Quality Markers of Functional Tomato Juice with Added Apple Phenolic Antioxidants
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Design
2.2. Apple Peels
2.3. Extraction of Phenolic Compounds
2.4. Determination of Phenolic Content
2.5. Physical-Chemical Analysis
2.5.1. pH
2.5.2. Turbidity
2.5.3. Total Phenolic Content
2.5.4. Colour
2.5.5. Ascorbic Acid Content
2.5.6. Total Carotenoid Content
2.6. Antioxidant Capacity Equivalent to Ascorbic Acid (AEAC)
2.6.1. Ferric Reducing Antioxidant Power (FRAP)
2.6.2. Radical Scavenging Capacity (DPPH)
2.6.3. Lipid Peroxidation Test
2.7. Microbiological Analysis
2.7.1. Microbial Load
2.7.2. Antimicrobial Capacity
| Strain | Reference |
|---|---|
| Bacteria | |
| Gram-positive | |
| Lactobacillus plantarum | ATCC 8014 |
| Staphylococcus aureus | ATCC 25923 |
| Gram-negative | |
| Escherichia coli | ATCC 25922 |
| Salmonella typhimurium | ATCC 14028 |
| Yeast | |
| Saccharomices cerevisiae | ATCC 9763 |
2.8. Statistical Analysis
3. Results and Discussion
3.1. Composition of Apple Peel Phenolics
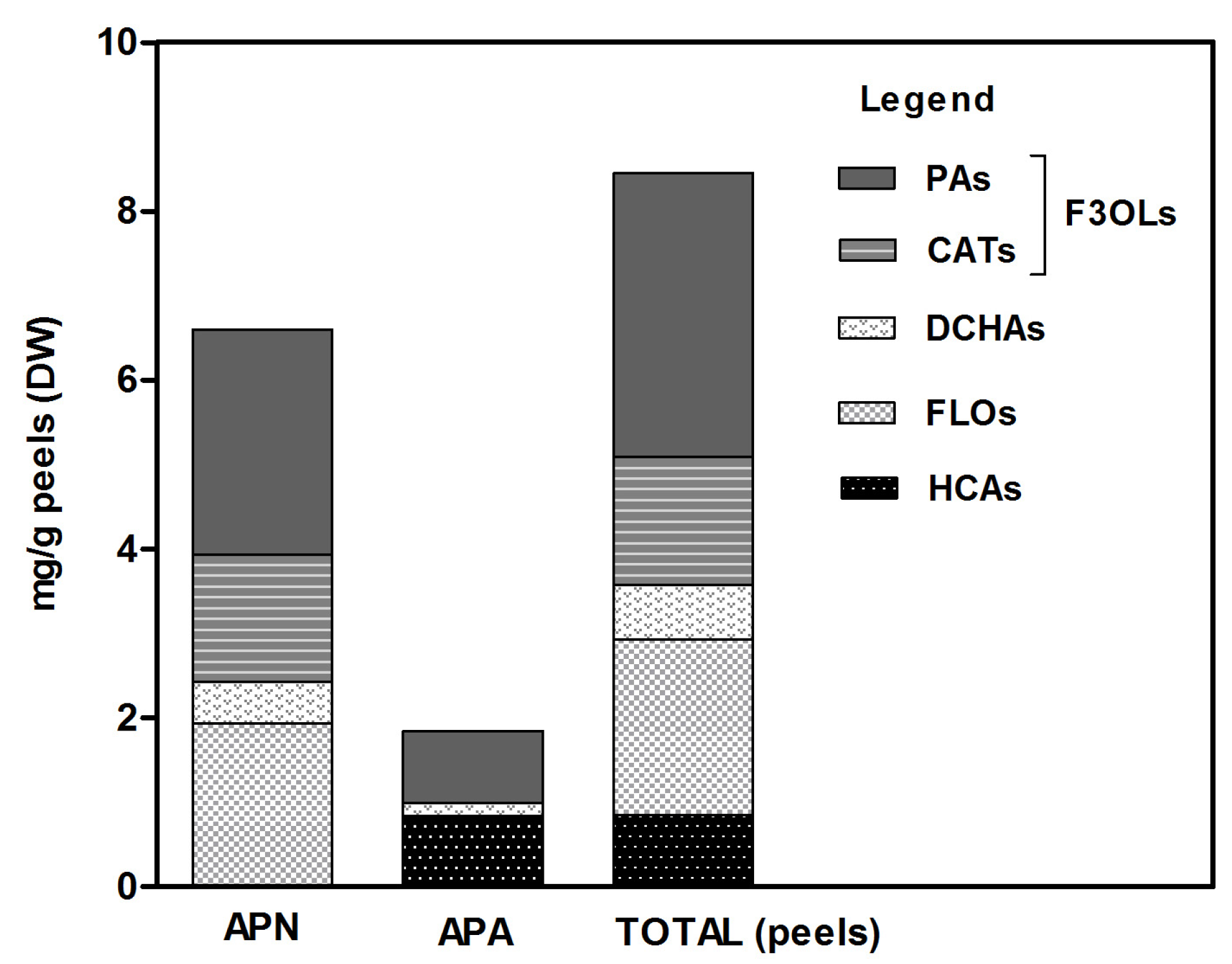
3.2. Quality Markers
3.3. Ascorbic Acid and Total Carotenoids
| Juice sample | Time (Days) | pH | Turbidity (FNU) | Colour | ||||
|---|---|---|---|---|---|---|---|---|
| L * | a * | b * | Chroma | ∆E | ||||
| Control | 0 | 4.10 ± 0.01 B | 232.87 ± 4.06 A | 26.77 ± 0.13 A | 31.04 ± 0.18 A | 30.36 ± 0.30 A | 43.79 ± 0.09 A | - |
| 4 | 4.19 ± 0.01 A | 242.08 ± 3.87 A | 26.52 ± 0.02 A | 30.51 ± 0.08 B | 30.89 ± 0.06 A | 43.03 ± 0.16 B | - | |
| Enriched (APA) | 0 | 4.07 ± 0.01 B | 268.76 ± 25.96 A | 26.95 ± 0.00 A | 31.02 ± 0.02 A | 30.37 ± 0.08 A | 43.92 ± 0.09 A | 0.28 ± 0.07 A |
| 4 | 4.14 ± 0.01 A | 255.65 ± 30.70 A | 26.85 ± 0.05 A | 30.28 ± 0.04 B | 31.08 ± 0.14 A | 42.89 ± 0.09 B | 0.44 ± 0.12 A | |
| Enriched (APN) | 0 | 4.10 ± 0.01 B | 256.28 ± 29.79 A | 27.29 ± 0.01 A | 30.88 ± 0.01 A | 30.41 ± 0.07 B | 44.01 ± 0.08 A | 0.74 ± 0.12 A |
| 4 | 4.18 ± 0.01 A | 250.44 ± 17.95 A | 27.19 ± 0.01 A | 30.19 ± 0.06 B | 31.36 ± 0.11 A | 42.85 ± 0.06 B | 0.79 ± 0.06 A | |
| Mean | ||||||||
| Control | 4.14 a | 237.48 a | 26.64 c | 30.77 a | 30.62 a | 43.39 a | - | |
| Enriched (APA) | 4.10 b | 240.13 a | 26.90 b | 30.65 a,b | 30.73a | 43.40 a | 0.34 b | |
| Enriched (APN) | 4.14 a | 253.36 a | 27.24 a | 30.53 b | 30.88 a | 43.42 a | 0.76 a | |
| F-test | *** | NS | ** | ** | NS | NS | * | |
| LSD0.05 | 0.01 | - | 0.25 | 0.13 | - | - | 0.22 | |
| Storage time | *** | NS | NS | *** | * | *** | NS | |
| Storage time × Sample | NS | NS | NS | NS | NS | NS | NS | |
| Juice sample | Time (Days) | AA (mg/100 mL) | TCAR (mg/100 mL) | AEAC (mg AAE/100 mL Juice) | |
|---|---|---|---|---|---|
| FRAP | Radical Scavenging Capacity (DPPH) | ||||
| Control | 0 | 5.97 ± 0.03 A | 5.72 ± 1.31 A | 47.70 ± 0.42 A | 34.31 ± 1.09 A |
| 4 | 4.47 ± 0.01 B | 4.56 ± 0.80 A | 39.00 ± 0.24 B | 30.18 ± 0.36 B | |
| Enriched (APA) | 0 | 6.06 ± 0.08 A | 5.21 ± 0.08 A | 56.15 ± 3.61 A | 34.85 ± 0.46 A |
| 4 | 4.62 ± 0.04 B | 4.67 ± 0.17 A | 47.71 ± 0.46 A | 36.19 ± 0.24 A | |
| Enriched (APN) | 0 | 5.83 ± 0.02 A | 5.61 ± 0.51 A | 86.20 ± 3.54 A | 48.03 ± 0.25 A |
| 4 | 4.70 ± 0.01 B | 5.29 ± 0.45 A | 65.23 ± 1.78 B | 47.25 ± 0.49 A | |
| Mean | |||||
| Control | 5.22 a | 5.12 a | 43.33 c | 32.24 c | |
| Enriched (APA) | 5.27 a | 5.14 a | 51.93 b | 35.52 b | |
| Enriched (APN) | 5.34 a | 5.62 a | 75.69 a | 47.64 a | |
| F-test | NS | NS | *** | *** | |
| LSD0.05 | - | - | 6.61 | 2.38 | |
| Storage time | *** | NS | *** | * | |
| Storage time × Sample | NS | NS | NS | * | |
3.4. Antioxidant Capacity
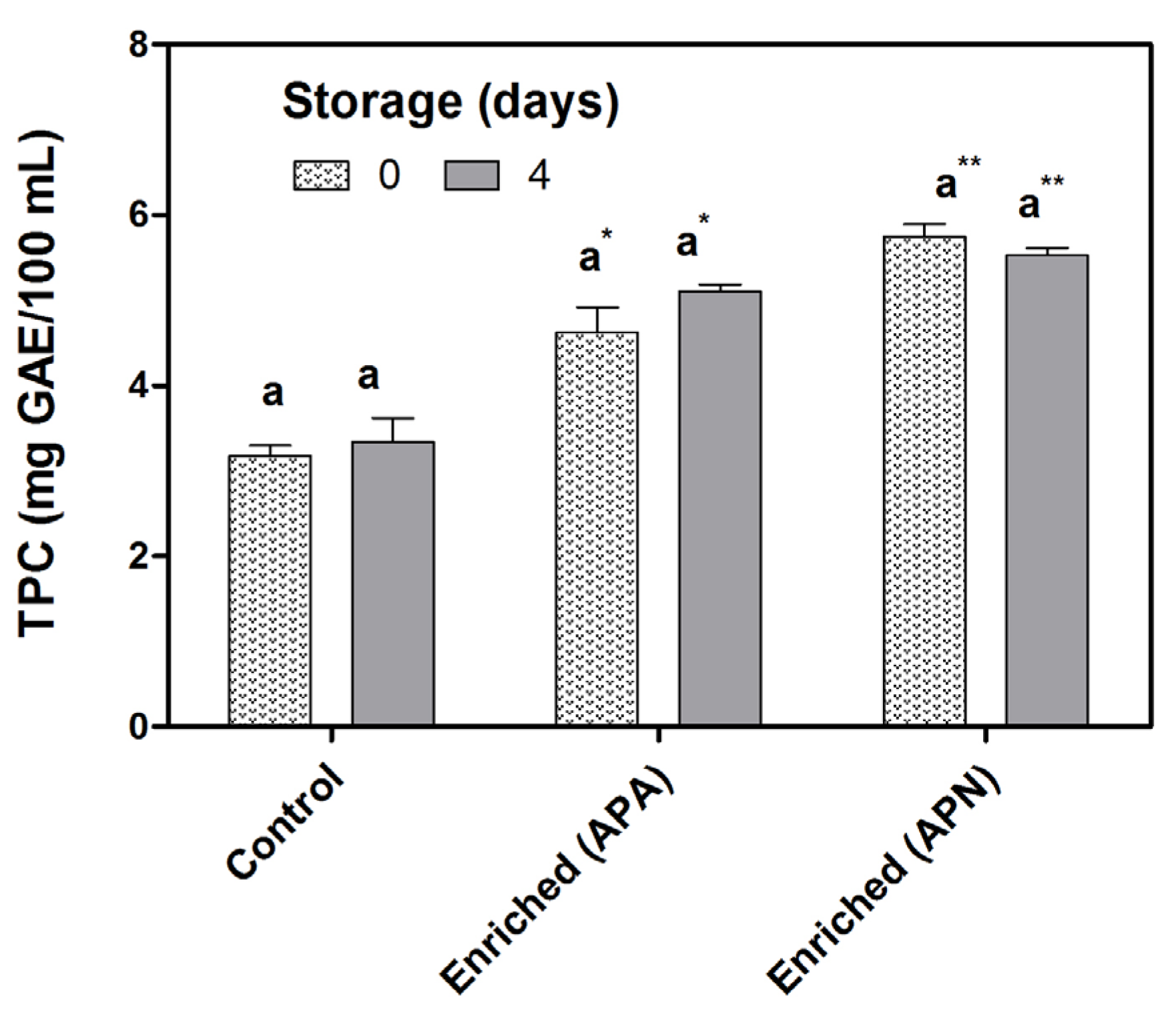
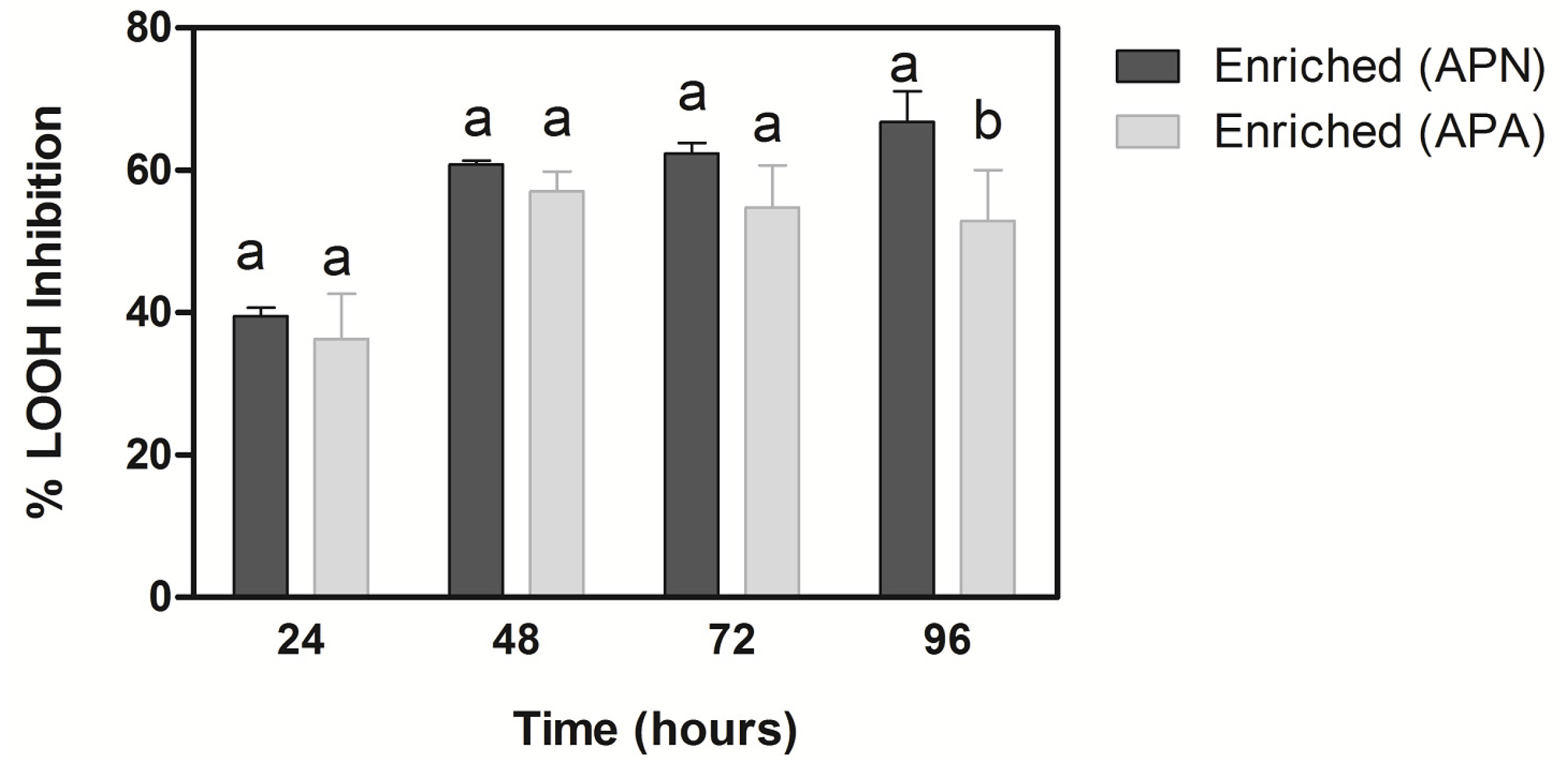
3.5. Microbiological Analysis

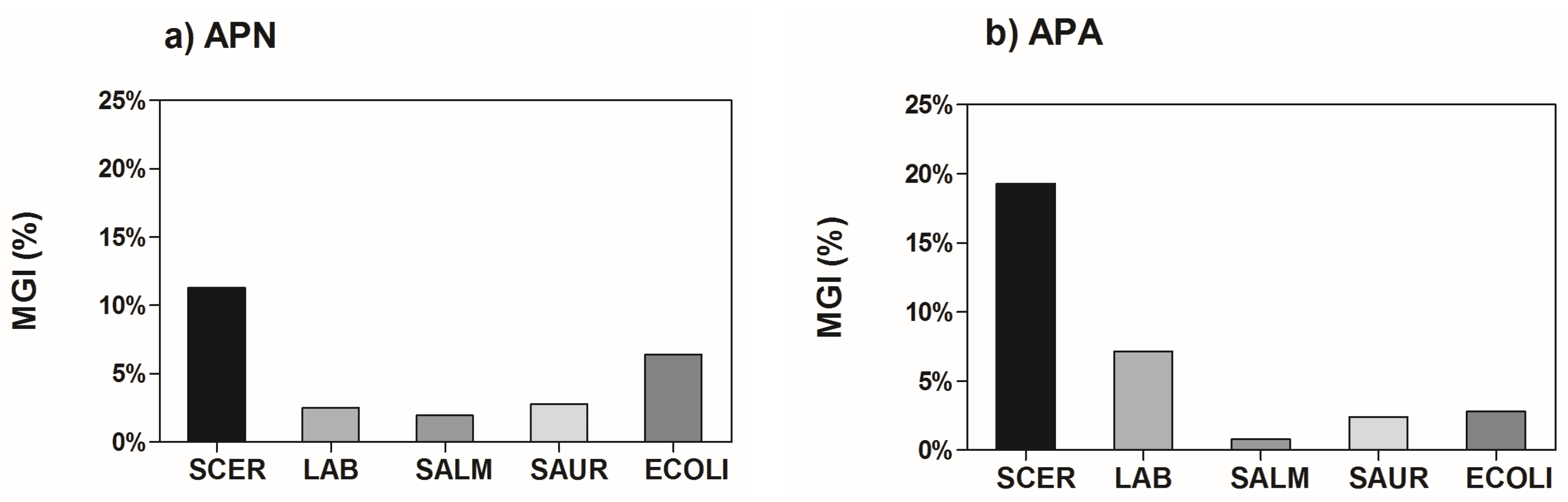
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massini, L.; Martín Diana, A.B.; Barry-Ryan, C.; Rico, D. Apple (Malus domestica Borkh. cv Bramley’s Seedling) peel waste as a valuable source of natural phenolic antioxidants. In Total Food—Sustainability of the Agri-Food Chain; Waldron, K.W., Moates, G.K., Faulds, C.B., Eds.; RCS: Cambridge, UK, 2010; pp. 90–95. [Google Scholar]
- Eussen, S.R.B.M.; Verhagen, H.; Klungel, O.H.; Garssen, J.; van Loveren, H.; van Kranen, H.J.; Rompelberg, C.J.M. Functional foods and dietary supplements: Products at the interface between pharma and nutrition. Eur. J. Pharmacol. 2011, 668, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Berdahl, D.R.; Nahas, R.I.; Barren, J.P. Synthetic and natural antioxidant additives in food stabilization: Current applications and future research. In Oxidation in Foods and Beverages and Antioxidant Applications, Volume 1—Understanding Mechanisms of Oxidation and Antioxidant Activity; Decker, E.A., Elias, R.J., McClements, D.J., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 272–313. [Google Scholar]
- Decker, E.A.; Elias, R.J.; McClements, D.J. Oxidation in Foods and Beverages and Antioxidant Applications, Volume 1—Understanding Mechanisms of Oxidation and Antioxidant Activity; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Medina, I.; González, M.J.; Pazos, M.; Della Medaglia, D.; Sacchi, R.; Gallardo, J.M. Activity of plant extracts for preserving functional food containing n-3-PUFA. Eur. Food Res. Technol. 2003, 217, 301–307. [Google Scholar] [CrossRef]
- Bech-Larsen, T.; Scholderer, J. Functional foods in Europe: Consumer research, market experiences and regulatory aspects. Trends Food Sci. Technol. 2007, 18, 231–234. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Lück, E.; Jager, M. Antimicrobial Food Additives: Characteristics, Uses, Effects, 2nd ed.; Springer: Berlin, Germany, 1997. [Google Scholar]
- Pokorný, J. Are natural antioxidants better—And safer—Than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Gruenwald, J. Novel botanical ingredients for beverages. Clin. Dermatol. 2009, 27, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Stability of phytochemicals as sources of anti-inflammatory nutraceuticals in beverages: A review. Food Res. Int. 2013, 50, 480–486. [Google Scholar] [CrossRef]
- Massini, L.; Rico, D.; Martin-Diana, A.; Barry-Ryan, C. Valorisation of apple peels. Eur. J. Food Res. Rev. 2013, 3, 1–15. [Google Scholar]
- Delage, E.; Bohuon, G.; Baron, A.; Drilleau, J.F. High-performance liquid chromatography of the phenolic compounds in the juice of some French cider apple varieties. J. Chromatogr. A 1991, 555, 125–136. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Hernández, Y.; Lobo, M.G.; González, M. Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chem. 2006, 96, 654–664. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A. Increase of antioxidant activity of tomato juice upon functionalisation with vegetable byproduct extracts. LWT Food Sci. Technol. 2002, 35, 532–542. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- CCFRA. Manual of Microbiological Methods for the Food and Drinks Industry—Guideline No. 43, 4th ed.; Campden & Chorleywood Food Research Association Group (CCFRA): Gloucestershire, UK, 2003. [Google Scholar]
- Adams, M.R.; Moss, M.O. Food Microbiology, 2nd ed.; RCS: Cambridge, UK, 2000. [Google Scholar]
- Casey, J.T.; O’Cleirigh, C.; Walsh, P.K.; O’Shea, D.G. Development of a robust microtiter plate-based assay method for assessment of bioactivity. J. Microbiol. Methods 2004, 58, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Patton, T.; Barrett, J.; Brennan, J.; and Moran, N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J. Microbiol. Methods 2006, 64, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- García-Alonso, F.J.; Bravo, S.; Casas, J.; Pérez-Conesa, D.; Jacob, K.; Periago, M.J. Changes in antioxidant compounds during the shelf life of commercial tomato juices in different packaging materials. J. Agric. Food Chem. 2009, 57, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Marete, E.; Jacquier, J.C.; O’Riordan, D. Effects of extraction temperature on the phenolic and parthenolide contents, and colour of aqueous feverfew (Tanacetum parthenium) extracts. Food Chem. 2009, 117, 226–231. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010. [Google Scholar] [CrossRef]
- Hsieh, C.W.; Ko, W.C. Effect of high-voltage electrostatic field on quality of carrot juice during refrigeration. LWT Food Sci. Technol. 2008, 41, 1752–1757. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Barranco, A.; Abad, B.; Berrueta, L.A.; Gallo, B.; Vicente, F. Polyphenolic profiles of Basque cider apple cultivars and their technological properties. J. Agric. Food Chem. 2004, 52, 2938–2952. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Sosnowska, D.; Anders, B. Antioxidative capacity of tomato products. Eur. Food Res. Technol. 2003, 217, 296–300. [Google Scholar] [CrossRef]
- Pacheco-palencia, L.A.; Hawken, P.; Talcott, S.T. Phytochemical, antioxidant and pigment stability of açai (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Res. Int. 2007, 40, 620–628. [Google Scholar] [CrossRef]
- Pacheco-palencia, L.A.; Hawken, P.; Talcott, S.T. Juice matrix composition and ascorbic acid fortification effects on the phytochemical, antioxidant and pigment stability of açai (Euterpe oleracea Mart.). Food Chem. 2007, 105, 28–35. [Google Scholar] [CrossRef]
- Pernice, R.; Borriello, G.; Ferracane, R.; Borrelli, R.C.; Cennamo, F.; Ritieni, A. Bergamot: A source of natural antioxidants for functionalized fruit juices. Food Chem. 2009, 112, 545–550. [Google Scholar] [CrossRef]
- Lo Scalzo, R. Organic acids influence on DPPH scavenging by ascorbic acid. Food Chem. 2008, 107, 40–43. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [PubMed]
- Yesil-Celiktas, O.; Isleten, M.; Vardar-Sukan, V.; Oyku Cetin, E. In vitro release kinetics of pine bark extract enriched orange juice and the shelf stability. Br. Food J. 2010, 112, 1063–1076. [Google Scholar] [CrossRef]
- Ashurst, P. Chemistry and Technology of Soft Drinks and Fruit Juices, 2nd ed.; Blackwell: Oxford, UK, 2004. [Google Scholar]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2009, 27, 27–40. [Google Scholar] [CrossRef]
- Cushnie, T.T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massini, L.; Rico, D.; Martín-Diana, A.B.; Barry-Ryan, C. Quality Markers of Functional Tomato Juice with Added Apple Phenolic Antioxidants. Beverages 2016, 2, 4. https://doi.org/10.3390/beverages2010004
Massini L, Rico D, Martín-Diana AB, Barry-Ryan C. Quality Markers of Functional Tomato Juice with Added Apple Phenolic Antioxidants. Beverages. 2016; 2(1):4. https://doi.org/10.3390/beverages2010004
Chicago/Turabian StyleMassini, Laura, Daniel Rico, Ana Belen Martín-Diana, and Catherine Barry-Ryan. 2016. "Quality Markers of Functional Tomato Juice with Added Apple Phenolic Antioxidants" Beverages 2, no. 1: 4. https://doi.org/10.3390/beverages2010004
APA StyleMassini, L., Rico, D., Martín-Diana, A. B., & Barry-Ryan, C. (2016). Quality Markers of Functional Tomato Juice with Added Apple Phenolic Antioxidants. Beverages, 2(1), 4. https://doi.org/10.3390/beverages2010004







