [Google Scholar] [CrossRef]
- Jackson, R. Wine Science. Principles and Applications, 3rd ed.; Academic Press/Elsevier: Boston, MA, USA, 2008. [Google Scholar]
- Walker, G.M.; Hughes, P.S. Distilled Spirits. New Horizons: Energy, Environment and Enlightenment; Nottingham University Press: Nottingham, UK, 2010. [Google Scholar]
- Walker, G.M.; Goodall, I.; Fotheringham, R.; Murray, D. Distilled Spirits. Science and Sustainability; Nottingham University Press: Nottingham, UK, 2012. [Google Scholar]
- Goodall, I.; Fotheringham, R.; Murray, D.; Speers, R.A.; Walker, G.M. Distilled Spirits: Future Challenges, New Solutions; Context Products Ltd.: Packington, UK, 2015. [Google Scholar]
- Walker, G.M.; Bringhurst, T.; Brosnan, J.; Jack, F. Selecting new distilling yeasts for improved fermentation and for sustainability. In Distilled Spirits: Science and Sustainability, Proceedings of the 4th Worldwide Conference on Distilled Spirits, Edinburgh, UK, 11–15 September 2011; Walker, G.M., Goodall, I., Fotheringham, R., Murray, D., Eds.; Nottingham University Press: Nottingham, UK, 2012; pp. 127–136. [Google Scholar]
- Querol, A.; Fleet, G.H. (Eds.) The Yeast Handbook. Volume 1. Biodiversity and Ecophysiology of Yeasts. Volume 2. Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006.
- Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology Volume 1. The Microbiology of Wine and Vinifications; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Hammond, J.R.M. Microscopes, microbes and manipulation: 35 years of brewing. J. Am. Soc. Brew. Chem. 2016, 74, 157–172. [Google Scholar]
| Beverage | Yeast Involved | Comments |
|---|---|---|
| Beer | Lager beer: Saccharomyces pastorianus Ale: Saccharomyces cerevisiae Lambic beer: Brettanomyces bruxellensis and other yeasts | Lager yeasts are likely a natural hybrid (S. cerevisiae & S. eubayanus). Relatively few strains employed in lager fermentations. Lager strains utilise maltotriose more efficiently than ale strains, and they ferment at cooler temperatures. Ale yeasts are polyploid strains. Numerous strains employed in ale brewing. Ale yeasts ferment at warmer temperatures compared with lager yeasts. Lambic beer traditionally produced via spontaneous fermentation, but some lambic and Belgian-style beers employ pure starter cultures of Brettanomyces spp. |
| Wine | Saccharomyces cerevisiae, Saccharomyces bayanus (pure cultures) and naturally-occurring yeasts | Traditional winemaking is characterised by spontaneous fermentations of grape must with naturally occurring microflora (the main yeast genera associated with grapes are: Kloeckera and Hanseniaspora, with lesser representations of Candida, Metchnikowia, Cryptococcus, Pichia and Kluyveromyces and very low populations of Saccharomyces cerevisiae). Modern, large-scale wineries use specially selected starter cultures of S. cerevisiae strains available in dried form (e.g., active dry yeast, ADY) from specialist yeast supply companies. Occasionally, secondary commercial non-Saccharomyces starter cultures (e.g., Candida stellata) may be employed to impart specific flavour and aroma to wine. |
| Whisky | Saccharomyces cerevisiae | Scotch whisky producers currently use selected distilling strains of S. cerevisiae in three main formats, cream yeast, pressed (cake) and dried yeast. Malt whisky distilleries traditionally use pressed yeast, but larger grain distillers have now adopted cream yeast. Dried yeasts are not as prevalent as pressed and cream formats in whisky fermentations. |
| Rum | Saccharomyces cerevisiae and Schizosaccharomyces pombe | S. cerevisiae strains in rum fermentations are developed as starter cultures and provide faster fermentation with more higher alcohols and fatty acids, but less esters resulting in lighter style rums. Schiz. pombe in rum fermentations provides slower fermentations leading to less higher alcohols and fatty acids, but more esters resulting in heavy, strong aroma rums. Growth of Schiz. pombe is favoured by low pH, higher sugar conc. |
| Tequila, Mezcal, Bacanora | Natural yeasts in artisanal Agave fermentations | Various yeasts have been isolated from such processes: S. cerevisiae, Kluyveromyces marxianus, Pichia spp., Brettanomyces spp., Rhodotorula spp., etc. |
| Brandies, Gin, Vodka, etc. | Saccharomyces cerevisiae | For brandies, cognac, etc. the base wine is produced by pure starter cultures of S. cerevisiae. For gin, vodka, etc. selected distilling strains of S. cerevisiae will be used. |
| Cheese whey-derived beverages | Kluyveromyces marxianus | Lactose-fermenting yeast to produce ethanol destined for gin, vodka and cream liqueurs, etc. |
| Media | Fermentable Sugars | Beverage |
|---|---|---|
| Barley malt wort | Glucose, maltose, maltotriose | Ale and lager beer. Scotch malt whisky |
| Cereal wort based on barley malt and exogenous enzymes plus un-malted starch from wheat, rye, maize, sorghum, etc. | Glucose, maltose, maltotriose | Some beers, Scotch grain whisky, Bourbon and Tennessee whiskey, Canadian rye whisky, Irish whiskey, grain neutral spirit (for gin, vodka, etc.) |
| Rice hydrolysate (from Koji enzymes) | Glucose | Sakē, Sochu, Arrack, Awamori |
| Potato hydrolysate (from amylolytic enzymes) | Glucose | Aquavit, vodka |
| Agave | Fructose | Tequila, mezcal, pulque |
| Sugar cane molasses | Sucrose | Rum |
| Sugar cane juice | Sucrose | Cachaça (Brazil), Rhum Agricole |
| Grape must, fruit juices, honey | Glucose, fructose | Wine, cognac, armagnac, brandy, grappa, kirsch, slivovich, cider, perry, mead |
| Cheese whey | Lactose | Gin, vodka, cream liquers |
| Component | Molasses | Malt Wort | Grape Must | Cheese Whey |
|---|---|---|---|---|
| Carbon source | Sucrose, glucose, fructose, raffinose | Glucose, maltose, maltotriose | Glucose, fructose | Lactose |
| Nitrogen source | Limiting (requires supplementation) | Amino acids | Amino acids | Amino and urea nitrogen, albumins, globulins |
| Minerals | P, S adequate, but K and Ca may be in excess. Mg and Mn may be limiting | Most minerals adequate, but Zn may be limiting | Most minerals adequate, but Zn, Mg may be limiting | Most minerals adequate |
| Vitamins | Most vitamins adequate, but biotin may be limiting | Most vitamins adequate, but biotin may be limiting | Range of vitamins present but yeast foods may supply extra | Biotin, pyridoxine and thiamine present |
| Minor components | Maillard reaction products, betaine, organic acids, waxes, pigments, silica | Maltodextrins (unfermentable) | Pentoses (unfermentable), organic and fatty acids | High levels of lactic acid, fat, and fibre |
| Metabolite Class | Examples of Compounds | Comments |
|---|---|---|
| Higher alcohols | Isoamyl alcohol, Phenylethanol, Isopropanol | Within certain concentration limits, higher alcohols (or fusel oils) impart desirable flavour and aromas to fermented beverages, notably in distilled spirits. |
| Esters | Ethyl acetate | These compound impart fruity and floral flavours and aromas to fermented beverages, especially beers and wines. |
| Carbonyl compounds | Acetaldehyde | Above its flavour threshold in beer, this compound can impart a “grassy” or “green apple” flavour but this can be removed by secondary yeast fermentation during conditioning. |
| Organic acids | Succinic acid, Citric acid, Acetic acid | These compounds contribute in a beneficial way to the astringency, or “sharpness”, of fermented beverages. The presence of some acids, notably lactic acid, indicate undesirable bacterial spoilage. |
| Polyols | Glycerol | This compound is produced during normal yeast metabolism, or when yeasts are confronted with osmotic stress. Glycerol may contribute desirable viscosity to fermented beverages, notably wines. |
| Vicinal diketones | Diacetyl, Pentane-2,3-dione | Diacetyl in most beers is undesirable, imparting a rancid-butter or “butterscotch” flavour, but levels can be reduced during beer conditioning. |
| Sulphur compounds | Hydrogen sulphide, Dimethyl sulphide, Sulphur dioxide, Thiols | These are important beverage flavour and aroma compounds. For example in beer, dimethyl sulphide (DMS) if present in low concentrations is a desirable attribute of lagers, but higher concentrations impart off-flavours. |
| Phenolic compounds | 4-Vinylguiacol | Some yeasts, including wild yeasts, that are POF+ (phenolic off-flavour) can produce undesirable phenolic flavours and aromas. However, the clove-like compound, 4-vinylguiacol, is desirable in certain beer styles and can be produced by hefe ale yeast strains of S. cerevisiae. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. https://doi.org/10.3390/beverages2040030
Walker GM, Stewart GG. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages. 2016; 2(4):30. https://doi.org/10.3390/beverages2040030
Chicago/Turabian StyleWalker, Graeme M, and Graham G Stewart. 2016. "Saccharomyces cerevisiae in the Production of Fermented Beverages" Beverages 2, no. 4: 30. https://doi.org/10.3390/beverages2040030
APA StyleWalker, G. M., & Stewart, G. G. (2016). Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages, 2(4), 30. https://doi.org/10.3390/beverages2040030






