Investigating the Volatiles of Kombucha During Storage Under Refrigerated Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Kombucha Tea
2.2. Determination of the Volatile Compounds
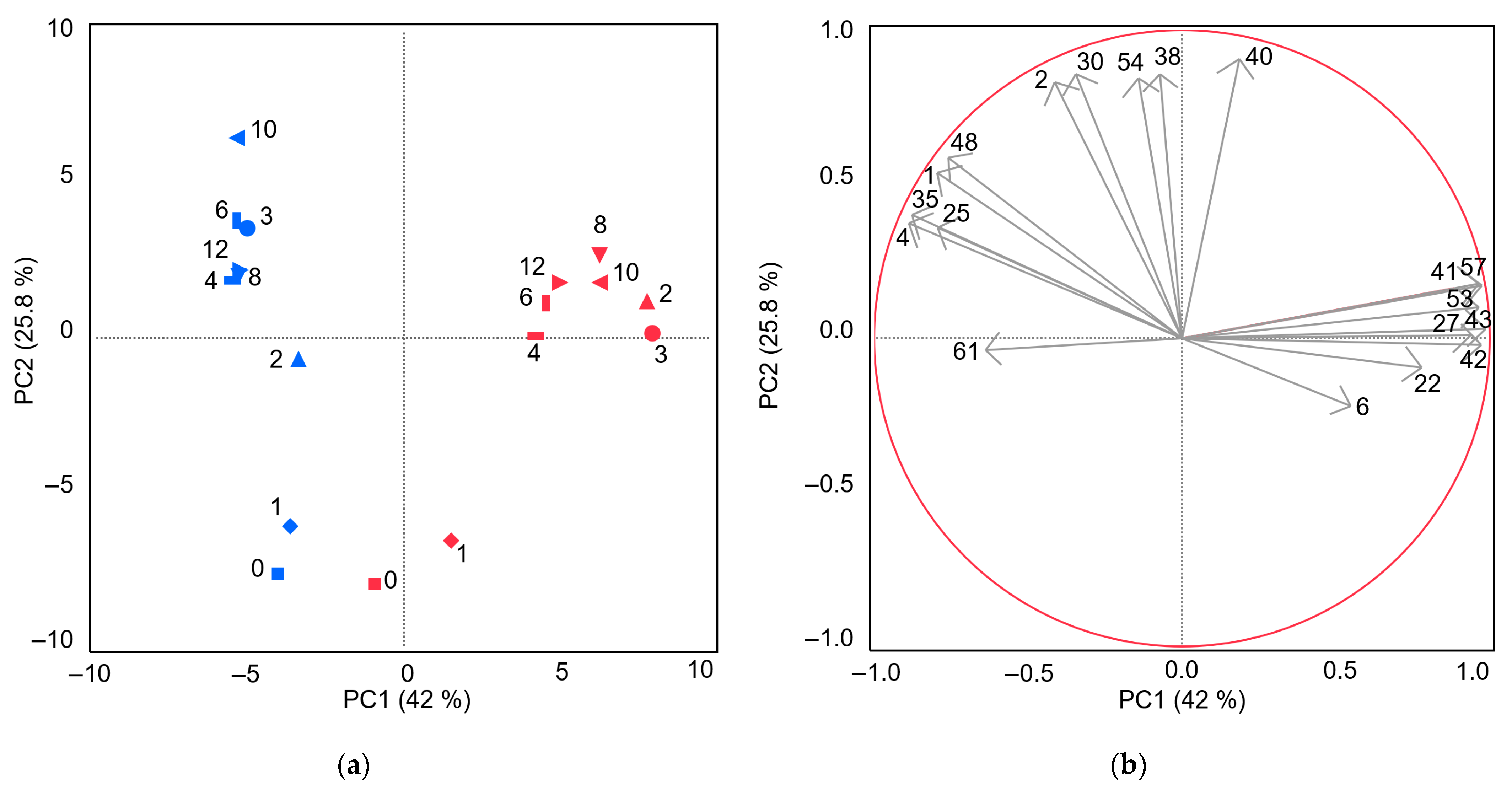
2.3. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAB | Acetic acid bacteria |
| ANOVA | Analysis of variance |
| CAR | Carboxen |
| DVB | Divinylbenzene |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| HS | Headspace |
| LAB | Lactic acid bacteria |
| PCA | Principal component analysis |
| PDMS | Polydimethylsiloxane |
| RI | Retention index (Kovats) |
| RT | Retention time |
| SBSE | Stir-bar sorptive extraction |
| SD | Standard deviation |
| SPE | Solid-phase extraction |
| SPME | Solid-phase microextraction |
| UFA | Unsaturated fatty acid |
Appendix A
| Peak # 1 | RT [min] | RI_cal | NIST Mass Spectral Library | Name | CAS Number | Chemical Family | Previously Identified by 2: | ||
|---|---|---|---|---|---|---|---|---|---|
| MF | RMF | RI_lit | |||||||
| 20 | 9.52 | 1004 | 830 | 919 | 1003 | Octanal | 124-13-0 | Aldehyde | [18,21,26,27,28,29,30,42,49,50,51] |
| 32 | 12.23 | 1105 | 839 | 848 | 1104 | Nonanal | 124-19-6 | Aldehyde | [18,21,26,27,28,29,30,31,32,42,43,44,46,48,49,50,51,52,54,55] |
| 1 | 1.89 | 954 | 954 | 427 | Ethanol | 64-17-5 | Alkanol | [1,18,26,27,28,31,33,35,36,37,40,41,44,45,46,47,48,49,50] | |
| 4 | 3.69 | 737 | 942 | 947 | 736 | 3-Methyl-1-butanol | 123-51-3 | Alkanol | [18,21,26,27,29,30,31,33,35,36,37,38,40,41,42,45,46,48,49,51,52] |
| 12 | 6.20 | 874 | 790 | 870 | 868 | 1-Hexanol | 111-27-3 | Alkanol | [21,26,27,34,35,37,39,43,46,50,51] |
| 23 | 10.20 | 1032 | 788 | 942 | 1030 | 2-Ethyl-1-hexanol | 104-76-7 | Alkanol | [18,26,27,28,29,30,31,33,34,35,37,38,39,40,45,47,51,53,54] |
| 34 | 12.49 | 1116 | 882 | 922 | 1119 | 2-Phenylethyl alcohol | 60-12-8 | Alkanol | [1,18,26,28,29,30,31,32,33,34,36,37,38,39,40,41,43,44,45,46,47,48,49,50,51,52,53,55] |
| 14 | 6.70 | 894 | 818 | 929 | 893 | Styrene | 100-42-5 | Alkenylbenzene | [31,33,46,55] |
| 64 | 22.74 | 1539 | 822 | 857 | 1532 | Dihydroactinidiolide | 17092-92-1 | Benzofuran | [28,31,36,41,46,49] |
| 50 | 17.60 | 1315 | 820 | 842 | 1314 | Edulan 1 | 41678-29-9 | Benzopyran | [29,30] |
| 3 | 2.67 | 660 | 910 | 916 | 610 | Acetic acid | 64-19-7 | Carboxylic acid | [1,18,26,28,31,33,34,35,36,37,38,40,41,42,43,44,45,46,48,49,50,51,52,54,55] |
| 5 | 4.13 | 767 | 911 | 913 | 765 | Propanoic acid, 2 methyl- | 79-31-2 | Carboxylic acid | [1,21,26,27,31,32,33,34,36,38,41,43,46,48,49,50,54,55] |
| 10 | 5.93 | 863 | 956 | 956 | 850 | Butanoic acid, 3-methyl- | 503-74-2 | Carboxylic acid | [18,21,26,27,28,29,31,32,34,36,37,39,41,43,44,47,48,49,50,51,54,55] |
| 11 | 6.08 | 869 | 942 | 947 | 861 | Butanoic acid, 2-methyl- | 116-53-0 | Carboxylic acid | [18,26,28,29,51,54] |
| 16 | 8.94 | 984 | 736 | 906 | 990 | n-Hexanoic acid | 142-62-1 | Carboxylic acid | [21,26,27,28,29,32,41,47,48] |
| 33 | 12.44 | 1114 | 738 | 825 | 1123 | Hexanoic acid, 2-ethyl- | 149-57-5 | Carboxylic acid | [21] |
| 38 | 14.08 | 1177 | 908 | 921 | 1180 | n-Octanoic acid | 124-07-2 | Carboxylic acid | [1,21,26,27,28,29,31,32,33,34,35,36,37,38,39,41,43,44,46,47,48,49,50,54] |
| 46 | 16.45 | 1269 | 916 | 935 | 1273 | n-Nonanoic acid | 112-05-0 | Carboxylic acid | [21,26,28,29,31,34,36,37,41,43,46,48,49] |
| 51 | 18.84 | 1367 | 930 | 941 | 1372 | n-Decanoic acid | 334-48-5 | Carboxylic acid | [1,21,26,27,28,31,32,33,34,35,36,37,38,40,41,43,44,45,46,48,49,50] |
| 2 | 2.46 | 640 | 743 | 786 | 612 | Ethyl acetate | 141-78-6 | Ester | [1,18,21,26,27,28,31,33,34,36,37,38,42,43,44,45,47,48,49,50,51,54,55] |
| 6 | 4.23 | 773 | 894 | 899 | 772 | Isobutyl acetate | 110-19-0 | Ester | [18,21,53,54] |
| 7 | 4.72 | 801 | 771 | 868 | 801 | Butanoic acid, ethyl ester | 66-25-1 | Ester | [21] |
| 8 | 5.71 | 850 | 832 | 915 | 849 | Butanoic acid, 2-methyl-, ethyl ester | 7452-79-1 | Ester | [1,28,38,48] |
| 9 | 5.79 | 853 | 733 | 800 | 853 | Butanoic acid, 3-methyl-, ethyl ester | 108-64-5 | Ester | [21,26,47,54] |
| 13 | 6.31 | 879 | 969 | 969 | 876 | 1-Butanol, 3-methyl-, acetate | 123-92-2 | Ester | [18,26,27,34,37,39,44,47,48,50,51,54,55] |
| 15 | 7.44 | 926 | 856 | 897 | 925 | Hexanoic acid, methyl ester | 106-70-7 | Ester | [46] |
| 19 | 9.36 | 998 | 916 | 926 | 999 | Hexanoic acid, ethyl ester | 123-66-0 | Ester | [1,18,21,33,34,38,39,43,45,46,47,50,55] |
| 21 | 9.73 | 1013 | 874 | 906 | 1014 | 1-Butanol, 3-methyl-, propanoate | 105-68-0 | Ester | |
| 30 | 12.00 | 1097 | 909 | 917 | 1098 | Heptanoic acid, ethyl ester | 106-30-9 | Ester | [48] |
| 39 | 14.12 | 1178 | 763 | 807 | 1181 | Butanedioic acid, diethyl ester | 123-25-1 | Ester | [21,55] |
| 40 | 14.63 | 1196 | 936 | 954 | 1196 | Octanoic acid, ethyl ester | 106-32-1 | Ester | [26,27,28,30,31,32,34,36,39,40,43,44,45,47,48,55] |
| 44 | 15.84 | 1246 | 880 | 918 | 1247 | Benzeneacetic acid, ethyl ester | 101-97-3 | Ester | [1,21,26,28,38,44,45,46,50,53,54,55] |
| 45 | 16.14 | 1257 | 925 | 952 | 1258 | Acetic acid, 2-phenylethyl ester | 103-45-7 | Ester | [1,18,21,26,29,30,31,33,34,37,38,39,43,46,47,50,53,54] |
| 48 | 16.97 | 1289 | 883 | 915 | 1283 | 3-Nonenoic acid, ethyl ester | 91213-30-8 | Ester | |
| 49 | 17.11 | 1294 | 943 | 945 | 1295 | Nonanoic acid, ethyl ester | 123-29-5 | Ester | [31,32,36,48] |
| 52 | 19.17 | 1380 | 798 | 845 | 1375 | 4-Decenoic acid, ethyl ester | 76649-16-6 | Ester | [21] |
| 54 | 19.30 | 1385 | 824 | 843 | 1388 | 9-Decenoic acid, ethyl ester | 67233-91-4 | Ester | [31,34,55] |
| 55 | 19.50 | 1393 | 946 | 951 | 1396 | Decanoic acid, ethyl ester | 110-38-3 | Ester | [1,26,28,31,32,33,34,36,40,43,44,45,47,48,49,50,55] |
| 58 | 20.69 | 1447 | 884 | 943 | 1446 | Octanoic acid, 3-methylbutyl ester | 2035-99-6 | Ester | [31] |
| 59 | 20.75 | 1449 | 763 | 785 | 1453 | Octanoic acid, 2-methylbutyl ester | 67121-39-5 | Ester | |
| 65 | 22.88 | 1546 | 671 | 753 | 1559 | 3-Methylbutyl nonanoate | 7779-70-6 | Ester | |
| 67 | 23.89 | 1592 | 922 | 935 | 1594 | Dodecanoic acid, ethyl ester | 106-33-2 | Ester | [1,21,28,30,31,32,33,38,40,44,45,48,55] |
| 68 | 24.96 | 1645 | 852 | 870 | 1645 | Decanoic acid, 3-methylbutyl ester | 2306-91-4 | Ester | [21,27,31,34,37,49] |
| 17 | 9.01 | 986 | 886 | 900 | 986 | 5-Hepten-2-one, 6-methyl- | 110-93-0 | Ketone | |
| 26 | 10.42 | 1041 | 765 | 837 | 1036 | 2,2,6-Trimethylcyclohexanone | 2408-37-9 | Ketone | [26] |
| 28 | 11.29 | 1073 | 717 | 901 | 1072 | (E,E)-3,5-Octadien-2-one | 38284-27-4 | Ketone | [21,43,46,50] |
| 53 | 19.24 | 1383 | 879 | 917 | 1386 | Damascenone | 23726-93-4 | Ketone | [21,26,27,28,39,44,45,47,51,53,54] |
| 56 | 20.24 | 1426 | 831 | 837 | 1426 | α-Ionone | 127-41-3 | Ketone | [31,34,54] |
| 57 | 20.37 | 1432 | 763 | 784 | 1485 | Dehydro-β-ionone | 1203-08-3 | Ketone | [27,29,46] |
| 61 | 21.41 | 1478 | 809 | 836 | 1480 | α-Isomethylionone | 127-51-5 | Ketone | |
| 62 | 21.53 | 1483 | 911 | 917 | 1486 | β-Ionone | 79-77-6 | Ketone | [21,26,28,29,31,34,36,46,50] |
| 18 | 9.16 | 991 | 871 | 924 | 991 | β-Myrcene | 123-35-3 | Monoterpene (hydrocarbon) | [29,31,34,35,36,40,47,51,55] |
| 22 | 10.14 | 1030 | 938 | 953 | 1022 | o-Cymene | 527-84-4 | Monoterpene (hydrocarbon) | [29,35] |
| 24 | 10.27 | 1035 | 923 | 925 | 1031 | D-Limonene | 5989-27-5 | Monoterpene (hydrocarbon) | [18,26,29,30,34,35,36,40,46,47,51,53,54,55] |
| 27 | 11.05 | 1064 | 790 | 870 | 1060 | γ-Terpinene | 99-85-4 | Monoterpene (hydrocarbon) | [26,28,29,31,36,46,47] |
| 43 | 15.60 | 1236 | 840 | 903 | 908 | Bornylene | 464-17-5 | Monoterpene (hydrocarbon) | [26,49,53,54] |
| 25 | 10.37 | 1039 | 808 | 827 | 1032 | Eucalyptol | 470-82-6 | Monoterpene (oxygenated) | [18,21,54] |
| 29 | 11.37 | 1075 | 806 | 811 | 1074 | Linalool oxide, (Z)- | 5989-33-3 | Monoterpene (oxygenated) | [28,46,47] |
| 31 | 12.10 | 1100 | 948 | 949 | 1099 | Linalool | 78-70-6 | Monoterpene (oxygenated) | [18,26,27,28,29,30,31,32,33,34,36,40,46,47,49,50,51,52,53,54] |
| 35 | 13.49 | 1155 | 794 | 889 | 1144 | D-Camphor | 464-49-3 | Monoterpene (oxygenated) | |
| 36 | 13.68 | 1162 | 792 | 907 | 1157 | Menthone | 14073-97-3 | Monoterpene (oxygenated) | |
| 41 | 14.71 | 1199 | 923 | 935 | 1198 | α-Terpineol | 98-55-5 | Monoterpene (oxygenated) | [27,29,31,32,33,35,37,38,39,40,42,43,45,46,47,51,52,54] |
| 42 | 15.33 | 1225 | 891 | 916 | 1220 | β-Cyclocitral | 432-25-7 | Monoterpene (oxygenated) | [21,46,51] |
| 37 | 13.89 | 1170 | 712 | 813 | 1169 | 4-Ethylphenol | 123-07-9 | Phenol | [1,28,33,34,38,40,43,46,47,50,51,55] |
| 47 | 16.65 | 1277 | 910 | 926 | 1282 | 4-Ethyl-2-methoxy-phenol | 2785-89-9 | Phenol | [1,27,28,31,33,34,36,38,39,40,43,46,50,51,54,55] |
| 63 | 22.06 | 1507 | 949 | 951 | 1514 | 2,4-Di-tert-butylphenol | 96-76-4 | Phenol | [21,26,29,30,31,34,36,39,43,50,51,53] |
| 60 | 20.87 | 1455 | 875 | 899 | 1457 | cis-β-Farnesene | 28973-97-9 | Sesquiterpene (hydrocarbon) | [31,53] |
| 66 | 23.28 | 1564 | 951 | 957 | 1564 | trans-Nerolidol | 40716-66-3 | Sesquiterpene (oxygenated) | [21,55] |
| Analyte | MT | ST | MT × ST |
|---|---|---|---|
| Octanal | *** | *** | *** |
| Nonanal | *** | *** | ** |
| Ethanol | *** | *** | *** |
| 3-Methyl-1-butanol | *** | *** | ** |
| 1-Hexanol | *** | *** | *** |
| 2-Ethyl-1-hexanol | *** | *** | *** |
| 2-Phenylethyl alcohol | *** | *** | *** |
| Styrene | *** | ||
| Dihydroactinidiolide | *** | *** | *** |
| Edulan 1 | *** | ||
| Acetic acid | *** | * | |
| Propanoic acid, 2 methyl- | *** | *** | *** |
| Butanoic acid, 3-methyl- | *** | ** | ** |
| Butanoic acid, 2-methyl- | *** | *** | |
| n-Hexanoic acid | *** | ||
| Hexanoic acid, 2-ethyl- | *** | *** | ** |
| n-Octanoic acid | ** | *** | * |
| n-Nonanoic acid | ** | ||
| n-Decanoic acid | *** | ||
| Ethyl acetate | *** | *** | * |
| Isobutyl acetate | *** | * | ** |
| Butanoic acid, ethyl ester | *** | *** | ** |
| Butanoic acid, 2-methyl-, ethyl ester | *** | ** | * |
| Butanoic acid, 3-methyl-, ethyl ester | *** | *** | |
| 1-Butanol, 3-methyl-, acetate | * | ||
| Hexanoic acid, methyl ester | |||
| Hexanoic acid, ethyl ester | ** | ||
| 1-Butanol, 3-methyl-, propanoate | *** | ||
| Heptanoic acid, ethyl ester | ** | *** | |
| Butanedioic acid, diethyl ester | ** | ||
| Octanoic acid, ethyl ester | *** | ||
| Benzeneacetic acid, ethyl ester | *** | *** | *** |
| Acetic acid, 2-phenylethyl ester | *** | *** | |
| 3-Nonenoic acid, ethyl ester | *** | * | * |
| Nonanoic acid, ethyl ester | * | ** | |
| 4-Decenoic acid, ethyl ester | ** | *** | |
| 9-Decenoic acid, ethyl ester | * | ||
| Decanoic acid, ethyl ester | * | ** | |
| Octanoic acid, 3-methylbutyl ester | * | * | |
| Octanoic acid, 2-methylbutyl ester | *** | * | |
| Nonanoic acid, 3-methylbutyl ester | * | ** | |
| Dodecanoic acid, ethyl ester | * | * | |
| Decanoic acid, 3-methylbutyl ester | * | ||
| 5-Hepten-2-one, 6-methyl- | *** | *** | *** |
| 2,2,6-Trimethylcyclohexanone | *** | ** | ** |
| (E,E)-3,5-Octadien-2-one | *** | *** | *** |
| Damascenone | *** | * | |
| α-Ionone | |||
| Dehydro-β-ionone | *** | *** | *** |
| α-Isomethylionone | *** | *** | *** |
| β-Ionone | *** | *** | * |
| β-Myrcene | * | ||
| o-Cymene | *** | * | |
| D-Limonene | *** | * | |
| γ-Terpinene | *** | ||
| Bornylene | *** | ||
| Eucalyptol | *** | ** | |
| Linalool oxide, (Z)- | *** | *** | * |
| Linalool | *** | *** | |
| D-Camphor | *** | * | * |
| Menthone | *** | * | * |
| α-Terpineol | *** | *** | *** |
| β-Cyclocitral | *** | ||
| 3-Ethylphenol | ** | *** | |
| 4-Ethyl-2-methoxy-phenol | *** | *** | |
| 2,4-Di-tert-butylphenol | * | *** | ** |
| cis-β-Farnesene | ** | *** | |
| trans-Nerolidol | *** | *** | * |
References
- Phung, L.T.; Kitwetcharoen, H.; Chamnipa, N.; Boonchot, N.; Thanonkeo, S.; Tippayawat, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Changes in the chemical compositions and biological properties of kombucha beverages made from black teas and pineapple peels and cores. Sci. Rep. 2023, 13, 7859. [Google Scholar] [CrossRef]
- Bortolomedi, B.M.; Paglarini, C.S.; Brod, F.C.A. Bioactive compounds in kombucha: A review of substrate effect and fermentation conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef] [PubMed]
- Barros, V.C.; Botelho, V.A.; Chisté, R.C. Alternative Substrates for the Development of Fermented Beverages Analogous to Kombucha: An Integrative Review. Foods 2024, 13, 1768. [Google Scholar] [CrossRef]
- Su, J.; Tan, Q.; Tang, Q.; Tong, Z.; Yang, M. Research progress on alternative kombucha substrate transformation and the resulting active components. Front. Microbiol. 2023, 14, 1254014. [Google Scholar] [CrossRef]
- Barakat, N.; Beaufort, S.; Rizk, Z.; Bouajila, J.; Taillandier, P.; El Rayess, Y. Kombucha analogues around the world: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10105–10129. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Yang, T.; Mac Regenstein, J.; Zhou, P. Functional properties and sensory characteristics of kombucha analogs prepared with alternative materials. Trends Food Sci. Technol. 2022, 129, 608–616. [Google Scholar] [CrossRef]
- Dutta, H.; Paul, S.K. 8-Kombucha Drink: Production, Quality, and Safety Aspects. In Production and Management of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 259–288. [Google Scholar] [CrossRef]
- Kim, J.; Adhikari, K. Current trends in kombucha: Marketing perspectives and the need for improved sensory research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Sarikaya Aydin, S.; Gültekin Subasi, B.; Erskine, E.; Gök, R.; Ibrahim, S.A.; Yilmaz, B.; Özogul, F.; Capanoglu, E. Additional advances related to the health benefits associated with kombucha consumption. Crit. Rev. Food Sci. Nutr. 2024, 64, 6102–6119. [Google Scholar] [CrossRef]
- Vargas, B.K.; Fabricio, M.F.; Záchia Ayub, M.A. Health effects and probiotic and prebiotic potential of Kombucha: A bibliometric and systematic review. Food Biosci. 2021, 44, 101332. [Google Scholar] [CrossRef]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and technological parameters impacting the chemical composition and sensory quality of kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in Kombucha Tea Fermentation: A Review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Nummer, B.A. Kombucha brewing under the food and drug administration model Food Code: Risk analysis and processing guidance. J. Environ. Health 2013, 76, 8–11. [Google Scholar] [PubMed]
- Jayabalan, R.; Marimuthu, S.; Thangaraj, P.; Sathishkumar, M.; Binupriya, A.R.; Swaminathan, K.; Sei, E.Y. Preservation of kombucha tea—Effect of temperature on tea components and free radical scavenging properties. J. Agric. Food Chem. 2008, 56, 9064–9071. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Steyer, D.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Field Investigation of Flavored Kombucha’s Shelf Life Unveils High Sensitivity of Microbial Dynamics Towards Assimilable Nitrogen. Food Bioprocess Technol. 2025, 18, 370–391. [Google Scholar] [CrossRef]
- Daneluz, J.; da Silva, G.F.; Duarte, J.; Turossi, T.C.; Santos, V.d.; Baldasso, C.; Daneluz, A.C. Membrane separation process of microfiltration applied to the filtration of kombuchas. Food Chem. Adv. 2023, 3, 100451. [Google Scholar] [CrossRef]
- Grassi, A.; Cristani, C.; Palla, M.; Di Giorgi, R.; Giovannetti, M.; Agnolucci, M. Storage time and temperature affect microbial dynamics of yeasts and acetic acid bacteria in a kombucha beverage. Int. J. Food Microbiol. 2022, 382, 109934. [Google Scholar] [CrossRef]
- Fabricio, M.F.; Vargas, B.K.; Tischer, B.; Wagner, R.; Ribeiro, S.R.; Cordeiro, N.; Flôres, S.H.; Záchia Ayub, M.A. Revamping kombucha production: Achieving consistency and probiotic potential through a tailor-made microbial consortium. Int. J. Gastron. Food Sci. 2023, 34, 100844. [Google Scholar] [CrossRef]
- Fu, C.; Yan, F.; Cao, Z.; Xie, F.; Lin, J. Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef]
- La Torre, C.; Fazio, A.; Caputo, P.; Plastina, P.; Caroleo, M.C.; Cannataro, R.; Cione, E. Effects of long-term storage on radical scavenging properties and phenolic content of kombucha from black tea. Molecules 2021, 26, 5474. [Google Scholar] [CrossRef]
- Mozzon, M.; Foligni, R.; Mannozzi, C. Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content. Foods 2020, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Foligni, R.; Mannozzi, C.; Ismaiel, L.; Capelli, F.; Laurita, R.; Tappi, S.; Dalla Rosa, M.; Mozzon, M. Impact of Cold Atmospheric Plasma (CAP) Treatments on the Oxidation of Pistachio Kernel Lipids. Foods 2022, 11, 419. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Liu, R.; Wang, L.; Wang, X.; Wei, J.; Yuan, Y.; Yue, T.; Cai, R.; Wang, Z. Exploring the dynamic characteristic of typical kombucha induced by symbiotic microbiota succession from four Chinese regions: A comprehensive analytical framework. Food Res. Int. 2024, 198, 115335. [Google Scholar] [CrossRef] [PubMed]
- Dartora, B.; Crepalde, L.T.; Hickert, L.R.; Fabricio, M.F.; Ayub, M.A.Z.; Veras, F.F.; Brandelli, A.; Perez, K.J.; Sant’Anna, V. Kombuchas from black tea, green tea, and yerba-mate decocts: Perceived sensory map, emotions, and physicochemical parameters. Int. J. Gastron. Food Sci. 2023, 33, 100789. [Google Scholar] [CrossRef]
- Sales, A.L.; Cunha, S.C.; Morgado, J.; Cruz, A.; Santos, T.F.; Ferreira, I.M.P.L.V.O.; Fernandes, J.O.; Miguel, M.A.L.; Farah, A. Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas. Foods 2023, 12, 2710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ahmad, W.; Zhu, A.; Geng, W.; Kang, W.; Ouyang, Q.; Chen, Q. Identification of volatile compounds and metabolic pathway during ultrasound-assisted kombucha fermentation by HS-SPME-GC/MS combined with metabolomic analysis. Ultrason. Sonochem. 2023, 94, 106339. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.-Y.; Chen, J.-X.; Wang, F.; Gao, Y.; Fu, Y.-Q.; Xu, Y.-Q.; Yin, J.-F. Zijuan tea-based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, J.; Lu, J.; Chen, D.; Song, S.; Wang, H.; Sun, M.; Feng, T. Revealing the influence of microbiota on the flavor of kombucha during natural fermentation process by metagenomic and GC-MS analysis. Food Res. Int. 2023, 169, 112909. [Google Scholar] [CrossRef]
- Zhang, J.; Van Mullem, J.; Dias, D.R.; Schwan, R.F. The chemistry and sensory characteristics of new herbal tea-based kombuchas. J. Food Sci. 2021, 86, 740–748. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Phannarangsee, Y.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Klanrit, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Functional kombucha production from fusions of black tea and Indian gooseberry (Phyllanthus emblica L.). Heliyon 2024, 10, e40939. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, X.; Li, Y.; Chen, J.; Chen, X. Microbial interactions and dynamic changes of volatile flavor compounds during the fermentation of traditional kombucha. Food Chem. 2024, 430, 137060. [Google Scholar] [CrossRef]
- Silva, T.O.; Costa, G.N.; dos Santos Lima, M.; Feihrmann, A.C.; Barão, C.E.; Magnani, M.; Pimentel, T.C. Chemical, microbial, and functional characterization of a new fruity probiotic kombucha. Food Res. Int. 2024, 198, 115398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, H.; Wang, H.; Sun, M.; Yu, C.; Liu, Q.; He, Z.; Song, S.; Feng, T.; Yao, L. Flavor and sensory profile of kombucha fermented with raw Pu-erh tea and evaluation of the antioxidant properties. LWT 2024, 200, 116220. [Google Scholar] [CrossRef]
- Tran, T.; Billet, K.; Torres-Cobos, B.; Vichi, S.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Use of a Minimal Microbial Consortium to Determine the Origin of Kombucha Flavor. Front. Microbiol. 2022, 13, 836617. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Tippayawat, P.; Klanrit, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Enhancing kombucha functionality: Utilizing dried pineapple peels and cores as an alternative ingredient for improved antioxidant and antimicrobial properties. LWT 2025, 216, 117358. [Google Scholar] [CrossRef]
- Venegas, C.A.; Saona, L.A.; Urbina, K.; Quintrel, P.; Peña, T.A.; Mardones, W.; Cubillos, F.A. Addition of Saccharomyces eubayanus to SCOBY fermentations modulates the chemical and volatile compound profiles in kombucha. Food Microbiol. 2023, 116, 104357. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, J.C.d.; Magnani, M.; Almeida da Costa, W.K.; Madruga, M.S.; Souza Olegário, L.; da Silva Campelo Borges, G.; Macedo Dantas, A.; Lima, M.d.S.; de Lima, L.C.; de Lima Brito, I.; et al. Traditional and flavored kombuchas with pitanga and umbu-cajá pulps: Chemical properties, antioxidants, and bioactive compounds. Food Biosci. 2021, 44, 101380. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Casimiro, L.K.S.; Martinez, S.J.; Dias, D.R.; Schwan, R.F. Kombucha with yam: Comprehensive biochemical, microbiological, and sensory characteristics. Food Res. Int. 2024, 192, 114762. [Google Scholar] [CrossRef]
- Kilmanoglu, H.; Yigit Cinar, A.; Durak, M.Z. Evaluation of microbiota-induced changes in biochemical, sensory properties and volatile profile of kombucha produced by reformed microbial community. Food Chem. X 2024, 22, 101469. [Google Scholar] [CrossRef] [PubMed]
- Njieukam, J.A.; Ciccone, M.; Gottardi, D.; Ricci, A.; Parpinello, G.P.; Siroli, L.; Lanciotti, R.; Patrignani, F. Microbiological, Functional, and Chemico-Physical Characterization of Artisanal Kombucha: An Interesting Reservoir of Microbial Diversity. Foods 2024, 13, 1947. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Xu, Q.; Wang, Y.; Wang, S.; Zou, Y.; Yang, Z.; Yuan, L. Addition of lactic acid bacteria modulates microbial community and promotes the flavor profiles of Kombucha. Food Biosci. 2024, 60, 104340. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Wang, Y.; Wang, S.; Zou, Y.; Sun, Z.; Yuan, L. Changes on physiochemical properties and volatile compounds of Chinese kombucha during fermentation. Food Biosci. 2023, 55, 103029. [Google Scholar] [CrossRef]
- Suffys, S.; Richard, G.; Burgeon, C.; Werrie, P.-Y.; Haubruge, E.; Fauconnier, M.-L.; Goffin, D. Characterization of Aroma Active Compound Production during Kombucha Fermentation: Towards the Control of Sensory Profiles. Foods 2023, 12, 1657. [Google Scholar] [CrossRef]
- Ferremi Leali, N.; Binati, R.L.; Martelli, F.; Gatto, V.; Luzzini, G.; Salini, A.; Slaghenaufi, D.; Fusco, S.; Ugliano, M.; Torriani, S.; et al. Reconstruction of Simplified Microbial Consortia to Modulate Sensory Quality of Kombucha Tea. Foods 2022, 11, 3045. [Google Scholar] [CrossRef]
- Savary, O.; Mounier, J.; Thierry, A.; Poirier, E.; Jourdren, J.; Maillard, M.-B.; Penland, M.; Decamps, C.; Coton, E.; Coton, M. Tailor-made microbial consortium for Kombucha fermentation: Microbiota-induced biochemical changes and biofilm formation. Food Res. Int. 2021, 147, 110549. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, S.; Zhang, J.; Bu, D.; Zang, C.; Fan, R.; Wang, J.; Guo, T.; Han, R.; Yang, Y. Dynamic changes in microbial communities and volatile compounds in kombucha fermentation using Flos sophorae and Elm fruits, compared to black and green tea. Food Res. Int. 2024, 197, 115233. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Zhang, B.; Tian, H.; Liang, Y.; Dang, H.; Zhao, Y. Dynamic Changes in Microbial Communities, Physicochemical Properties, and Flavor of Kombucha Made from Fu-Brick Tea. Foods 2023, 12, 4242. [Google Scholar] [CrossRef]
- Kang, W.; Lin, H.; Ahmad, W.; Li, H.; Chen, Q. Determination of active constituents in kombucha fermentation broth using nano-composite colorimetric sensor based on selected volatile markers determined by GC–MS. Microchem. J. 2023, 195, 109493. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Sui, Y.-C.; Wu, H.-W.; Zhou, C.-B.; Hu, X.-C.; Zhang, J. Flavour chemical dynamics during fermentation of kombucha tea. EJFA 2018, 30, 732–741. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Zou, C.; Gao, Y.; Yin, J.-F.; Contursi, P.; Zhang, S.; Gong, Y.-S.; Liu, J.-J.; Xu, Y.-Q. Kombucha beverages made from Camellia nitidissima Chi and Camellia sinensis flowers—Physicochemical properties, sensory properties and bioactivity. Int. J. Gastron. Food Sci. 2024, 37, 100964. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Wang, L.; Liu, X.; Wang, X.; Cai, R.; Yuan, Y.; Yue, T.; Wang, Z. Unraveling symbiotic microbial communities, metabolomics and volatilomics profiles of kombucha from diverse regions in China. Food Res. Int. 2023, 174, 113652. [Google Scholar] [CrossRef]
- Xu, W.; Tong, Y.; Tong, Q.; Liu, Y.; Wang, Z. Effects of different re-fermentation methods on the quality characteristics of kombucha beverages. J. Food Sci. Technol. 2023, 60, 1414–1424. [Google Scholar] [CrossRef]
- Talebi, M.; Frink, L.A.; Patil, R.A.; Armstrong, D.W. Examination of the Varied and Changing Ethanol Content of Commercial Kombucha Products. Food Anal. Methods 2017, 10, 4062–4067. [Google Scholar] [CrossRef]
- Vitas, J.; Malbaša, R.; Vukmanović, S. Volatile Compounds Formation in Kombucha. In Volatile Compounds Formation in Specialty Beverages, 1st ed.; Richter Reis, F., Eleutério dos Santos, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 185–208. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Garces Daza, F.; Haitz, F.; Born, A.; Boles, E. An optimized reverse β-oxidation pathway to produce selected medium-chain fatty acids in Saccharomyces cerevisiae. Biotechnol. Biofuels 2023, 16, 71. [Google Scholar] [CrossRef]
- Yuan, S.; Jin, Z.; Ali, A.; Wang, C.; Liu, J. Caproic Acid-Producing Bacteria in Chinese Baijiu Brewing. Front. Microbiol. 2022, 13, 883142. [Google Scholar] [CrossRef]
- Baumann, L.; Doughty, T.; Siewers, V.; Nielsen, J.; Boles, E.; Oreb, M. Transcriptomic Response of Saccharomyces Cerevisiae to Octanoic Acid Production. FEMS Yeast Res. 2021, 21, foab011. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Wang, H.; Jiao, S.; Wu, J.; Hou, Y.; Sun, J.; Yuan, J. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. LWT 2022, 168, 113931. [Google Scholar] [CrossRef]
- van Wyk, N.; Binder, J.; Ludszuweit, M.; Köhler, S.; Brezina, S.; Semmler, H.; Pretorius, I.S.; Rauhut, D.; Senz, M.; von Wallbrunn, C. The Influence of Pichia kluyveri Addition on the Aroma Profile of a Kombucha Tea Fermentation. Foods 2023, 12, 1938. [Google Scholar] [CrossRef] [PubMed]
- Assad, M.; Ashaolu, T.J.; Khalifa, I.; Baky, M.H.; Farag, M.A. Dissecting the role of microorganisms in tea production of different fermentation levels: A multifaceted review of their action mechanisms, quality attributes and future perspectives. World J. Microbiol. Biotechnol. 2023, 39, 265. [Google Scholar] [CrossRef] [PubMed]


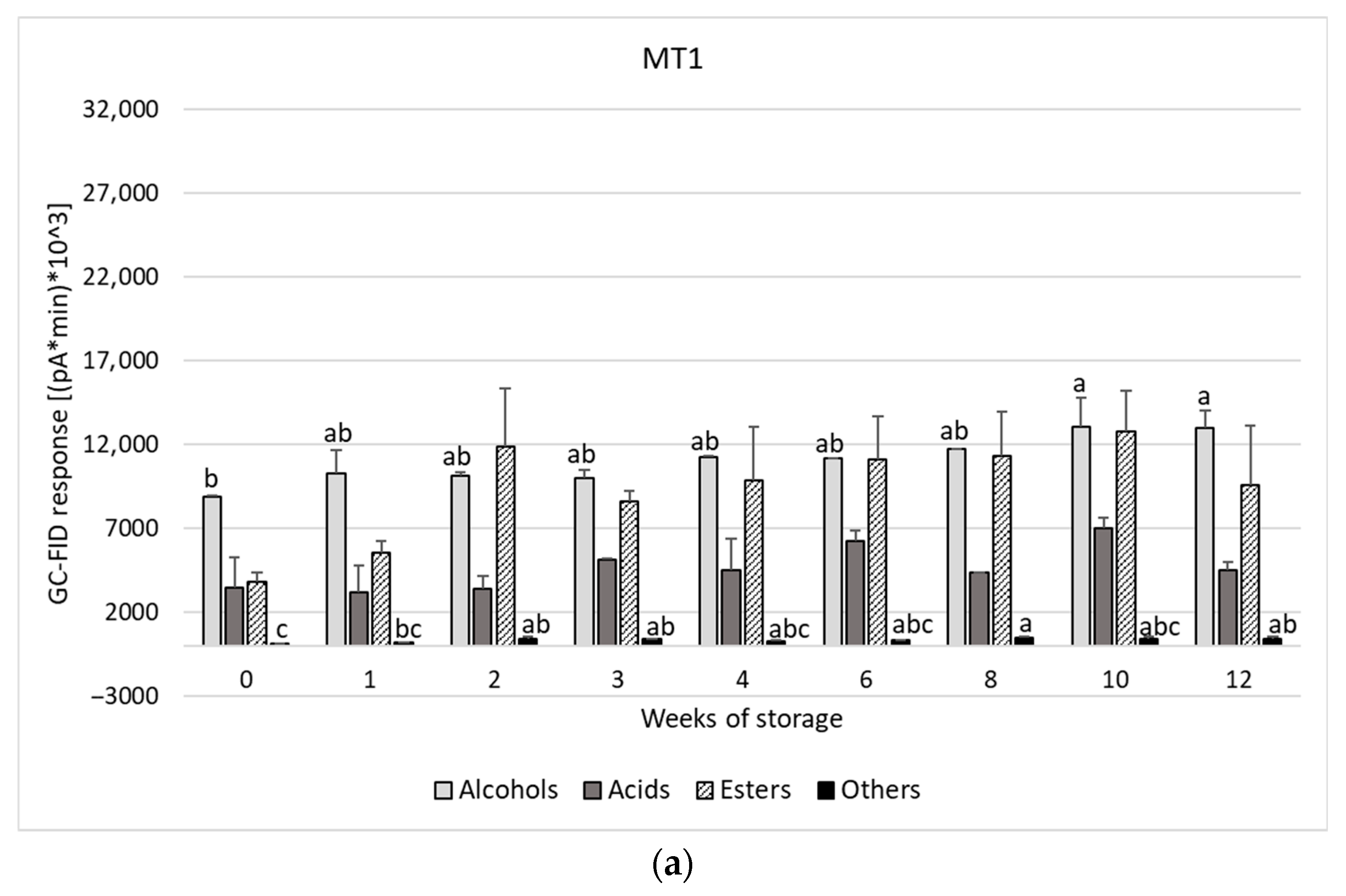

| Weeks of Storage at 4 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 6 | 8 | 10 | 12 | |
| Aldehydes | |||||||||
| Nonanal | 0 ± 0 b | 0 ± 0 b | 3 ± 1 ab | 3 ± 2 ab | 4 ± 1 ab | 6 ± 1 ab | 18 ± 5 a | 17 ± 9 a | 8 ± 7 ab |
| Alkanols | |||||||||
| Ethanol | 11,936 ± 476 c | 14,014 ± 257 bc | 14,270 ± 1524 bc | 17,256 ± 2195 abc | 20,545 ± 1523 ab | 21,327 ± 1948 a | 22,174 ± 759 a | 23,106 ± 1291 a | 23,300 ± 3094 a |
| 3-Methyl-1-butanol | 3576 ± 214 b | 3612 ± 226 b | 3984 ± 384 b | 4303 ± 153 b | 4591 ± 527 ab | 4856 ± 720 ab | 4656 ± 141 ab | 5808 ± 250 a | 4660 ± 149 ab |
| 2-Ethyl-1-hexanol | 30 ± 2 abc | 0 ± 0 c | 8 ± 2 c | 25 ± 0 bc | 10 ± 0 c | 45 ± 2 abc | 64 ± 6 abc | 109 ± 4 ab | 116 ± 68 a |
| 2-Phenylethyl alcohol | 39 ± 11 bc | 0 ± 0 c | 34 ± 14 c | 297 ± 117 abc | 350 ± 25 ab | 424 ± 117 a | 364 ± 11 a | 413 ± 10 a | 526 ± 199 a |
| Carboxylic acids | |||||||||
| Acetic acid | 1738 ± 11 | 1513 ± 79 | 1155 ± 214 | 1283 ± 44 | 2097 ± 1004 | 3517 ± 2281 | 2066 ± 434 | 1635 ± 49 | 3080 ± 258 |
| Propanoic acid, 2 methyl- | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c | 30 ± 10 a | 15 ± 3 abc | 19 ± 1 ab | 11 ± 1 abc | 22 ± 11 ab | 9 ± 3 bc |
| Butanoic acid, 3-methyl- | 209 ± 51 | 301 ± 24 | 404 ± 76 | 354 ± 209 | 222 ± 24 | 231 ± 66 | 222 ± 14 | 272 ± 31 | 218 ± 28 |
| Butanoic acid, 2-methyl- | 48 ± 7 | 61 ± 4 | 87 ± 19 | 90 ± 34 | 76 ± 2 | 82 ± 11 | 78 ± 2 | 76 ± 5 | 83 ± 20 |
| n-Hexanoic acid | 13 ± 4 | 25 ± 2 | 27 ± 2 | 21 ± 5 | 21 ± 0 | 35 ± 29 | 26 ± 5 | 44 ± 7 | 33 ± 13 |
| Hexanoic acid, 2-ethyl- | 0 ± 0 | 0 ± 0 | 2 ± 0 | 2 ± 0 | 3 ± 2 | 4 ± 3 | 1 ± 0 | 1 ± 0 | 0 ± 1 |
| n-Octanoic acid | 34 ± 11 e | 63 ± 17 de | 274 ± 29 bc | 259 ± 111 bc | 213 ± 10 cde | 289 ± 51 bc | 239 ± 33 bcd | 489 ± 53 a | 410 ± 32 ab |
| n-Nonanoic acid | 0 ± 0 | 0 ± 0 | 124 ± 32 | 75 ± 76 | 37 ± 3 | 37 ± 24 | 25 ± 15 | 45 ± 15 | 71 ± 58 |
| n-Decanoic acid | 0 ± 0 | 0 ± 0 | 102 ± 39 | 175 ± 56 | 105 ± 0 | 188 ± 66 | 68 ± 1 | 48 ± 35 | 280 ± 212 |
| Esters | |||||||||
| Ethyl acetate | 2882 ± 557 d | 4571 ± 10 cd | 5329 ± 1115 bcd | 7518 ± 1168 abc | 7738 ± 1827 abc | 8059 ± 113 abc | 7980 ± 70 abc | 8732 ± 852 ab | 8969 ± 489 a |
| Isobutyl acetate | 6 ± 5 | 19 ± 1 | 7 ± 4 | 14 ± 7 | 16 ± 9 | 17 ± 8 | 15 ± 0 | 27 ± 6 | 25 ± 0 |
| Butanoic acid, ethyl ester | 0 ± 0 c | 0 ± 0 c | 2 ± 1 bc | 5 ± 3 abc | 9 ± 2 ab | 11 ± 1 a | 12 ± 2 a | 11 ± 2 a | 8 ± 2 ab |
| Butanoic acid, 2-methyl-, ethyl ester | 0 ± 0 | 2 ± 0 | 12 ± 9 | 70 ± 84 | 102 ± 9 | 109 ± 20 | 87 ± 3 | 79 ± 9 | 63 ± 17 |
| Butanoic acid, 3-methyl-, ethyl ester | 0 ± 0 | 0 ± 0 | 3 ± 0 | 6 ± 4 | 7 ± 0 | 7 ± 3 | 6 ± 1 | 6 ± 0 | 6 ± 3 |
| 1-Butanol, 3-methyl-, acetate | 316 ± 115 | 677 ± 45 | 559 ± 312 | 786 ± 299 | 669 ± 245 | 735 ± 190 | 599 ± 31 | 750 ± 79 | 520 ± 216 |
| Hexanoic acid, methyl ester | 0 ± 0 | 0 ± 0 | 4 ± 0 | 1 ± 1 | 9 ± 13 | 5 ± 7 | 10 ± 8 | 0 ± 0 | 3 ± 4 |
| Hexanoic acid, ethyl ester | 3 ± 1 | 11 ± 4 | 47 ± 30 | 168 ± 169 | 158 ± 42 | 189 ± 50 | 142 ± 4 | 146 ± 7 | 108 ± 67 |
| 1-Butanol, 3-methyl-, propanoate | 0 ± 0 | 0 ± 0 | 1 ± 1 | 4 ± 5 | 5 ± 1 | 4 ± 1 | 3 ± 1 | 3 ± 0 | 2 ± 2 |
| Heptanoic acid, ethyl ester | 0 ± 0 | 0 ± 0 | 12 ± 7 | 30 ± 25 | 26 ± 11 | 40 ± 18 | 36 ± 2 | 37 ± 4 | 24 ± 15 |
| Octanoic acid, ethyl ester | 18 ± 9 b | 33 ± 4 b | 1295 ± 778 ab | 2290 ± 2388 ab | 1451 ± 997 ab | 2254 ± 600 ab | 1816 ± 725 ab | 4265 ± 288 a | 1395 ± 334 ab |
| Acetic acid, 2-phenylethyl ester | 2 ± 1 b | 6 ± 2 ab | 23 ± 8 ab | 32 ± 9 a | 29 ± 6 ab | 30 ± 7 a | 22 ± 2 ab | 24 ± 0 ab | 24 ± 14 ab |
| 3-Nonenoic acid, ethyl ester | 0 ± 0 | 0 ± 0 | 1 ± 1 | 3 ± 3 | 4 ± 2 | 5 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 4 |
| Nonanoic acid, ethyl ester | 0 ± 0 b | 0 ± 0 b | 279 ± 162 ab | 237 ± 186 ab | 170 ± 132 ab | 222 ± 110 ab | 120 ± 9 ab | 939 ± 623 a | 130 ± 58 ab |
| 4-Decenoic acid, ethyl ester | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 2 ± 2 ab | 1 ± 0 ab | 2 ± 0 ab | 2 ± 0 ab | 4 ± 1 a | 1 ± 0 ab |
| 9-Decenoic acid, ethyl ester | 0 ± 0 | 0 ± 0 | 13 ± 7 | 53 ± 68 | 19 ± 10 | 41 ± 2 | 24 ± 10 | 67 ± 21 | 12 ± 3 |
| Decanoic acid, ethyl ester | 14 ± 7 | 23 ± 3 | 312 ± 97 | 873 ± 970 | 483 ± 228 | 714 ± 47 | 562 ± 89 | 1676 ± 880 | 376 ± 91 |
| Octanoic acid, 3-methylbutyl ester | 0 ± 0 | 0 ± 0 | 23 ± 4 | 41 ± 47 | 27 ± 21 | 31 ± 11 | 23 ± 4 | 70 ± 24 | 14 ± 1 |
| Octanoic acid, 2-methylbutyl ester | 0 ± 0 c | 0 ± 0 c | 3 ± 0 a | 1 ± 1 bc | 1 ± 0 bc | 1 ± 0 bc | 1 ± 0 bc | 2 ± 0 ab | 1 ± 1 bc |
| Nonanoic acid, 3-methylbutyl ester | 0 ± 0 b | 0 ± 0 b | 11 ± 2 ab | 9 ± 6 ab | 9 ± 5 ab | 7 ± 2 ab | 4 ± 1 ab | 20 ± 11 a | 4 ± 1 ab |
| Dodecanoid acid, ethyl ester | 2 ± 0 | 2 ± 0 | 19 ± 5 | 75 ± 82 | 51 ± 13 | 42 ± 6 | 26 ± 1 | 43 ± 12 | 33 ± 17 |
| Decanoic acid, 3-methylbutyl ester | 0 ± 0 | 0 ± 0 | 7 ± 0 | 27 ± 33 | 23 ± 9 | 20 ± 0 | 9 ± 1 | 22 ± 6 | 7 ± 1 |
| Ketones | |||||||||
| 5-Hepten-2-one, 6-methyl- | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 2 ± 1 | 0 ± 0 | 0 ± 0 |
| Damascenone | 0 ± 0 | 1 ± 0 | 2 ± 0 | 2 ± 2 | 1 ± 0 | 0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| α-Ionone | 0 ± 0 | 5 ± 5 | 2 ± 0 | 2 ± 0 | 1 ± 0 | 1 ± 0 | 2 ± 0 | 1 ± 0 | 1 ± 0 |
| α-Isomethylionone | 1 ± 1 ab | 3 ± 0 a | 3 ± 1 a | 2 ± 0 a | 2 ± 0 ab | 0 ± 0 b | 1 ± 0 ab | 2 ± 0 ab | 0 ± 0 b |
| β-Ionone | 0 ± 0 | 0 ± 0 | 4 ± 4 | 1 ± 0 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Phenols | |||||||||
| 3-Ethylphenol | 0 ± 0 | 0 ± 0 | 14 ± 6 | 21 ± 9 | 8 ± 6 | 12 ± 5 | 6 ± 2 | 17 ± 9 | 8 ± 11 |
| 4-Ethyl-2-methoxy-phenol | 0 ± 0 b | 0 ± 0 b | 5 ± 1 ab | 7 ± 3 ab | 3 ± 0 ab | 7 ± 2 ab | 7 ± 0 ab | 7 ± 1 ab | 10 ± 5 a |
| 2,4-Di-tert-butylphenol | 11 ± 3 c | 19 ± 1 bc | 27 ± 7 abc | 45 ± 3 ab | 48 ± 12 ab | 41 ± 8 ab | 30 ± 4 abc | 52 ± 12 a | 37 ± 10 abc |
| Terpenes | |||||||||
| β-Myrcene | 0 ± 0 | 0 ± 0 | 51 ± 11 | 11 ± 14 | 63 ± 89 | 119 ± 84 | 70 ± 58 | 1 ± 0 | 12 ± 17 |
| o-Cymene | 0 ± 0 | 0 ± 0 | 3 ± 0 | 2 ± 3 | 3 ± 4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| D-Limonene | 2 ± 2 b | 4 ± 1 b | 11 ± 2 b | 4 ± 2 b | 15 ± 11 b | 7 ± 7 b | 47 ± 11 a | 4 ± 1 b | 5 ± 5 b |
| Eucalyptol | 7 ± 1 | 13 ± 0 | 12 ± 7 | 15 ± 0 | 9 ± 2 | 12 ± 4 | 18 ± 2 | 17 ± 1 | 12 ± 4 |
| Linalool oxide, (Z)- | 5 ± 6 b | 17 ± 0 ab | 23 ± 12 ab | 32 ± 3 ab | 24 ± 9 ab | 27 ± 1 ab | 35 ± 1 a | 37 ± 1 a | 34 ± 14 a |
| Linalool | 23 ± 5 b | 39 ± 3 ab | 51 ± 21 ab | 67 ± 3 ab | 54 ± 14 ab | 63 ± 10 ab | 82 ± 6 a | 76 ± 4 a | 64 ± 22 ab |
| D-Camphor | 1 ± 1 b | 3 ± 1 ab | 4 ± 1 ab | 5 ± 1 a | 4 ± 2 ab | 5 ± 1 ab | 4 ± 0 ab | 5 ± 0 a | 4 ± 0 ab |
| Menthone | 0 ± 1 | 2 ± 0 | 2 ± 1 | 3 ± 0 | 2 ± 1 | 2 ± 0 | 2 ± 0 | 1 ± 1 | 0 ± 1 |
| α-Terpineol | 0 ± 0 | 2 ± 0 | 2 ± 0 | 3 ± 3 | 2 ± 1 | 2 ± 0 | 3 ± 1 | 1 ± 0 | 2 ± 2 |
| cis-β-Farnesene | 0 ± 0 | 0 ± 0 | 2 ± 2 | 2 ± 1 | 2 ± 0 | 3 ± 0 | 2 ± 0 | 4 ± 1 | 5 ± 3 |
| Others | |||||||||
| trans-Nerolidol | 1 ± 0 b | 3 ± 0 ab | 9 ± 3 ab | 12 ± 1 a | 10 ± 2 ab | 10 ± 1 ab | 9 ± 0 ab | 11 ± 3 ab | 12 ± 6 a |
| Styrene | 2 ± 2 | 6 ± 0 | 15 ± 9 | 15 ± 3 | 10 ± 7 | 9 ± 2 | 14 ± 5 | 20 ± 9 | 29 ± 25 |
| Edulan 1 | 0 ± 0 b | 2 ± 0 b | 2 ± 1 ab | 2 ± 0 ab | 1 ± 1 ab | 1 ± 0 ab | 2 ± 0 a | 2 ± 0 a | 2 ± 1 |
| Weeks of Storage at 4 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 6 | 8 | 10 | 12 | |
| Aldehydes | |||||||||
| Octanal | 0 ± 0 b | 0 ± 0 b | 2 ± 2 b | 2 ± 0 b | 1 ± 1 b | 1 ± 1 b | 15 ± 5 ab | 3 ± 2 b | 7 ± 3 ab |
| Nonanal | 7 ± 4 b | 3 ± 1 b | 10 ± 3 b | 15 ± 4 b | 3 ± 1 b | 4 ± 2 b | 51 ± 5 a | 12 ± 7 b | 30 ± 20 ab |
| Alkanols | |||||||||
| Ethanol | 7668 ± 7 b | 9005 ± 1384 ab | 8758 ± 130 ab | 8594 ± 382 ab | 9818 ± 175 ab | 9869 ± 69 ab | 10,284 ± 57 ab | 11,263 ± 1502 a | 11,142 ± 931 a |
| 3-Methyl-1-butanol | 1157 ± 141 b | 1275 ± 22 ab | 1373 ± 84 ab | 1392 ± 139 ab | 1397 ± 121 ab | 1245 ± 45 ab | 1360 ± 77 ab | 1695 ± 232 a | 1708 ± 112 a |
| 1-Hexanol | 3 ± 0 bc | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c | 1 ± 2 c | 6 ± 0 ab | 7 ± 1 a | 8 ± 1 a |
| 2-Ethyl-1-hexanol | 13 ± 5 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 5 ± 4 ab | 10 ± 0 ab | 10 ± 7 ab |
| 2-Phenylethyl alcohol | 1 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 22 ± 10 cd | 37 ± 11 bc | 56 ± 3 ab | 75 ± 16 a | 85 ± 11 a |
| Carboxylic acids | |||||||||
| Acetic acid | 3086 ± 1736 | 2667 ± 1503 | 2124 ± 1030 | 3716 ± 31 | 3247 ± 2050 | 5058 ± 517 | 3201 ± 65 | 5690 ± 661 | 2923 ± 339 |
| Propanoic acid, 2 methyl- | 31 ± 10 abc | 35 ± 10 abc | 82 ± 13 abc | 92 ± 5 a | 45 ± 7 abc | 43 ± 3 abc | 13 ± 1 c | 30 ± 42 bc | 0 ± 0 c |
| Butanoic acid, 3-methyl- | 249 ± 31 c | 348 ± 15 bc | 580 ± 100 ab | 608 ± 4 ab | 718 ± 190 a | 554 ± 52 abc | 589 ± 2 ab | 650 ± 76 ab | 719 ± 72 a |
| Butanoic acid, 2-methyl- | 56 ± 5 c | 75 ± 10 bc | 126 ± 30 ab | 129 ± 3 ab | 145 ± 33 a | 110 ± 9 abc | 122 ± 3 ab | 133 ± 9 ab | 145 ± 14 a |
| n-Hexanoic acid | 7 ± 2 c | 15 ± 4 bc | 16 ± 0 bc | 24 ± 2 bc | 41 ± 19 ab | 30 ± 2 bc | 42 ± 2 ab | 43 ± 11 ab | 61 ± 6 a |
| Hexanoic acid, 2-ethyl- | 2 ± 0 c | 6 ± 1 bc | 11 ± 3 a | 13 ± 1 a | 8 ± 1 ab | 10 ± 1 ab | 9 ± 0 ab | 6 ± 1 bc | 9 ± 1 ab |
| n-Octanoic acid | 34 ± 8 c | 53 ± 4 c | 249 ± 23 b | 261 ± 22 b | 144 ± 36 bc | 219 ± 44 b | 206 ± 60 b | 240 ± 3 b | 401 ± 56 a |
| n-Nonanoic acid | 0 ± 0 b | 0 ± 0 b | 62 ± 30 ab | 104 ± 38 a | 44 ± 18 ab | 45 ± 21 ab | 44 ± 7 ab | 75 ± 22 ab | 68 ± 45 ab |
| n-Decanoic acid | 0 ± 0 b | 0 ± 0 b | 143 ± 51 a | 203 ± 20 a | 102 ± 30 ab | 154 ± 10 a | 135 ± 18 a | 108 ± 31 ab | 158 ± 41 a |
| Esters | |||||||||
| Ethyl acetate | 3104 ± 141 b | 4326 ± 615 ab | 5678 ± 1553 ab | 5381 ± 135 ab | 5253 ± 812 ab | 5926 ± 137 ab | 5273 ± 795 ab | 6320 ± 1143 a | 4886 ± 412 ab |
| Isobutyl acetate | 42 ± 20 ab | 73 ± 24 a | 49 ± 8 ab | 40 ± 8 ab | 67 ± 2 ab | 26 ± 7 ab | 34 ± 13 ab | 27 ± 2 ab | 19 ± 6 b |
| Butanoic acid, ethyl ester | 0 ± 0 c | 0 ± 0 c | 19 ± 2 abc | 15 ± 6 abc | 7 ± 6 bc | 42 ± 6 ab | 51 ± 28 a | 9 ± 7 bc | 22 ± 1 abc |
| Butanoic acid, 2-methyl-, ethyl ester | 0 ± 0 | 2 ± 0 | 10 ± 3 | 8 ± 1 | 16 ± 7 | 9 ± 10 | 14 ± 4 | 10 ± 3 | 8 ± 5 |
| Butanoic acid, 3-methyl-, ethyl ester | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 4 ± 1 | 4 ± 0 | 4 ± 1 | 4 ± 2 | 3 ± 2 |
| 1-Butanol, 3-methyl-, acetate | 653 ± 340 | 1110 ± 69 | 1153 ± 182 | 1028 ± 249 | 691 ± 320 | 608 ± 178 | 792 ± 297 | 931 ± 384 | 572 ± 333 |
| Hexanoic acid, methyl ester | 0 ± 0 c | 0 ± 0 c | 2 ± 0 bc | 2 ± 0 bc | 4 ± 1 bc | 8 ± 2 a | 4 ± 1 ab | 4 ± 1 bc | 4 ± 1 bc |
| Hexanoic acid, ethyl ester | 3 ± 2 | 5 ± 1 | 142 ± 156 | 20 ± 3 | 48 ± 17 | 49 ± 17 | 44 ± 20 | 29 ± 11 | 20 ± 14 |
| Heptanoic acid, ethyl ester | 0 ± 0 b | 0 ± 0 b | 13 ± 1 ab | 9 ± 1 ab | 22 ± 7 a | 21 ± 9 a | 23 ± 9 a | 15 ± 5 ab | 8 ± 5 ab |
| Butanedioic acid, diethyl ester | 0 ± 0 | 0 ± 0 | 9 ± 4 | 9 ± 0 | 6 ± 3 | 15 ± 16 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Octanoic acid, ethyl ester | 9 ± 5 | 14 ± 1 | 2561 ± 600 | 1433 ± 253 | 2037 ± 943 | 2270 ± 1056 | 2765 ± 766 | 2850 ± 716 | 1892 ± 1212 |
| Benzeneacetic acid, ethyl ester | 0 ± 0 b | 0 ± 0 b | 3 ± 1 a | 3 ± 0 a | 2 ± 1 ab | 2 ± 1 ab | 3 ± 0 a | 2 ± 0 ab | 2 ± 1 ab |
| Acetic acid, 2-phenylethyl ester | 0 ± 0 c | 2 ± 0 bc | 13 ± 3 a | 14 ± 0 a | 11 ± 3 ab | 11 ± 4 ab | 11 ± 1 ab | 10 ± 3 ab | 10 ± 3 ab |
| Nonanoic acid, ethyl ester | 0 ± 0 | 4 ± 4 | 711 ± 460 | 322 ± 163 | 434 ± 10 | 524 ± 441 | 513 ± 285 | 1011 ± 155 | 734 ± 764 |
| 4-Decenoic acid, ethyl ester | 0 ± 0 c | 0 ± 0 c | 2 ± 1 abc | 1 ± 1 bc | 4 ± 2 abc | 4 ± 2 ab | 5 ± 1 a | 4 ± 0 bc | 3 ± 1 bc |
| 9-Decenoic acid, ethyl ester | 0 ± 0 | 0 ± 0 | 23 ± 8 | 7 ± 1 | 22 ± 12 | 29 ± 17 | 32 ± 7 | 26 ± 1 | 22 ± 15 |
| Decanoic acid, ethyl ester | 8 ± 4 | 9 ± 1 | 1356 ± 751 | 283 ± 51 | 1088 ± 921 | 1456 ± 852 | 1633 ± 404 | 1370 ± 15 | 1249 ± 773 |
| Octanoic acid, 3-methylbutyl ester | 0 ± 0 | 0 ± 0 | 22 ± 16 | 6 ± 0 | 21 ± 19 | 19 ± 12 | 23 ± 9 | 21 ± 3 | 16 ± 9 |
| Octanoic acid, 2-methylbutyl ester | 0 ± 0 | 0 ± 0 | 6 ± 5 | 4 ± 0 | 6 ± 5 | 6 ± 3 | 7 ± 2 | 7 ± 2 | 5 ± 2 |
| Nonanoic acid, 3-methylbutyl ester | 0 ± 0 | 0 ± 0 | 5 ± 3 | 3 ± 1 | 6 ± 3 | 5 ± 3 | 5 ± 3 | 8 ± 1 | 5 ± 4 |
| Dodecanoid acid, ethyl ester | 0 ± 0 | 0 ± 0 | 59 ± 27 | 28 ± 1 | 102 ± 76 | 91 ± 27 | 79 ± 22 | 80 ± 10 | 62 ± 12 |
| Decanoic acid, 3-methylbutyl ester | 0 ± 0 | 0 ± 0 | 5 ± 2 | 3 ± 0 | 13 ± 12 | 9 ± 3 | 9 ± 3 | 8 ± 1 | 5 ± 1 |
| Ketones | |||||||||
| 5-Hepten-2-one, 6-methyl- | 2 ± 1 c | 4 ± 1 bc | 9 ± 0 a | 8 ± 3 ab | 3 ± 1 bc | 4 ± 1 abc | 5 ± 1 abc | 3 ± 1 c | 3 ± 1 c |
| 2,2,6-Trimethylcyclohexanone | 0 ± 0 b | 2 ± 0 ab | 3 ± 0 a | 3 ± 0 a | 1 ± 1 ab | 1 ± 0 ab | 1 ± 0 ab | 2 ± 1 ab | 1 ± 1 ab |
| (E,E)-3,5-Octadien-2-one | 0 ± 0 b | 0 ± 0 b | 6 ± 3 a | 5 ± 0 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Damascenone | 1 ± 0 b | 4 ± 0 ab | 6 ± 1 a | 7 ± 0 a | 4 ± 1 ab | 4 ± 1 ab | 5 ± 1 ab | 6 ± 1 ab | 5 ± 2 ab |
| α-Ionone | 0 ± 0 c | 2 ± 0 bc | 4 ± 1 ab | 4 ± 1 a | 2 ± 1 bc | 2 ± 1 ab | 3 ± 0 ab | 2 ± 0 ab | 2 ± 1 ab |
| Dehydro-β-ionone | 0 ± 0 c | 2 ± 0 bc | 5 ± 1 a | 6 ± 0 a | 4 ± 1 ab | 4 ± 1 ab | 5 ± 0 ab | 4 ± 1 ab | 4 ± 1 ab |
| β-Ionone | 0 ± 0 d | 3 ± 0 c | 5 ± 0 ab | 6 ± 0 a | 4 ± 0 bc | 3 ± 1 bc | 5 ± 0 ab | 5 ± 0 ab | 4 ± 1 bc |
| Phenols | |||||||||
| 3-Ethylphenol | 1 ± 0 c | 3 ± 1 c | 22 ± 9 ab | 33 ± 1 a | 12 ± 7 bc | 16 ± 3 abc | 16 ± 3 abc | 20 ± 4 ab | 23 ± 1 ab |
| 4-Ethyl-2-methoxy-phenol | 0 ± 0 b | 5 ± 0 ab | 15 ± 4 a | 17 ± 1 a | 10 ± 0 ab | 9 ± 5 ab | 15 ± 0 a | 14 ± 7 a | 16 ± 0 a |
| 2,4-Di-tert-butylphenol | 8 ± 3 b | 24 ± 2 ab | 45 ± 14 a | 36 ± 9 ab | 33 ± 10 ab | 33 ± 6 ab | 28 ± 14 ab | 13 ± 1 ab | 39 ± 3 ab |
| Terpenes | |||||||||
| β-Myrcene | 0 ± 0 c | 0 ± 0 c | 24 ± 2 bc | 23 ± 6 bc | 4 ± 3 c | 65 ± 28 a | 47 ± 10 ab | 6 ± 3 bc | 40 ± 1 bc |
| o-Cymene | 5 ± 3 | 8 ± 0 | 14 ± 4 | 7 ± 2 | 4 ± 1 | 7 ± 2 | 5 ± 2 | 7 ± 7 | 3 ± 1 |
| D-Limonene | 18 ± 5 | 29 ± 6 | 45 ± 21 | 27 ± 4 | 29 ± 2 | 27 ± 1 | 90 ± 29 | 75 ± 79 | 50 ± 20 |
| γ-Terpinene | 5 ± 0 | 7 ± 0 | 11 ± 4 | 8 ± 1 | 6 ± 3 | 8 ± 0 | 9 ± 1 | 15 ± 12 | 10 ± 4 |
| Bornylene | 2 ± 0 | 3 ± 0 | 5 ± 1 | 5 ± 1 | 4 ± 2 | 3 ± 2 | 5 ± 1 | 5 ± 1 | 4 ± 2 |
| Eucalyptol | 0 ± 0 b | 2 ± 2 b | 2 ± 0 b | 3 ± 0 b | 2 ± 0 b | 1 ± 0 b | 8 ± 2 a | 1 ± 0 b | 1 ± 1 b |
| Linalool oxide, (Z)- | 3 ± 0 bc | 3 ± 1 c | 8 ± 2 bc | 8 ± 0 b | 5 ± 3 bc | 5 ± 1 bc | 7 ± 0 bc | 8 ± 0 a | 10 ± 1 a |
| Linalool | 33 ± 5 b | 62 ± 4 ab | 101 ± 14 a | 107 ± 0 a | 70 ± 14 ab | 72 ± 19 ab | 113 ± 5 a | 102 ± 18 a | 86 ± 34 ab |
| α-Terpineol | 5 ± 0 b | 8 ± 0 b | 22 ± 4 a | 21 ± 1 a | 20 ± 1 a | 20 ± 1 a | 19 ± 0 a | 22 ± 2 a | 24 ± 4 a |
| β-Cyclocitral | 2 ± 1 | 4 ± 0 | 6 ± 1 | 6 ± 1 | 4 ± 1 | 4 ± 3 | 5 ± 1 | 5 ± 1 | 3 ± 1 |
| cis-β-Farnesene | 0 ± 0 b | 0 ± 0 b | 4 ± 1 a | 6 ± 0 a | 3 ± 1 ab | 3 ± 1 a | 4 ± 0 a | 4 ± 1 a | 5 ± 1 a |
| trans-Nerolidol | 5 ± 1 b | 6 ± 1 b | 31 ± 12 a | 35 ± 2 a | 30 ± 8 a | 31 ± 4 a | 30 ± 2 a | 35 ± 6 a | 34 ± 6 a |
| Others | |||||||||
| Dihydroactinidiolide | 0 ± 0 b | 0 ± 0 b | 3 ± 1 a | 2 ± 0 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Edulan 1 | 0 ± 0 b | 2 ± 0 ab | 3 ± 0 a | 3 ± 0 a | 2 ± 0 ab | 2 ± 1 ab | 2 ± 1 ab | 2 ± 0 ab | 2 ± 1 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozzon, M.; Rinaldi, L.; Ahmed, A.E.M.; Kovács, B.; Foligni, R. Investigating the Volatiles of Kombucha During Storage Under Refrigerated Conditions. Beverages 2025, 11, 143. https://doi.org/10.3390/beverages11050143
Mozzon M, Rinaldi L, Ahmed AEM, Kovács B, Foligni R. Investigating the Volatiles of Kombucha During Storage Under Refrigerated Conditions. Beverages. 2025; 11(5):143. https://doi.org/10.3390/beverages11050143
Chicago/Turabian StyleMozzon, Massimo, Luigi Rinaldi, Abdelhakam Esmaeil Mohamed Ahmed, Béla Kovács, and Roberta Foligni. 2025. "Investigating the Volatiles of Kombucha During Storage Under Refrigerated Conditions" Beverages 11, no. 5: 143. https://doi.org/10.3390/beverages11050143
APA StyleMozzon, M., Rinaldi, L., Ahmed, A. E. M., Kovács, B., & Foligni, R. (2025). Investigating the Volatiles of Kombucha During Storage Under Refrigerated Conditions. Beverages, 11(5), 143. https://doi.org/10.3390/beverages11050143









