Advanced Multimodeling for Isotopic and Elemental Content of Fruit Juices
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. IRMS Measurements
2.3. ICP-MS Measurements
2.4. Data Acquisition and Chemometric Processing
3. Results and Discussion
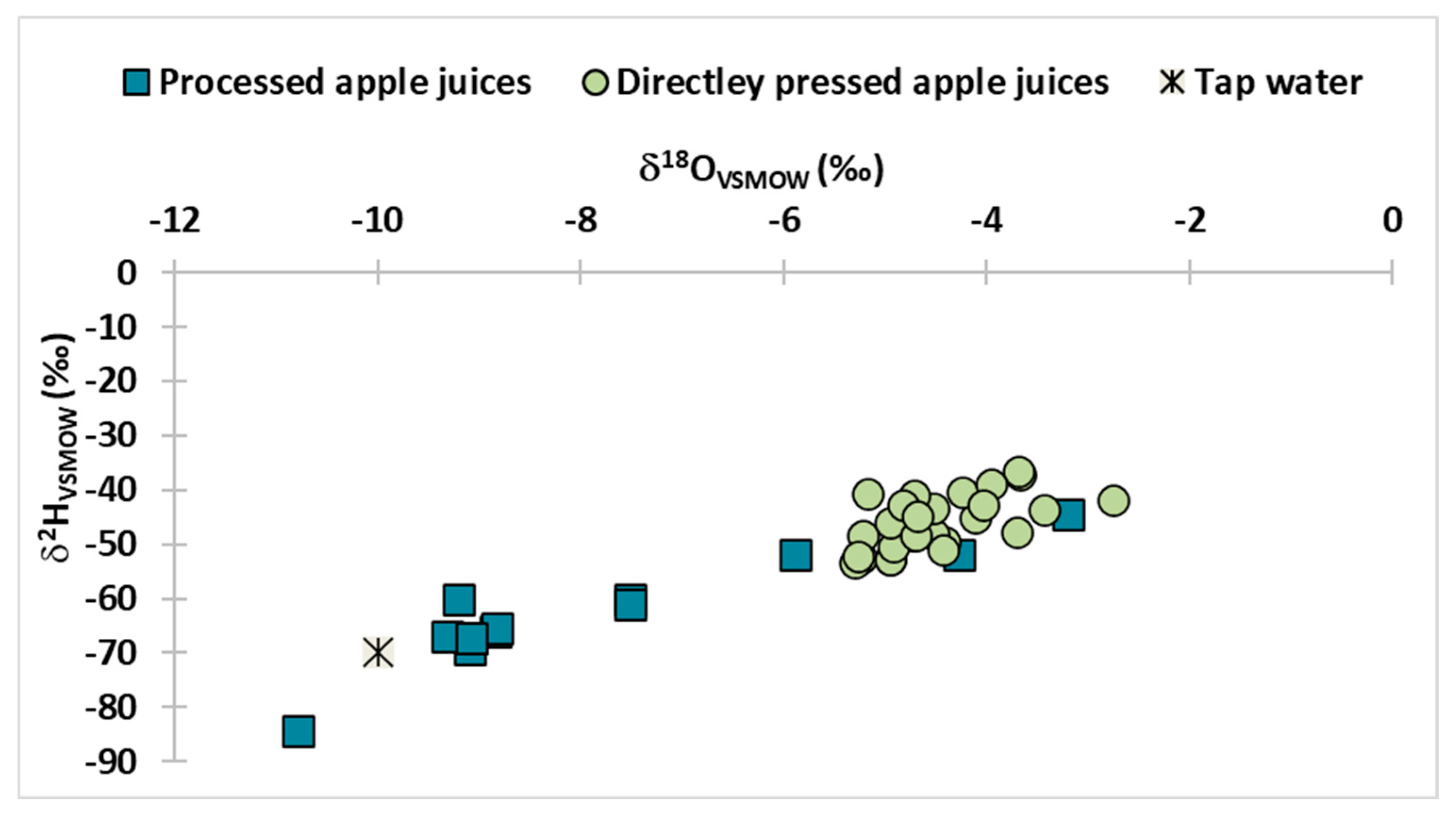
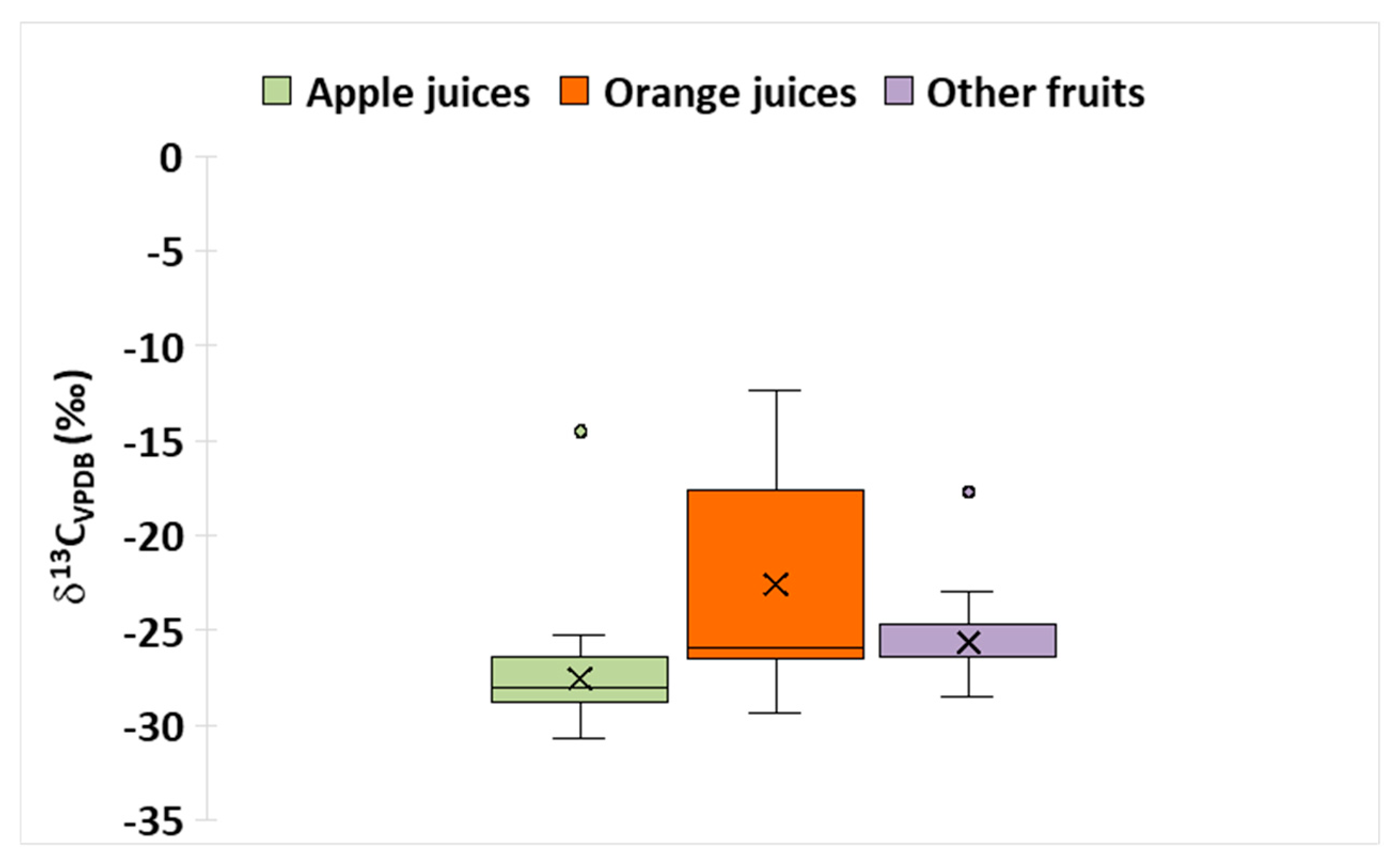
3.1. Isotopic Fingerprint of Fruit Juices
3.2. Distribution of Macro-, Micro- and Trace Elements in Fruit Juices
| Elements | Type of Fruit Juice | Water Quality Standards | |||||
|---|---|---|---|---|---|---|---|
| Apple (n = 13) | Orange (n = 37) | Others (n = 23) | Directly Pressed Juice (Apple) (n = 28) | USEPA [34] 2018 | WHO [35] 2017 | N (%) | |
| Min–Max Values (Mean Value ± SD) | |||||||
| Na (mg/L) | 1.13–53.50 (20.30 ± 13.81) | 1.82–206.19 (51.70 ± 55.97) | 5.08–1324.98 (125.09 ± 267.12) | 0.32–1.42 (0.84 ± 0.30) | nm | nm | - |
| Mg (mg/L) | 23.79–50.43 (38.10 ± 7.91) | 7.48–135.89 (60.06 ± 37.58) | 13.80–142.16 (46.63 ± 36.83) | 9.76–30.39 (18.54 ± 5.42) | nm | nm | - |
| K (mg/L) | 332.11–812.18 (514.86 ± 171.40) | 48.96–1471.70 (623.10 ± 466.88) | 52.52–824.83 (297.68 ± 206.73) | 324.74–533.52 (399.65 ± 65.78) | nm | nm | - |
| Ca (mg/L) | 22.00–72.44 (43.11 ± 16.79) | 10.31–407.80 (80.24 ± 78.15) | 0.43–107.41 (40.70 ± 28.47) | 6.03–32.47 (15.70 ± 6.52) | nm | nm | - |
| Cr (µg/L) | 16.41–1055.20 (308.99 ± 344.02) | 8.44–1121.78 (203.09 ± 220.85) | 30.70–554.50 (147.17 ± 133.62) | 6.04–144.84 (50.59 ± 34.45) | 100 | 50 | 45 (44.6%) [34] |
| Mn (µg/L) | 5.92–391.23 (256.05 ± 106.63) | 6.44–483.38 (210.68 ± 154.88) | 59.60–2240.12 (416.20 ± 476.67) | 54.10–262.18 (143.11 ± 57.89) | 50 | nm | 94 (93.1%) [34] |
| Fe (µg/L) | 54.45–2719.02 (824.22 ± 796.16) | 2.46–3167.88 (425.76 ± 638.80) | 60.30–6413.26 (1037.72 ± 1731.16) | 338.06–1720.96 (940.11 ± 375.29) | 300 | nm | 55 (54.5%) [34] |
| Co (µg/L) | 0.26–11.37 (4.89 ± 3.36) | 0.66–13.92 (4.63 ± 3.78) | 1.22–76.00 (23.63 ± 26.48) | 0.22–5.06 (0.89 ± 0.95) | nm | nm | 0 (0%) |
| Ni (µg/L) | 13.75–1083.98 (137.17 ± 289.66) | 0.83–492.50 (70.22 ± 105.95) | 1.52–321.40 (70.46 ± 77.26) | 0.10–69.16 (15.44 ± 17.02) | nm | 70 | 20 (19.8%) [35] |
| Cu (µg/L) | 37.58–800.61 (139.92 ± 201.65) | 12.86–1297.40 (231.41 ± 229.31) | 162.60–2143.00 (536.22 ± 429.16) | 109.86–1215.10 (302.23 ± 216.00) | 1300 | 2000 | 1 (1.0%) [35] |
| Zn (µg/L) | 12.36–379.10 (163.19 ± 128.98) | 6.78–830.57 (299.36 ± 223.96) | 112.62–1186.06 (358.93 ± 259.66) | 83.06–573.06 (180.74 ± 117.67) | nm | nm | 0 (0%) |
| As (µg/L) | 0.01–0.44 (0.20 ± 0.13) | 0.07–3.02 (0.35 ± 0.52) | 0.02–0.38 (0.17 ± 0.11) | 0.02–2.52 (0.85 ± 0.77) | 10 | 10 | 0 (0%) |
| Rb (µg/L) | 422.74–2247.23 (771.91 ± 490.57) | 57.82–2135.37 (901.15 ± 702.89) | 147.10–1279.04 (620.49 ± 343.98) | 87.76–1748.48 (552.79 ± 437.18) | nm | nm | 0 (0%) |
| Sr (µg/L) | 71.46–771.63 (228.64 ± 193.33) | 28.73–1658.44 (435.39 ± 309.09) | 121.62–878.74 (400.89 ± 209.05) | 63.80–705.80 (233.11 ± 173.42) | nm | nm | 0 (0%) |
| Pb (µg/L) | 0.03–0.78 (0.27 ± 0.22) | 0.10–1.92 (0.32 ± 0.32) | 0.12–0.67 (0.33 ± 0.19) | 0.01–3.73 (0.69 ± 0.99) | 15 | 10 | 0 (0%) |
3.3. Chemometric Modeling Based on Isotopic and Multielemental Data
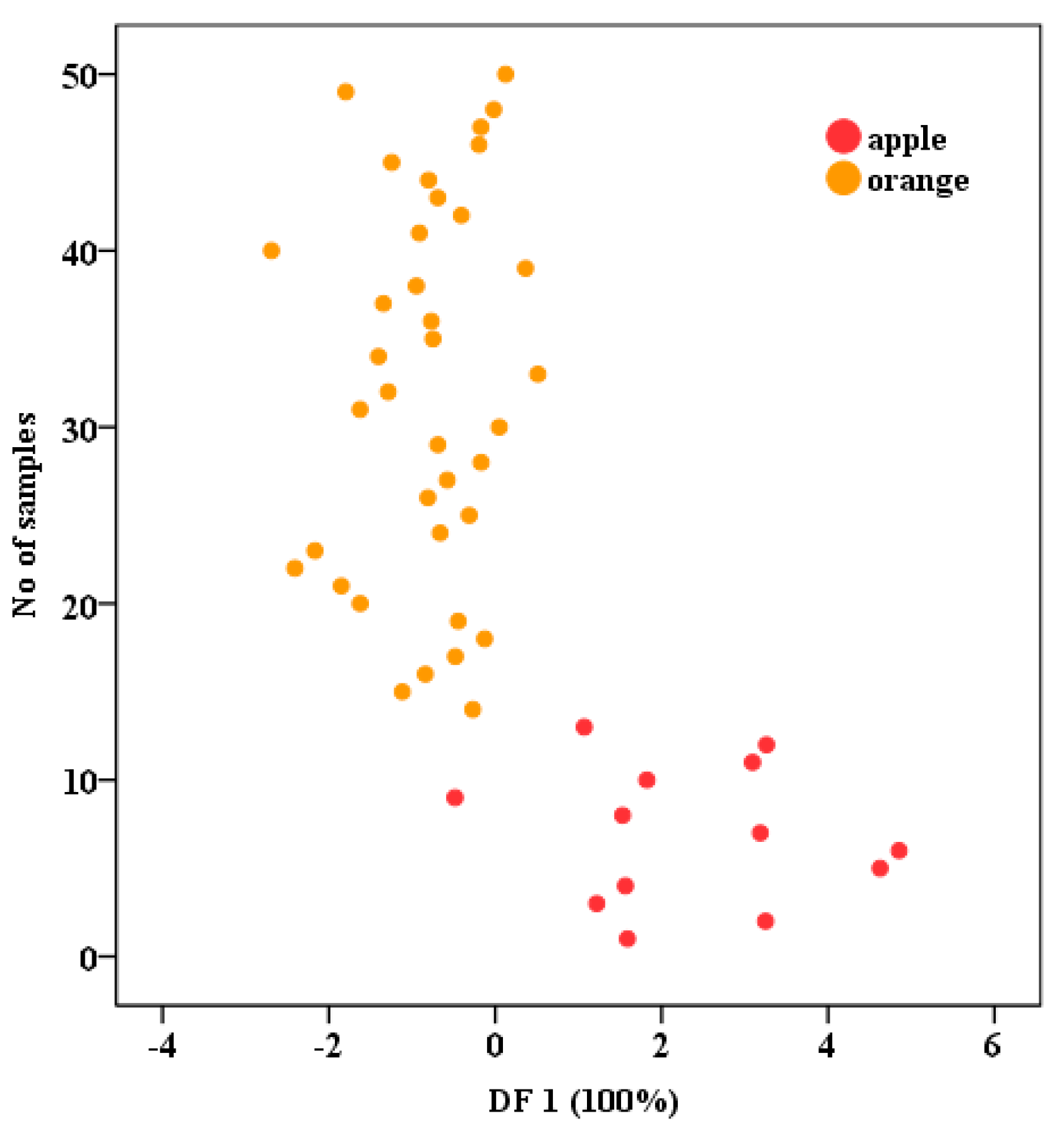
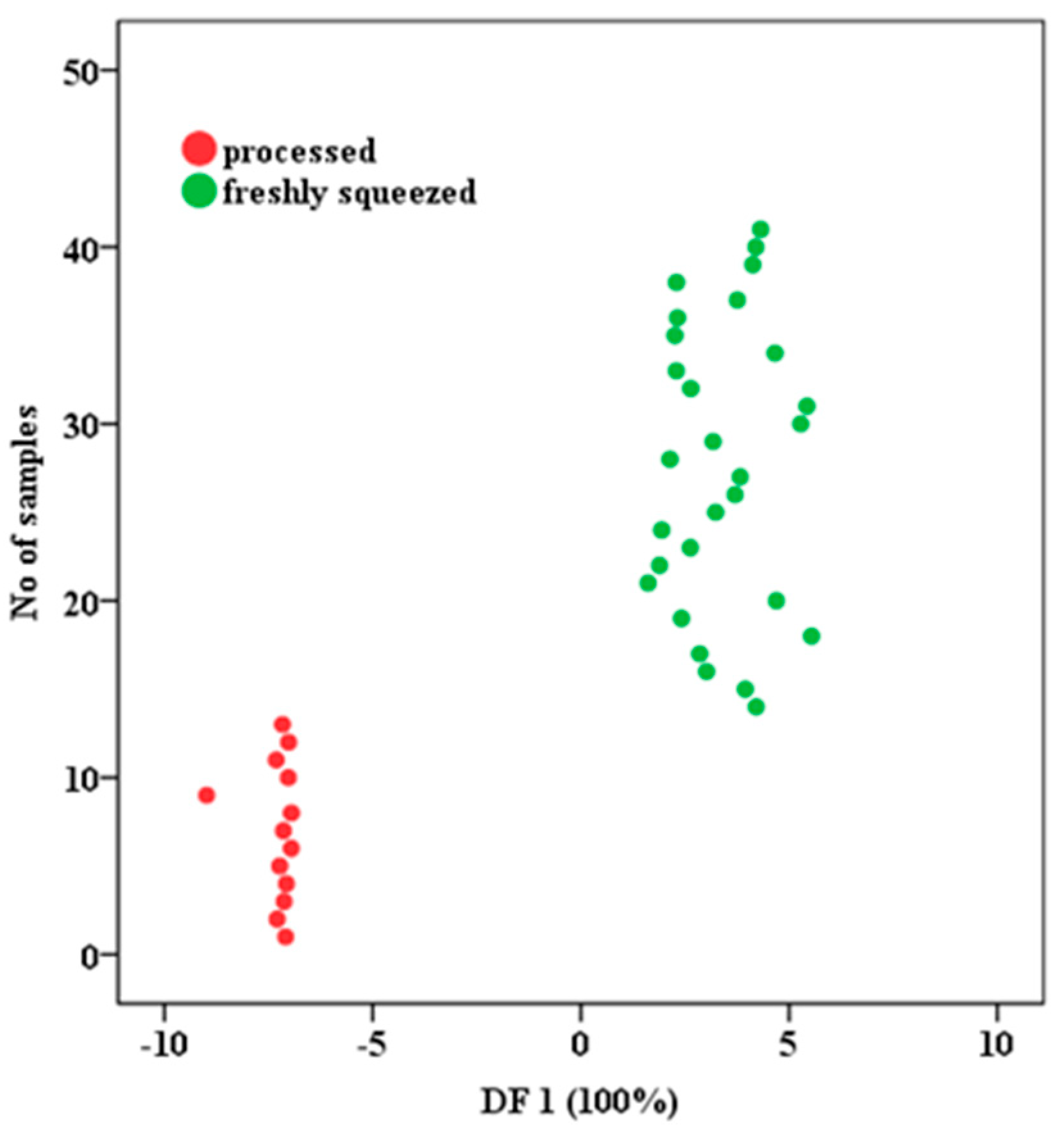
3.3.1. Development of LDA for Fruit Juices Classifications
3.3.2. Development of k-NN Algorithm for Fruit Juice Classifications
3.3.3. Development of ANNs for Fruit Juices Classifications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDA | Linear discriminant analysis |
| k-NN | K-nearest neighbor |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| RSD | Relative standard deviation |
| DF | Discriminant function |
| ANN | Artificial neural network |
| IRMS | Isotope ratio mass spectrometry |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| LOOCV | Leave-one-out cross-validation |
| SHAP | Shapley additive explanation |
| AUC | Area under the curve |
References
- Grand View Research. Fruit Beverages Market Size, Share & Trends Analysis Report. Available online: https://www.grandviewresearch.com/industry-analysis/fruit-beverages-market (accessed on 10 April 2025).
- Cheng, J.; Wang, Q.; Yu, J. Life cycle assessment of concentrated apple juice production in China: Mitigation options to reduce the environmental burden. Sustain. Prod. Consum. 2022, 32, 15–26. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Quality and Authenticity Control of Fruit Juices-A Review. Molecules 2019, 24, 1014. [Google Scholar] [CrossRef]
- Vlad, I.M.; Butcaru, A.C.; Fîntîneru, G.; Bădulescu, L.; Stănică, F.; Toma, E. Mapping the Preferences of Apple Consumption in Romania. Horticulturae 2023, 9, 35. [Google Scholar] [CrossRef]
- Eurostat. APRO_CPSH1—Custom Dataset. Available online: https://ec.europa.eu/eurostat/databrowser/view/apro_cpsh1__custom_16005223/default/bar?lang=en (accessed on 2 April 2025).
- Xu, L.; Xu, Z.; Liao, X. A review of fruit juice authenticity assessments: Targeted and untargeted analyses. Crit. Rev. Food. Sci. Nutr. 2021, 22, 6081–6102. [Google Scholar] [CrossRef] [PubMed]
- Katerinopoulou, K.; Kontogeorgos, A.; Salmas, C.E.; Patakas, A.; Ladavos, A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mu, J.; Tan, D.; Mao, K.; Zhang, J.; Ahmed Sadiq, F.; Sang, Y.; Zhang, A. Application of stable isotopic and mineral elemental fingerprints in identifying the geographical origin of concentrated apple juice in China. Food Chem. 2022, 391, 133269. [Google Scholar] [CrossRef]
- Cavdaroglu, C.; Altug, N.; Serpen, A.; Őztop, B. Comparative performance of artificial neural networks and support vector machines in detecting adulteration of apple juice concentrate using spectroscopy and time domain NMR. Food Res. Int. 2025, 201, 115616. [Google Scholar] [CrossRef]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, M.; Yu, Q.; Liu, Y. Status and trends of artificial intelligence in the R&D of future fruit & vegetable juices. Innov. Food Sci. Emerg. Technol. 2024, 97, 103796. [Google Scholar] [CrossRef]
- Calle, J.L.P.; Ferreiro-González, M.; Ruiz-Rodríguez, A.; Fernández, D.; Palma, M. Detection of Adulterations in Fruit Juices Using Machine Learning Methods over FT-IR Spectroscopic Data. Agronomy 2022, 12, 683. [Google Scholar] [CrossRef]
- Mac, H.X.; Pham, T.T.; Ha, N.T.T.; Nguyen, L.L.P.; Baranyai, L.; Friedrich, L. Current Techniques for Fruit Juice and Wine Adulterant Detection and Authentication. Beverages 2023, 9, 84. [Google Scholar] [CrossRef]
- Harmankaya, M.; Gezgin, S.; Özcan, M.M. Comparative evaluation of some macro- and micro-element and heavy metal contents in commercial fruit juices. Environ. Monit. Assess. 2012, 184, 5415–5420. [Google Scholar] [CrossRef]
- Dehelean, A.; Magdas, D.A. Analysis of mineral and heavy metal content of some commercial fruit juices by inductively coupled plasma mass spectrometry. Sci. World J. 2013, 2013, 215423. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Zhong, Q.; Feng, D.; An, H.; Yue, H.; Wu, Z.; Zhang, L.; Guo, X.; Wang, S.F. Application of gas chromatography-stable isotope mass spectrometry to determine the oxygen isotope ratio of water in concentrated fruit juice. J. Agric. Food Res. 2024, 15, 100940. [Google Scholar] [CrossRef]
- Magdas, D.A.; Cristea, G.; Puscas, R.; Tusa, F. The use of isotope ratios in commercial fruit juices authentication. Rom. J. Phys. 2013, 59, 355–359. [Google Scholar]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C. Food authentication: Techniques, trends & emerging approaches. Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data Fusion Methodologies for Food and Beverage Authentication and Quality Assessment—A Review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef]
- Messai, H.; Farman, M.; Sarraj-Laabidi, A.; Hammami-Semmar, A.; Semmar, N. Chemometrics Methods for Specificity, Authenticity and Traceability Analysis of Olive Oils: Principles, Classifications and Applications. Foods 2016, 5, 77. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H. E-nose coupled with an artificial neural network to detection of fraud in pure and industrial fruit juices. Int. J. Food Prop. 2021, 24, 592–602. [Google Scholar] [CrossRef]
- Granato, D.; Putnik, P.; Kovačević, D.B.; Sousa Santos, J.; Calado, V.; Silva Rocha, R.; Gomes Da Cruz, A.; Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in Chemometrics: Food Authentication, Microbiology, and Effects of Processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677. [Google Scholar] [CrossRef]
- Funes, E.; Allouche, Y.; Beltrán, G.; Jiménez, A. A Review: Artificial Neural Networks as Tool for Control Food Industry Process. J. Sens. Technol. 2015, 5, 28–43. [Google Scholar] [CrossRef]
- Goyal, S. Artificial Neural Networks in Fruits: A Comprehensive Review. Int. J. Image Graph. Signal Process. 2014, 6, 53–63. [Google Scholar] [CrossRef]
- Oerter, E.; Malone, M.; Putman, A.; Drits-Esser, D.; Stark, L.; Bowen, G. Every Apple Has a Voice: Using Stable Isotopes to Teach about Food Sourcing and the Water Cycle. Hydrol. Earth Syst. Sci. 2017, 21, 3799–3810. [Google Scholar] [CrossRef]
- Brencic, M.; Vreca, P. Identification of Sources and Production Processes of Bottled Waters by Stable Hydrogen and Oxygen Isotope Ratios. Rapid Commun. Mass Spectrom. 2006, 20, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Cristea, G.; Dehelean, A.; Puscas, R.; Covaciu, F.-D.; Hategan, A.R.; Müller Molnár, C.; Magdas, D.A. Characterization and Differentiation of Wild and Cultivated Berries Based on Isotopic and Elemental Profiles. Appl. Sci. 2023, 13, 2980. [Google Scholar] [CrossRef]
- Available online: https://wikifarmer.com/library/en/article/orange-tree-climate-and-soil-requirements (accessed on 8 April 2025).
- Cristea, G.; Dehelean, A.; Voica, C.; Feher, I.; Puscas, R.; Magdas, D.A. Isotopic and Elemental Analysis of Apple and Orange Juice by Isotope Ratio Mass Spectrometry (IRMS) and Inductively Coupled Plasma—Mass Spectrometry (ICP-MS). Anal. Lett. 2021, 54, 212–226. [Google Scholar] [CrossRef]
- Magdas, D.A.; Puscas, R. Stable isotopes determination in some Romanian fruit juices. Isot. Environ. Health Stud. 2011, 47, 372–378. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Piasentier, E. Stable Isotope Ratio Analysis for Assessing the Authenticity of Food of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2016, 15, 868–877. [Google Scholar] [CrossRef]
- Simpkins, W.A.; Louie, H.; Wub, M.; Harrison, M.; Goldberg, D. Trace elements in Australian orange juice and other products. Food Chem. 2000, 71, 423–433. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius Commission (2024). General Standard for Contaminants in Food and Feed (GSCTFF). Joint FAO/WHO Food Standards Programmer, Contaminants in foods. Codex Alimentarius. Available online: https://www.fao.org/fao-who-codexalimentarius/thematic-areas/contaminants/en/ (accessed on 15 April 2025).
- US Environmental Protection Agency (EPA). National Primary Drinking Water Regulations; US Environmental Protection Agency (EPA): Washington, DC, USA, 2018.
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2017.
- Bao, S.X.; Wang, Z.H.; Liu, J.S. X-ray fluorescence analysis of trace elements in fruit juice. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1893–1897. [Google Scholar] [CrossRef]
- Demir, F.; Kipcak, A.S.; Dere Ozdemir, O.; Moroydor Derun, E. Determination of essential and non-essential element concentrations and health risk assessment of some commercial fruit juices in Turkey. J. Food Sci. Technol. 2020, 57, 4432–4442. [Google Scholar] [CrossRef]
- Madeja, A.S.; Welna, M. Evaluation of a simple and fast method for the multi-elemental analysis in commercial fruit juice samples using atomic emission spectrometry. Food Chem. 2013, 141, 3466–3472. [Google Scholar] [CrossRef]
- Farid, S.M.; Enani, M.A. Levels of trace elements in commercial fruit juices in Jeddah, Saudi Arabia. Med. J. Islam. World Acad. Sci. 2010, 18, 31–38. [Google Scholar]
- Krejpcio, Z.; Sionkowski, S.; Bartela, J. Safety of fresh fruits and juices available on the polish market as determined by heavy metal residues. Pol. J. Environ. Stud. 2005, 14, 877–881. [Google Scholar]
- Velimirović, D.S.; Mitić, S.S.; Tošić, S.B.; Kaličanin, B.M.; Pavlović, A.N.; Mitić, M.N. Levels of major and minor elements in some commercial fruit juices available in Serbia. Trop. J. Pharm. Res. 2013, 12, 805–811. [Google Scholar] [CrossRef]
- Demir, N.; Acar, J. An investigation on the mineral contents of some fruit juices marketed in Ankara. GIDA 1995, 20, 305–311. [Google Scholar]
- Barners, K.W. The Analysis of Trace Metals in Fruit, Juice and Juice Production Using a Dual-View Plasma. Perkin Elmer Instruments. 2017. Available online: https://www.perkinelmer.com.cn/PDFs/downloads/APP_TraceMetalsinFruitJuicesViewPlasma.pdf (accessed on 13 April 2025).
- Akhtar, S.; Ali, J.; Javed, B.; Khan, F.A. Studies on the preparation and storage stability of pomegranate juice based drink. Middle-East J. Sci. Res. 2013, 16, 91–195. [Google Scholar]
- Bayızıt, A.A. Analysis of mineral content in pomegranate juice by ICP-OES. Asian J. Chem. 2010, 8, 6542–6546. [Google Scholar]
- Cámara, M.; Domínguez, L.; Medina, S.; Mena, P.; García-Viguera, C. A Comparative Analysis of Folate and Mineral Contents in Freshly Squeezed and Commercial 100% Orange Juices Available in Europe. Nutrients 2024, 16, 3605. [Google Scholar] [CrossRef]
- Cristea, G.; Covaciu, F.-D.; Feher, I.; Puscas, R.; Voica, C.; Dehelean, A. Multivariate Modelling Based on Isotopic, Elemental, and Fatty Acid Profiles to Distinguish the Backyard and Barn Eggs. Foods 2024, 13, 3240. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Palenzuela, A.Z.; Crevillén, A.G. Untargeted authentication of fruit juices based on electrochemical fingerprints combined with chemometrics. Adulteration of orange juice as case of study. LWT 2024, 209, 116797. [Google Scholar] [CrossRef]
- James, V.R.; Panchal, H.J.; Shah, A.P. Estimation of selected elemental impurities by inductively coupled plasma-mass spectroscopy (ICP-MS) in commercial and fresh fruit juices. Environ. Monit. Assess. 2023, 195, 1390–1404. [Google Scholar] [CrossRef]
- Fortunato de Carvalho Rocha, W.; Bezerra do Prado, C.; Blonder, N. Comparison of chemometric problems in food analysis using non-linear methods. Molecules 2020, 25, 3025. [Google Scholar] [CrossRef]


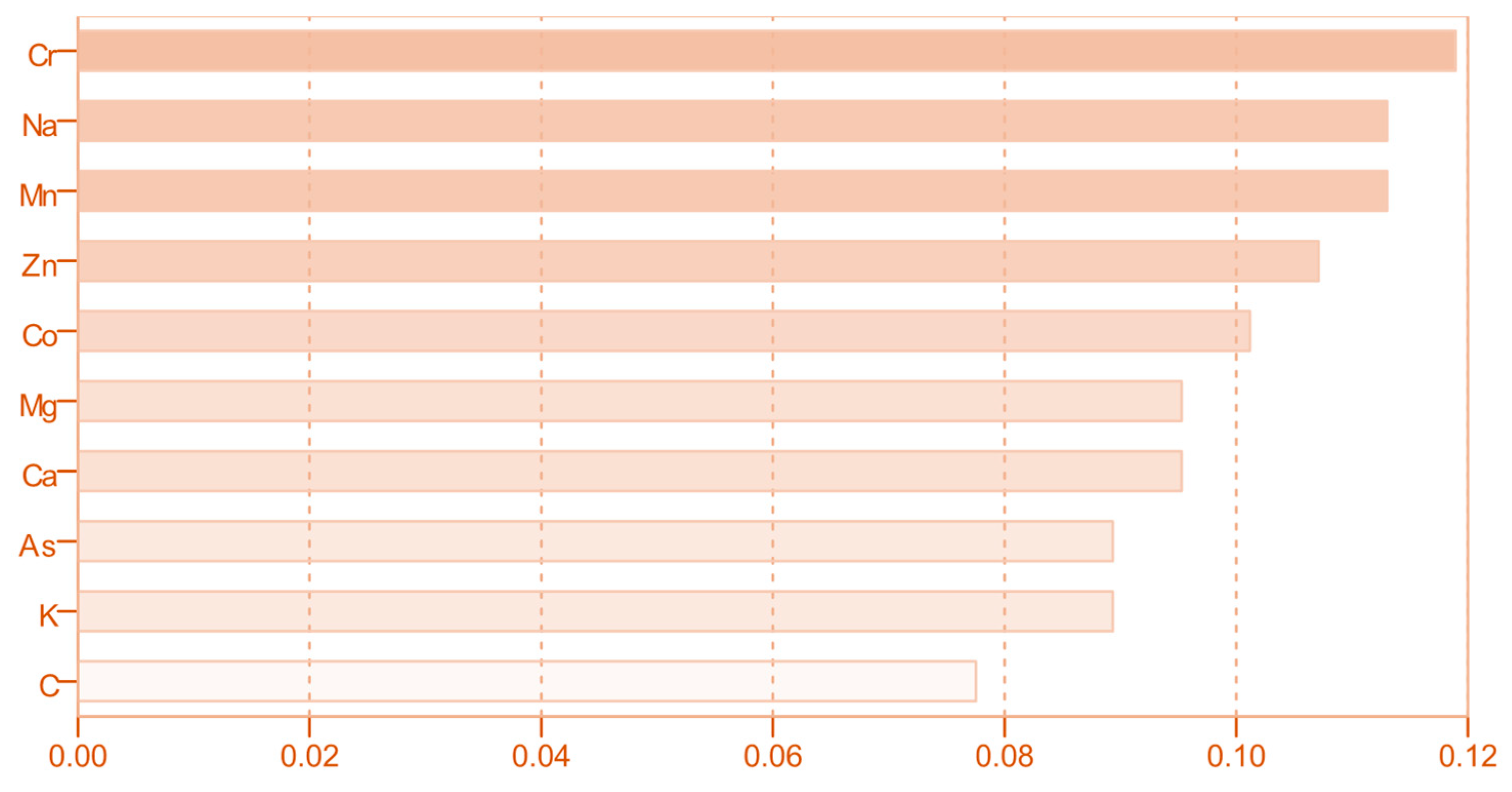
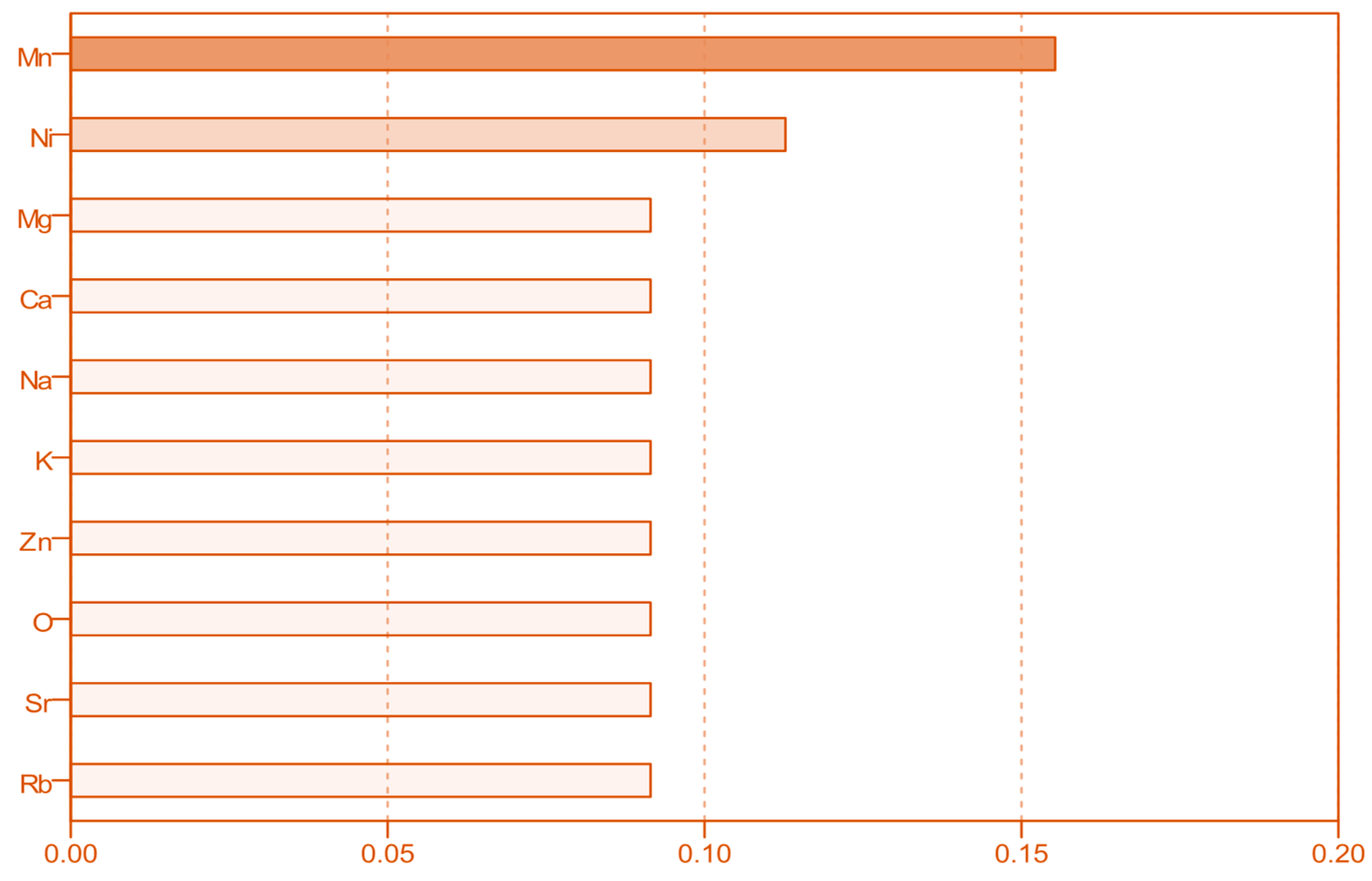
| Element | Concentration (mg/L) | ||
|---|---|---|---|
| Apple | Orange | Refs. | |
| Na | - | 88.00 | [36] |
| 20.00 | 39.80 | [15] | |
| 22.90 | 7.70 | [37] | |
| 20.30 | 51.70 | This study | |
| Mg | - | 29.00 | [36] |
| 44.30 | 71.20 | [38] | |
| 44.70 | 51.20 | [15] | |
| 33.04 | 73.30 | [37] | |
| 38.10 | 60.06 | This study | |
| K | - | 825 | [36] |
| 371.00 | 277.20 | [15] | |
| 896.00 | 1350.00 | [37] | |
| 514.9 | 623.1 | This study | |
| Ca | - | 52.00 | [36] |
| 83.40 | 1082.00 | [38] | |
| 51.30 | 43.30 | [15] | |
| 64.10 | - | [37] | |
| 43.11 | 80.24 | This study | |
| Concentration (μg/L) | |||
| Cr | 6.30 | 5.90 | [39] |
| 22.00 | 9.00 | [38] | |
| 20.60 | 14.20 | [15] | |
| 12.00 | - | [37] | |
| 308.99 | 203.09 | This study | |
| Mn | 23.40 | 20.90 | [39] |
| - | 200.00 | [36] | |
| 406.00 | 316.00 | [38] | |
| 242.03 | 120.27 | [15] | |
| 256.05 | 120.68 | This study | |
| Fe | 325.00 | 361.00 | [39] |
| - | 4900.00 | [36] | |
| 1790.00 | 549.00 | [38] | |
| 227.00 | 455.00 | [37] | |
| 824.22 | 425.76 | This study | |
| Co | 8.00 | 7.90 | [39] |
| 13.77 | 2.47 | [15] | |
| 4.89 | 4.63 | This study | |
| Cu | 317.00 | 500.00 | [39] |
| 283.00 | 245.00 | [40] | |
| - | 130.00 | [36] | |
| 83.00 | 198.00 | [38] | |
| 86.17 | 132.83 | [15] | |
| 7.00 | 48.00 | [37] | |
| 139.92 | 231.41 | This study | |
| Zn | 524.00 | 895.00 | [39] |
| 550.00 | 1177.00 | [40] | |
| - | 4700.00 | [36] | |
| 210.00 | 235.00 | [38] | |
| 230.00 | 180.56 | [15] | |
| 15.00 | 49.00 | [37] | |
| 163.19 | 299.36 | This study | |
| Ni | 6.20 | 5.70 | [39] |
| - | 100.00 | [36] | |
| 69.00 | 63.00 | [38] | |
| 80.64 | 79.23 | [15] | |
| BDL * | BDL * | [37] | |
| 137.17 | 70.22 | This study | |
| As | 1.73 | 1.04 | [15] |
| 0.20 | 0.35 | This study | |
| Pb | 130.00 | 95.00 | [40] |
| 670.00 | - | [38] | |
| 22.71 | 3.28 | [15] | |
| 58.00 | BDL * | [37] | |
| 0.27 | 0.32 | This study | |
| Element | Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|
| Cherry | Apricot | Peach | Grape | Pomegranate | Refs. | |
| Na | - | 79.80 | 52.00 | - | - | [15] |
| 75.80 | - | 50.58 | 88.20 | - | [41] | |
| 68.40 | 68.30 | 50.51 | - | - | [42] | |
| - | 30.00 | - | - | - | [43] | |
| - | - | - | - | 96.02 | [44] | |
| - | - | - | - | 133.00 | [45] | |
| 23.00 | 3.07 | 4.76 | 17.01 | 16.00 | [37] | |
| 193.80 | 79.80 | 52.00 | - | 132.70 | This study | |
| Mg | - | - | - | 48.80 | - | [38] |
| - | 34.80 | 32.30 | - | - | [15] | |
| 39.90 | - | 24.60 | 32.20 | - | [41] | |
| 91.00 | 150.90 | 110.70 | - | - | [42] | |
| - | 50.00 | - | - | - | [43] | |
| - | - | - | - | 67.20 | [44] | |
| - | - | - | - | 13.80 | [45] | |
| 31.40 | 30.81 | 27.70 | 71.01 | 61.70 | [37] | |
| 142.20 | 34.80 | 32.30 | - | 13.80 | This study | |
| K | - | 339.70 | 191.80 | - | - | [15] |
| 157.00 | 185.00 | 144.00 | [41] | |||
| 264.00 | 1046.00 | 679.00 | [42] | |||
| 1140.00 | [43] | |||||
| 1283.00 | [44] | |||||
| 207.00 | [45] | |||||
| 565.00 | 1038.00 | 842.00 | 1080.00 | 941.00 | [37] | |
| 756.90 | 339.70 | 191.80 | - | 207.50 | This study | |
| Ca | 123.00 | [38] | ||||
| - | 48.70 | 32.90 | - | - | [15] | |
| 42.80 | 38.60 | 49.40 | [41] | |||
| 54.70 | 102.05 | 42.90 | [42] | |||
| 70.00 | [43] | |||||
| 107.53 | [44] | |||||
| 0.42 | [45] | |||||
| 68.80 | 66.09 | 53.80 | 177.00 | 162.00 | [37] | |
| 107.40 | 48.70 | 32.90 | - | 0.40 | This study | |
| Concentration (μg/L) | ||||||
| Cr | - | - | - | 25.00 | - | [38] |
| - | 41.20 | 25.23 | - | - | [15] | |
| 246.00 | - | 377.00 | 330.00 | - | [41] | |
| 7.00 | 7.00 | 10.00 | 7.00 | BDL * | [37] | |
| 64.30 | 41.20 | 25.23 | - | 82.0 | This study | |
| Mn | 886.00 | [38] | ||||
| - | 225.72 | 162.77 | - | - | [15] | |
| 272.00 | 346.00 | 284.00 | [41] | |||
| 96.00 | [44] | |||||
| 15.00 | 47.00 | 90.00 | 87.00 | 13.00 | [37] | |
| 538.74 | 225.72 | 162.77 | - | 122.72 | This study | |
| Fe | - | - | - | 2750.00 | - | [38] |
| 5150.00 | - | 7370.00 | 5300.00 | - | [41] | |
| 9110.00 | 10,250.00 | 10,290.00 | - | - | [42] | |
| - | 3800.00 | - | - | - | [43] | |
| - | - | - | - | 1810.00 | [44] | |
| 195.00 | 894.00 | 205.00 | 343.00 | 211.00 | [37] | |
| 129.27 | 3157.24 | 3635.69 | - | 60.30 | This study | |
| Co | - | 4.10 | 4.81 | - | - | [15] |
| 21.00 | - | 17.00 | 22.00 | - | [41] | |
| 8.80 | 4.10 | 4.81 | - | 1.23 | This study | |
| Cu | - | - | - | 1680.00 | - | [38] |
| - | 336.78 | 747.54 | - | - | [15] | |
| 284.00 | - | 1360.00 | 321.00 | - | [41] | |
| - | 730.00 | - | - | - | [43] | |
| - | - | - | - | 100.00 | [44] | |
| 13.00 | 74.00 | 83.00 | 83.00 | 82.00 | [37] | |
| 493.00 | 336.78 | 747.54 | - | 1120.60 | This study | |
| Zn | - | - | - | 351.00 | - | [38] |
| - | 481.08 | 663.97 | - | - | [15] | |
| 158.00 | - | 536.00 | 322.00 | - | [41] | |
| - | 900.00 | - | - | - | [43] | |
| 6.00 | 81.00 | 830.00 | 94.00 | 80.00 | [37] | |
| 261.98 | 481.08 | 663.97 | - | 112.62 | This study | |
| Ni | - | - | - | 55.00 | - | [38] |
| - | 90.82 | 34.88 | - | - | [15] | |
| 15.30 | - | 331.00 | 41.30 | - | [41] | |
| - | - | - | - | 40.00 | [45] | |
| BDL * | 18.00 | 3.00 | BDL * | BDL * | [37] | |
| 65.52 | 90.82 | 34.88 | - | 9.10 | This study | |
| Pb | 106.00 | [38] | ||||
| 3.00 | [45] | |||||
| BDL * | 121.00 | 135.00 | 32.00 | 55.00 | [37] | |
| 0.18 | 0.44 | 0.33 | - | 0.17 | This study | |
| LDA Algorithm | Apple | Orange | Other Fruits | |
|---|---|---|---|---|
| Training (Accuracy = 0.941) | Apple | 2 (22.22%) | 1 (11.11%) | 6 (66.67%) |
| Orange | 26 (100%) | 0 (0%) | 0 (0%) | |
| Other fruits | 0 (0%) | 16 (100%) | 0 (0%) | |
| Recall | F1-score | AUC | Precision | |
| Macro | 0.957 | 0.911 | 0.999 | 0.889 |
| Micro | 0.941 | 0.941 | 0.997 | 0.941 |
| Testing (Accuracy = 0.636) | Apple | 2 (50%) | 1 (25%) | 1 (25%) |
| Orange | 10 (90.91%) | 0 (0%) | 1 (9.09%) | |
| Other fruits | 4 (57.14%) | 3 (42.86%) | 0 (0%) | |
| Recall | F1-score | AUC | Precision | |
| Macro | 0.625 | 0.540 | 0.778 | 0.529 |
| Micro | 0.636 | 0.636 | 0.806 | 0.636 |
| LDA Algorithm | Apple | Orange | |
|---|---|---|---|
| Training (Accuracy = 0.941) | Apple | 9 (100%) | 0 (0%) |
| Orange | 0 (0%) | 26 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Testing (Accuracy = 0.636) | Apple | 3 (75%) | 1 (25%) |
| Orange | 3 (27.27%) | 8 (72.73%) | |
| Precision | Recall | F1-score | |
| Macro | 0.694 | 0.739 | 0.700 |
| Micro | 0.733 | 0.733 | 0.733 |
| LDA Algorithm | Processed | Freshly Squeezed | |
|---|---|---|---|
| Training (Accuracy = 1) | Processed | 9 (100%) | 0 (0%) |
| Freshly squeezed | 0 (0%) | 19 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Testing (Accuracy = 1) | Processed | 4 (100%) | 0 (0%) |
| Freshly squeezed | 0 (0%) | 9 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Partition | Predicted | ||||
|---|---|---|---|---|---|
| Apple | Orange | Other Fruits | Classifications | ||
| Training | Apple | 7 | 1 | 0 | 87.5% |
| Orange | 2 | 21 | 3 | 80.8% | |
| Other fruits | 0 | 3 | 14 | 82.4% | |
| Overall classifications | 17.7% | 49.0% | 33.3% | 82.4% | |
| Testing | Apple | 3 | 0 | 2 | 60.0% |
| Orange | 1 | 9 | 1 | 81.8% | |
| Other fruits | 0 | 0 | 6 | 100% | |
| Overall classifications | 18.2% | 40.9% | 40.9% | 81.8% | |
| k-NN Partition | Apple | Orange | Other Fruits | |
|---|---|---|---|---|
| Training (Accuracy = 1) | Apple | 9 (100%) | 0 (0%) | 0 (0%) |
| Orange | 0 (0%) | 26 (100%) | 0 (0%) | |
| Other fruits | 0 (0%) | 0 (0%) | 16 (100%) | |
| Precision | Recall | F1-score | AUC | |
| Macro | 1 | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 | 1 |
| Testing (Accuracy = 0.455) | Apple | 0 (0%) | 3 (75%) | 1 (25%) |
| Orange | 0 (0%) | 8 (72.73%) | 3 (27.27%) | |
| Other fruits | 0 (0%) | 5 (71.43%) | 2 (28.57%) | |
| Precision | Recall | F1-score | AUC | |
| Macro | 0.278 | 0.338 | 0.300 | 0.503 |
| Micro | 0.455 | 0.455 | 0.455 | 0.591 |
| Partition | Predicted | |||
|---|---|---|---|---|
| Apple | Orange | Classifications | ||
| Training | Apple | 8 | 1 | 88.9% |
| Orange | 0 | 24 | 100% | |
| Overall classifications | 24.3% | 75.8% | 97.0% | |
| Testing | Apple | 1 | 3 | 25.0% |
| Orange | 0 | 13 | 100.0% | |
| Overall classifications | 5.9% | 94.1% | 82.4% | |
| k-NN Partition | Apple | Orange | |
|---|---|---|---|
| Training (Accuracy = 1) | Apple | 9 (100%) | 0 (0%) |
| Orange | 0 (0%) | 26 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Testing (Accuracy = 0.733) | Apple | 1 (25%) | 3 (75%) |
| Orange | 1 (9.09%) | 10 (90.91%) | |
| Precision | Recall | F1-score | |
| Macro | 0.635 | 0.580 | 0.583 |
| Micro | 0.733 | 0.733 | 0.733 |
| k-NN Partition | Processed | Freshly Squeezed | |
|---|---|---|---|
| Training (Accuracy = 1) | Processed | 9 (100%) | 0 (0%) |
| Freshly squeezed | 0 (0%) | 19 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Testing (Accuracy = 0.733) | Processed | 4 (25%) | 0 (75%) |
| Freshly squeezed | 0 (9.09%) | 9 (90.91%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| ANN Stage | Apple | Orange | Other Fruits | |
|---|---|---|---|---|
| Training (Accuracy = 0.863) | Apple | 6 (66.67%) | 2 (22.22%) | 1 (11.11%) |
| Orange | 0 (0%) | 24 (92.31%) | 2 (7.69%) | |
| Other fruits | 0 (0%) | 2 (12.5%) | 14 (87.5%) | |
| Precision | Recall | F1-score | AUC | |
| Macro | 0.894 | 0.822 | 0.846 | 0.977 |
| Micro | 0.863 | 0.863 | 0.863 | 0.976 |
| Testing (Accuracy = 0.682) | Apple | 1 (25%) | 2 (50%) | 1 (25%) |
| Orange | 0 (0%) | 10 (90.91%) | 1 (9.09%) | |
| Other fruits | 0 (0%) | 3 (42.86%) | 4 (57.14%) | |
| Precision | Recall | F1-score | AUC | |
| Macro | 0.778 | 0.577 | 0.595 | 0.867 |
| Micro | 0.682 | 0.682 | 0.682 | 0.857 |
| Sample | Groups | Apple Juices | Orange Juices | Classifications |
|---|---|---|---|---|
| Training | Apple juices | 12 | 0 | 100% |
| Orange juices | 0 | 26 | 100% | |
| Overall percent | 31.6% | 68.4% | 100% | |
| Testing | Apple juices | 1 | 0 | 100% |
| Orange juices | 1 | 10 | 90.9% | |
| Overall percent | 16.7% | 83.3% | 91.7% |
| ANN Stage | Apple | Orange | |
|---|---|---|---|
| Training (Accuracy = 1) | Apple | 9 (100%) | 0 (0%) |
| Orange | 0 (0%) | 26 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Testing (Accuracy = 0.800) | Apple | 1 (25%) | 3 (75%) |
| Orange | 0 (0%) | 11 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 0.893 | 0.625 | 0.640 |
| Micro | 0.800 | 0.800 | 0.800 |
| Sample | Groups | Processed Juice | Directly Pressed Juice | Classifications |
|---|---|---|---|---|
| Training | Processed juice | 9 | 0 | 100% |
| Directly pressed juice | 0 | 24 | 100% | |
| Overall percent | 27.3% | 72.7% | 100% | |
| Testing | Processed juice | 4 | 0 | 100% |
| Directly pressed juice | 0 | 4 | 100% | |
| Overall percent | 50% | 50% | 100% |
| ANN Stage | Processed | Freshly Squeezed | |
|---|---|---|---|
| Training (Accuracy = 1) | Processed | 9 (100%) | 0 (0%) |
| Freshly squeezed | 0 (0%) | 19 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Testing (Accuracy = 1) | Processed | 4 (100%) | 0 (0%) |
| Freshly squeezed | 0 (0%) | 9 (100%) | |
| Precision | Recall | F1-score | |
| Macro | 1 | 1 | 1 |
| Micro | 1 | 1 | 1 |
| Parameter | Commercial Juices | Commercial Apple and Orange Juices | Processed and Freshly Squeezed Apple Juices | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LDA | k-NN | ANNs | LDA | k-NN | ANNs | LDA | k-NN | ANNs | |
| Accuracy | 0.669 ± 0.090 | 0.617 ± 0.087 | 0.698 ± 0.085 | 0.820 ± 0.075 | 0.820 ± 0.040 | 0.820 ± 0.075 | 1.000 | 1.000 | 1.000 |
| Sensitivity | - | - | - | 0.868 ± 0.137 | 0.918 ± 0.067 | 0.918 ± 0.067 | 1.000 | 1.000 | 1.000 |
| Specificity | - | - | - | 0.667 ± 0.365 | 0.467 ± 0.125 | 0.700 ± 0.267 | 1.000 | 1.000 | 1.000 |
| AUC | 0.814 ± 0.054 | 0.674 ± 0.073 | 0.835 ± 0.090 | 0.858 ± 0.095 | 0.837 ± 0.135 | 0.927 ± 0.075 | 1.000 | 1.000 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feher, I.; Dehelean, A.; Puscas, R.; Magdas, D.A.; Tamas, V.; Cristea, G. Advanced Multimodeling for Isotopic and Elemental Content of Fruit Juices. Beverages 2025, 11, 145. https://doi.org/10.3390/beverages11050145
Feher I, Dehelean A, Puscas R, Magdas DA, Tamas V, Cristea G. Advanced Multimodeling for Isotopic and Elemental Content of Fruit Juices. Beverages. 2025; 11(5):145. https://doi.org/10.3390/beverages11050145
Chicago/Turabian StyleFeher, Ioana, Adriana Dehelean, Romulus Puscas, Dana Alina Magdas, Viorel Tamas, and Gabriela Cristea. 2025. "Advanced Multimodeling for Isotopic and Elemental Content of Fruit Juices" Beverages 11, no. 5: 145. https://doi.org/10.3390/beverages11050145
APA StyleFeher, I., Dehelean, A., Puscas, R., Magdas, D. A., Tamas, V., & Cristea, G. (2025). Advanced Multimodeling for Isotopic and Elemental Content of Fruit Juices. Beverages, 11(5), 145. https://doi.org/10.3390/beverages11050145










