Olive Leaf Extracts as a Medicinal Beverage: Origin, Physico-Chemical Properties, and Bio-Functionality
Abstract
1. Introduction
Consumption of Herbal Drinks in Several Countries and Their Positive Effect on Human Health
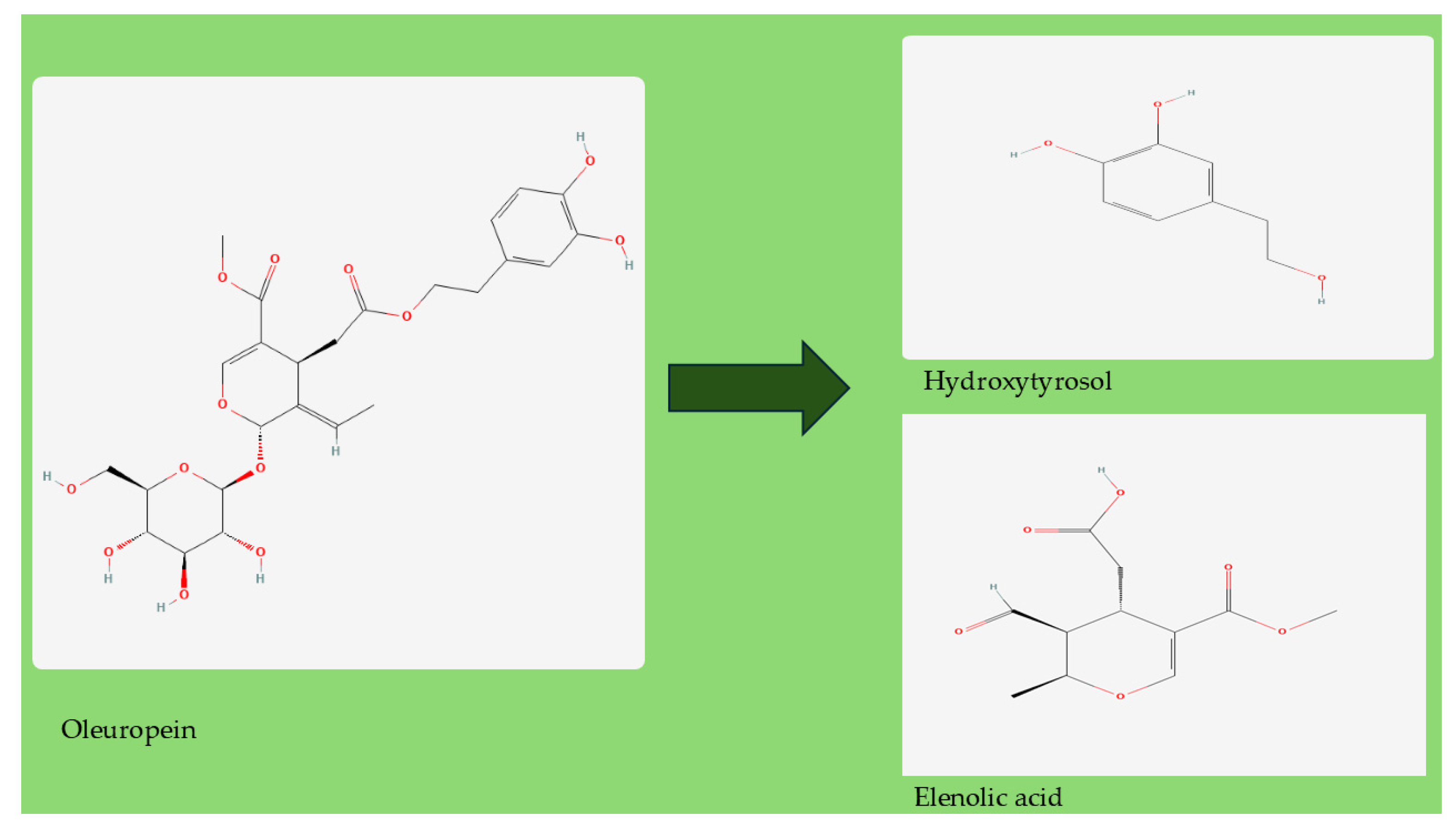
2. Olive Leaf Extracts
2.1. Antioxidant Activity
2.2. Anti-Inflammatory Activity
2.3. Hypoglycemic Activity
2.4. Anticholesterolemic Activity
2.5. Antihypertensive Activity
2.6. Anticancer Activity
3. Additional Anticancer, Antiviral, and Antimicrobial Properties of Oleuropein and Olive Leaf Extracts
3.1. Antiviral Activity
3.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPP-4 | Dipeptidyl peptidase-4 inhibitor |
| EVOO | Extra virgin olive oil |
| OLE | Olive leaf extract |
| iNOS | Nitric oxide synthase |
| LDL | Low-density lipoprotein |
| 8-iso-PGF2α | 8-iso Prostaglandin F2α |
| NrF2 | Nuclear factor erythroid 2-related factor 2 |
| HaCaT cells | aneuploid immortal keratinocyte cell line |
| IL-1β | Interleukin-1 beta (IL-1β) |
| NLRP3 | NLR family pyrin containing NACHT—NAIP neuronal apoptosis inhibitor protein, LRR—“leucine-rich repeat” and PYD—“PYRIN domain” |
| ROS | Reactive oxygen species |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin 6 |
| IL-17 | Interleukin 17 |
| TNF-a | Tumor necrosis factor-a |
| IFN-γ | Interferon gamma |
| MCP-1 | Monocyte chemoattractant proteins |
| COX | Cyclooxygenase |
| MMP | Metalloproteinases |
| NF-kβ | nuclear factor Kappa β |
| CRP | C-reactive protein endothelial and monocyte adhesion molecules |
| ICAM-1 | Intercellular adhesion molecule 1 |
| VCAM-1 | Vascular cell adhesion protein 1 |
| JNK | c-Jun N-terminal kinases |
| MMP-9 | Matrix metalloproteinase-9 |
| p38 MAPK | p38 mitogen-activated protein kinases |
| GLUT2 | Glucose transporter 2 |
| MAPK | Mitogen-activated protein kinase |
| DPP-4 | Dipeptidyl-peptidase 4 |
| GLP1 | Glucagon-like peptide-1 |
| NADPH oxidase | Nicotinamide adenine dinucleotide phosphate oxidase |
| PPARα | Proliferator-activated-receptor alpha |
| PPARγ | Proliferator-activated-receptor gamma |
| MCF-7 | Michigan Cancer Foundation-7 |
| HIF1A | Hypoxia-inducible factor 1-alpha |
| MIA PaCa-2 | Pancreatic cancer cell line |
| HCT116 | Human colon cancer cell line |
| HL-60 | Human leukemia cell line |
| GBM | Glioblastoma multiforme |
| HDAC2 and HDAC3 | Histone deacetylase 2 and Histone deacetylase 3 |
| TIMP | Metallopeptidase inhibitor 1 |
| PC3 | Human prostate cancer cell line |
| GBM | Glioblastoma multiforme |
| VEGF-A | Vascular endothelial growth factor A |
| PI3Ks | Phosphoinositide 3-kinases |
| Akt | Protein kinase B (PKB) |
| mTOR | Mammalian target of rapamycin |
| HER2 | Human epidermal growth factor receptor 2 |
| NGF | Nerve growth factor |
| HCC | Hepatocellular carcinoma |
| VHSV | Viral hemorrhagic septicemia virus |
| HSV-1 | Herpes simplex type 1 |
| HIV | Human immunodeficiency viruses |
| HBV | Hepatitis B virus |
| DHBV | Duck hepatitis B virus |
| IC50 | Half maximal inhibitory concentration |
| MIC | Minimum inhibitory concentration |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SOLE | Standardized olive leaf extract |
| TMPRSS2 | Transmembrane protease, serine 2 |
| BiPS | Binding immunoglobulin protein |
| ACE2 | Angiotensin-converting enzyme 2 |
| TLRs | Toll-like receptors |
| PLPro | Papain-like protease protein |
| 3CLpro | 3C-like protease |
| Nsp12 | Non-structural protein |
| ATCC | American Type Culture Collection |
| OESA | Olea europea L. var. sativa |
| OESY | Olea europea var. sylvestris |
| ACE | Angiotensin-converting enzyme |
References
- Li, Q.; Tu, Y.; Zhu, C.; Luo, W.; Huang, W.; Liu, W.; Li, Y. Cholinesterase, β-amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crop. Prod. 2017, 108, 512–519. [Google Scholar] [CrossRef]
- Nollet, L.M.; Gutierrez-Uribe, J.A. Phenolic Compounds in Food: Characterization and Analysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 167. [Google Scholar]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed]
- Etkin, N.L. Plants and Indigenous Medicine and Diet: Biobehavioral Approaches eBook; Taylor & Francis: Abingdon, UK, 2019; 336p. [Google Scholar]
- Watson, R.R.; Preedy, V.R. Fruits, Vegetables, and Herbs: Bioactive Foods in Health Promotion, 1st ed.; Elsevier Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Alavi, M.S.; Fanoudi, S.; Ghasemzadeh Rahbardar, M.; Mehri, S.; Hosseinzadeh, H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother. Res. 2021, 35, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia ofcinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, G.; Rahman, L.-U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)—A review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef]
- Teschke, R.; Eickhoff, A. Herbal hepatotoxicity in traditional and modern medicine: Actual key issues and new encouraging steps. Front. Pharmacol. 2015, 6, 72. [Google Scholar] [CrossRef]
- Adeeyo, A.O.; Ndou, T.M.; Alabi, M.A.; Mkoyi, H.D.; Enitan, E.M.; Beswa, D.; Makungo, R.; Odiyo, J.O. Structure: Activity and Emerging Applications of Spices and Herbs. Herbs and spices—New Processing Technologies; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef]
- Asmaey, M.A.; Elsoghiar, A.A.M.; Shaaban, M.; Moharram, A.M.; El-Gaby, M.S.A. Phenolics and Other Structural Compounds from Leaves of Olea europaea L.: Extraction Techniques and Pharmacological Activities. Chem. Afr. 2024, 7, 5123–5148. [Google Scholar] [CrossRef]
- Rahmanian, N.; Ali, S.H.B.; Homayoonfard, M.; Ali, N.J.; Rehan, M.M.; Sadef, Y.; Nizami, A.S. Analysis of physiochemical parameters to evaluate the drinking water quality in the state of Perak, Malaysia. J. Chem. 2015, 2015, 716125. [Google Scholar] [CrossRef]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of A herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef]
- Cahyawati, P.N.; Lestari, A.; Subrata, T.; Dewi, N.W.E.S.; Wiadnyana, I.G.P. Phytochemical test on herbal drinks loloh cemcem at Penglipuran Village, Bali. J. Phys. Conf. Ser. 2019, 1402, 055030. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández Gutiérrez, A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.M.; Prochaska, J.O. A philosophy of health: Life as reality, health as a universal value. Palgrave Commun. 2020, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Rashid, N.; Gbedomon, R.C.; Ahmad, M.; Salako, V.K.; Zafar, M.; Malik, K. Traditional knowledge on herbal drinks among indigenous communities in Azad Jammu and Kashmir, Pakistan. J. Ethnobiol. Ethnomed. 2018, 14, 16. [Google Scholar] [CrossRef]
- Ob’on, C.; Rivera, D.; Fonoll’a, E.; Alcaraz, F.; Attieh, L. A comparison study on traditional mixtures of herbal teas used in eastern mediterranean area. Front. Pharmacol. 2021, 12, 632692. [Google Scholar] [CrossRef]
- Martin. Top 10 Tea Drinking Countries in the World. 2019. Available online: https://www.storiesabouttea.com/top-10-tea-drinking-countries-in-the-world/ (accessed on 11 May 2022).
- Hara, Y. Tea catechins and their applications as supplements and pharmaceutics. Pharmacol. Res. 2011, 64, 100–104. [Google Scholar] [CrossRef]
- Long, T.; Hu, R.; Cheng, Z.; Xu, C.; Hu, Q.; Liu, Q.; Gu, R.; Huang, Y.; Long, C. Ethnobotanical study on herbal tea drinks in Guangxi, China. J. Ethnobiol. Ethnomed. 2023, 19, 10. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 November 2024).
- Cercato, L.M.; White, P.A.S.; Nampo, F.K.; Santos, M.R.V.; Camargo, E.A. A systematic review of medicinal plants used for weight loss in Brazil: Is there potential for obesity treatment? J. Ethnopharmacol. 2015, 176, 286–296. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO/UN). Current Market Situation and Medium-Term Outlook. In Proceedings of the Twenty-Third Session of the Intergovernmental Group on Tea, Hangzhou, China, 17–20 May 2018; pp. 13–16. Available online: http://www.fao.org/3/a-i4480e.pdf (accessed on 1 September 2024).
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Carbone, R.; Caracciolo, F.; Di Vita, G.; D’Amico, M.; Spina, D. Consumer Trends in the Herbal Tea Market: A Systematic Literature Review. Food Rev. Int. 2025, 1–19. [Google Scholar] [CrossRef]
- Cavalheiro, C.V.; Picoloto, R.S.; Cichoski, A.J.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Da Croce, D.M.; Barin, J.S. Olive leaves offer more than phenolic compounds—Fatty acids and mineral composition of varieties from Southern Brazil. Ind. Crop. Prod. 2015, 71, 122–127. [Google Scholar] [CrossRef]
- Kabbash, E.M.; Ayoub, I.M.; Gad, H.A.; Abdel-Shakour, Z.T.; El-Ahmady, S.H. Quality assessment of leaf extracts of 12 olive cultivars and impact of seasonal variation based on UV spectroscopy and phytochemcial content using multivariate analyses. Phytochem. Anal. 2021, 32, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive leaf addition increases olive oil nutraceutical properties. Molecules 2019, 24, 545. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Chammem, N.; Reyes-Batlle, M.; Mejri, M.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Activity of olive leaf extracts against the promastigote stage of Leishmania species and their correlation with the antioxidant activity. Exp. Parasitol. 2014, 141, 106–111. [Google Scholar] [CrossRef]
- Salem, M.; Affes, H.; Ksouda, K.; Sahnoun, Z.; Zeghal, K.; Hammami, S. Pharmacological activities of Olea europaea leaves. J. Food Process. Preserv. 2014, 39, 3128–3136. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.; Wani, T. A comprehensive review on the bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; E Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Ferreira, D.M.; de Oliveira, N.M.; Chéu, M.H.; Meireles, D.; Lopes, L.; Oliveira, M.B.; Machado, J. Updated Organic Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea europaea. Plants 2023, 12, 688. [Google Scholar] [CrossRef]
- Bouaziz, M.; Sayadi, S. Isolation and evaluation of antioxidants from leaves of a Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque de Castro, M.D. Static–dynamic superheated liquid extraction of hydroxytyrosol and other biophenols from alperujo (a semisolid residue of the olive oil industry). J. Agric. Food Chem. 2007, 55, 3629–3634. [Google Scholar] [CrossRef] [PubMed]
- Žugčić, T.; Abdelkebir, R.; Baena, C.; Collado, M.C.; García-Pérez, J.; Meléndez-Martínez, A.J.; Režek Jambrak, A.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2018, 83, 63–77. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Poudyal, H.; Campbell, F.; Brown, L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate–, high fat–fed rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef]
- Samet, I.; Villareal, M.O.; Motojima, H.; Han, J.; Sayadi, S.; Isoda, H. Olive leaf components apigenin 7-glucoside and luteolin 7-glucoside direct human hematopoietic stem cell differentiation towards erythroid lineage. Differentiation 2015, 89, 146–155. [Google Scholar] [CrossRef]
- Rostamzadeh, A.; Amini-Khoei, H.; Mardani Korani, M.J.; Rahimi-Madiseh, M. Comparison effects of olive leaf extract and oleuropein compounds on male reproductive function in cyclophosphamide exposed mice. Heliyon 2020, 6, e03785. [Google Scholar] [CrossRef]
- Nunes, M.; Pimentel, F.; Costa, A.; Alves, R.; Oliveira, M. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Yaqoob, P.; Spencer, J.; Rowland, I. Olive leaf phenolics and cardiovascular risk reduction: Physiological effects and mechanisms of action. Nutr. Aging 2012, 1, 125–140. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Cuomo, F.; Lopez, F. Evidence of oleuropein degradation by olive leaf protein extract. Food Chem. 2015, 175, 568–574. [Google Scholar] [CrossRef]
- Guinda Garín, M.Á.; Castellano, J.M.; Sántos-Lozano, J.M.; Delgado Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of Major Bioactive Compounds from Olive Leaf. J. Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Karakaya, S. Studies of olive tree leaf extract indicate seveal potential health benefits. Nutr. Rev. 2009, 67, 632–639. [Google Scholar]
- Dekanski, D.; Ristic, S.; Mitrovic, D. Antioxidant effect of dry olive (Olea europaea L.) leaf extract on ethanol-induced gastric lesions in rats. Mediterr. J. Nutr. Metab. 2009, 2, 205–211. [Google Scholar] [CrossRef]
- Carito, V.; Venditti, A.; Bianco, A.; Ceccanti, M.; Serrilli, A.M.; Chaldakov, G.; Tarani, L.; De Nicolò, S.; Fiore, M. Effects of olive leaf polyphenols on male mouse brain NGF, BDNF and their receptors TrkA, TrkB and p75. Nat. Prod. Res. 2014, 28, 1970–1984. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Galli, G.; Caruso, D. Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002, 104, 677–684. [Google Scholar] [CrossRef]
- Visioli, F.; Bogani, P.; Galli, C. Healthful properties of olive oil minor components. In Olive Oil, Chemistry and Technology; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2006; pp. 173–190. [Google Scholar] [CrossRef]
- De la Puerta, R.; Dominguez, M.E.M.; Ruiz-Guttierrez, V.; Flavill, J.A.; Hoult, J.R.S. Effects of olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001, 69, 1213–1222. [Google Scholar] [CrossRef]
- Coni, E.; Benedetto, R.; Pasquale, M.; Masella, R.; Modesti, D.; Mattei, R.; Carline, E.A. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids 2000, 35, 45–54. [Google Scholar] [CrossRef]
- Manna, C.; Migliardi, V.; Golino, P.; Scognamiglio, A.; Galletti, P.; Chiariello, M.; Zappia, V. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J. Nutr. Biochem. 2004, 15, 461–466. [Google Scholar] [CrossRef]
- De la Puerta, R.; Ruiz Gutierrez, V.; Hoult, J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Visioli, F.; Caruso, D.; Galli, C.; Viappiani, S.; Galli, G.; Sala, A. Olive Oils Rich in Natural Catecholic Phenols Decrease Isoprostane Excretion in Humans. Biochem. Biophys. Res. Commun. 2000, 278, 797–799. [Google Scholar] [CrossRef]
- Parzonko, A.; Czerwińska, M.E.; Kiss, A.K.; Naruszewicz, M. Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine 2013, 20, 1088–1094. [Google Scholar] [CrossRef]
- Al-Azzawie, H.F.; Alhamdani, M.-S.S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006, 78, 1371–1377. [Google Scholar] [CrossRef]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and Antioxidant Effects of Hydroxytyrosol and Oleuropein from Olive Leaves in Alloxan-Diabetic Rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Kotyzová, D.; Hodková, A.; Eybl, V. The effect of olive oil phenolics—Hydroxytyrosol and oleuropein on antioxidant defence status in acute arsenic exposed rats. Toxicol. Lett. 2011, 205, S222. [Google Scholar] [CrossRef]
- Li, H.; Deng, N.; Yang, J.; Zhao, Y.; Jin, X.; Cai, A.; Seeram, N.P.; Ma, H.; Li, D.; Yang, H.; et al. Anti-inflammatory and antioxidant properties of oleuropein in human keratinocytes characterized by bottom-up proteomics. Front. Pharmacol. 2025, 15, 1496078. [Google Scholar] [CrossRef]
- Dessì, M.; Noce, A.; Agnoli, A.; De Angelis, S.; Fuiano, L.; Tozzo, C.; Taccone-Gallucci, M.; Fuiano, G.; Federici, G. The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 811–815. [Google Scholar] [CrossRef]
- Castejon, M.L.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Montoya, T.; Martín-LaCave, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary oleuropein and its new acyl-derivate attenuate murine lupus nephritis through HO-1/Nrf2 activation and suppressing JAK/STAT, NF-κB, MAPK and NLRP3 inflammasome signaling pathways. J. Nutr. Biochem. 2019, 74, 108229. [Google Scholar] [CrossRef]
- Jacob, K.D.; Noren Hooten, N.; Trzeciak, A.R.; Evans, M.K. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 2013, 134, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Bandeen-Roche, K.; Walston, J.D.; Huang, Y.; Semba, R.D.; Ferrucci, L. Measuring Systemic Inflammatory Regulation in Older Adults: Evidence and Utility. Rejuvenat. Res. 2009, 12, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, J.I.; Blaum, C.; Moore, T.; Xue, Q.L.; Hirsch, C.H.; Walston, J.D.; Fried, L.P. Insulin Resistance and Inflammation as Precursors of Frailty: The Cardiovascular Health Study. Arch. Intern. Med. 2007, 167, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Collerton, J.; Martin-Ruiz, C.; Davies, K.; Hilkens, C.M.; Isaacs, J.; Kolenda, C.; Parker, C.; Dunn, M.; Catt, M.; Jagger, C.; et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech. Ageing Dev. 2012, 133, 456–466. [Google Scholar] [CrossRef]
- Fried, L.P.; Xue, Q.-L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D.; et al. Nonlinear Multisystem Physiological Dysregulation Associated With Frailty in Older Women: Implications for Etiology and Treatment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Miles, E.A.; Zoubouli, P.; Calder, P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef]
- Ryu, S.J.; Choi, H.S.; Yoon, K.Y.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Oleuropein Suppresses LPS-Induced Inflammatory Responses in RAW 264.7 Cell and Zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Giner, R.M. Oleuropein Protects against Dextran Sodium Sulfate-Induced Chronic Colitis in Mice. J. Nat. Prod. 2013, 76, 1113–1120. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free Radical-Scavenging Properties of Olive Oil Polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Morittu, V.M.; Procopio, A.; Britti, D.; Cuzzocrea, S. The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clin. Nutr. 2011, 30, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effect of oleuropein on cognitive deficits and changes in hippocampal brain-derived neurotrophic factor and cytokine expression in a rat model of post-traumatic stress disorder. J. Nat. Med. 2018, 72, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Khalatbary, A.; Zarrinjoei, G. Anti-Inflammatory Effect of Oleuropein in Experimental Rat Spinal Cord Trauma. Iran. Red. Crescent Med. J. 2012, 14, 229–234. [Google Scholar] [PubMed]
- Puel, C.; Mathey, J.; Agalias, A.; Kati-coulibaly, S.; Mardon, J.; Obled, C.; Davicco, M.J.; Lebecque, P.; Horcajada, M.N.; Skaltsounis, A.L.; et al. Dose–response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin. Nutr. 2006, 25, 859–868. [Google Scholar] [CrossRef]
- Larussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef]
- WHO. Classification of Diabetes Mellitus 2019; WHO: Geneva, Switzerland, 2019.
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. Antioxidative potential of antidiabetic agents: A possible protective mechanism against vascular complications in diabetic patients. J. Cell. Physiol. 2019, 234, 2436–2446. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Mohammadi, M.T.; Sahebkar, A. Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomed. Pharmacother. 2018, 98, 333–337. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Mohammadi, M.T.; Sahebkar, A. PPAR-α agonist improves hyperglycemia-induced oxidative stress in pancreatic cells by potentiating antioxidant defense system. Drug Res. 2018, 68, 355–360. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Asgary, S.; Naderi, G.A.; Sarraf Zadegan, N.; Vakili, R. The inhibitory effects of pure flavonoids on in vitro protein glycosylation. J. Herb. Pharmacother. 2002, 2, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A.; Galli, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.M.; Abad, M.J.; Fernandez, L.; Recuero, C.; Villaescusa, L.; Silvan, A.M.; Bermejo, P. In vitro anti-inflammatory activity of iridoids and triterpenoid compounds isolated from Phillyrea latifolia L. Biol. Pharm. Bull. 2000, 23, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.I.; Shode, F.O.; Mipando, M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine 2004, 11, 121–129. [Google Scholar] [CrossRef]
- Gonzalez, M.; Zarzuelo, A.; Gamez, M.J.; Utrilla, M.P.; Jimenez, J.; Osuna, I. Hypoglycemic activity of olive leaf. Planta Med. 1992, 58, 513–515. [Google Scholar] [CrossRef]
- Romani, A.; Mulinacci, N.; Pinelli, P.; Vincieri, F.; Cimato, A. Polyphenolic content in five Tuscany cultivars of Olea europaea L. J. Agric. Food Chem. 1999, 47, 964–967. [Google Scholar] [CrossRef]
- Ahmadvand, H.; Noori, A.; Dehnoo, M.G.; Bagheri, S.; Cheraghi, R.A. Hypoglycemic, hypolipidemic and antiatherogenic effects of oleuropein in alloxan-induced Type 1 diabetic rats. Asian Pac. J. Trop. Dis. 2014, 4, S421–S425. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [CrossRef]
- Kerimi, A.; Nyambe-Silavwe, H.; Pyner, A.; Oladele, E.; Gauer, J.S.; Stevens, Y.; Williamson, G. Nutritional implications of olives and sugar: Attenuation of post-prandial glucose spikes in healthy volunteers by inhibition of sucrose hydrolysis and glucose transport by oleuropein. Eur. J. Nutr. 2019, 58, 1315–1330. [Google Scholar] [CrossRef]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive Component Oleuropein Promotes β-Cell Insulin Secretion and Protects β-Cells from Amylin Amyloid-Induced Cytotoxicity. Biochemistry 2017, 56, 5035–5039. [Google Scholar] [CrossRef]
- Chaari, A. Inhibition of human islet amyloid polypeptide aggregation an cellular toxicity by oleuropein and derivatives from olive oil. Int. J. Biol. Macromol. 2020, 162, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, H.; Wang, A. Oleuropein alleviated gestational diabetes mellitus by activating AMPK signalling. Endocr. Connect. 2021, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Y.; Fang, J.; Geng, R.; Li, M.; Zhao, Y.; Kang, S.G.; Huang, K.; Tong, T. Oleuropein ameliorates advanced stage of type 2 diabetes in db/db mice by regulating gut microbiota. Nutrients 2021, 13, 2131. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; de Cassia Freitas, K.; Figueiredo Santana, L.; Pott, A.; Rodrigues Donadon, J.; de Cassia Avellaneda Guimares, R. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Filesi, C.; Vari, R.; Scazzocchio, B.; Filardi, T.; Fogliano, V.; D’Archivio, M.; Giovannini, C.; Lenzi, A.; Morano, S.; et al. Consumption of extra-virgin oil rich in phenolic compounds improves metabolic control in patients with type 2 diabetes mellitus: A possible involvement of reduced levels of circulating visfatin. J. Endocrinol. Invest. 2016, 39, 1295–1301. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Lampousi, A.M.; Portillo, M.P.; Romaguera, D.; Hoffmann, G.; Boeing, H. Olive oil in the prevention and management of type 2 diabetes mellitus: A systematic review and meta-analysis of cohort studies and intervention trials. Nutr. Diabetes 2017, 7, e262. [Google Scholar] [CrossRef]
- De Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Hermans, M.P.; Lempereur, P.; Salembier, J.P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation effect of a combination of olive (Olea europea L.) leaf and fruit extracts in the clinical management of hypertension and metabolic syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes 2015, 5, e172. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Del Ben, M.; Angelico, F.; Nocella, C.; Petruccioli, A.; Bartimoccia, S.; Monticolo, R.; Cava, E.; Violi, F. Extra virgin olive oil improves post-prandial glycemic and lipid profile in patients with impaired fasting glucose. Clin. Nutr. 2017, 36, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Nocella, C.; Loffredo, L.; Bartimoccia, S.; Cammisotto, V.; Mancinella, M.; Angelico, F.; Valenti, V.; Cavarretta, E.; Carnevale, R.; et al. Oleuropein-enriched chocolate by extra virgin olive oil blunts hyperglycaemia in diabetic patients: Results from a one-time 2-hour post-prandial cross over study. Clin. Nutr. 2020, 39, 2187–2191. [Google Scholar] [CrossRef]
- Malliou, F.; Andreadou, I.; Gonzalez, F.J.; Lazou, A.; Xepapadaki, E.; Vallianou, I.; Lambrinidis, G.; Mikros, E.; Marselos, M.; Skaltsounmis, A.L.; et al. The olive constituent oleuropein, as PPARα agonist, markedly reduces serum triglycerides. J. Nutr. Biochem. 2018, 59, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Mahmoudi, A.; Bouallagui, Z.; Feki, I.; Isoda, H.; Feve, B.; Sayadi, S. Evaluation of hypocholesterolemic effect of oleuropein in cholesterol-fed rats. Chem. Biol. Interact. 2016, 252, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Manceau, P.; Netien, G.; Jardon, P. Hypoglycemic action of extracts of olive leaves. Comptes Rendues Soc. Biol. 1942, 136, 810–811. [Google Scholar]
- Capretti, G.; Bonaconza, E. Effects of infusions or decoctions of olive leaves, (O. europaea) on some physical constants of blood and components of metabolism. G. Clin. Med. 1949, 30, 630–642. [Google Scholar]
- Ribeiro, R.A.; Fiuza de Melo, M.M.; De Barros, F.; Gomes, C.; Trolin, G. Acute antihypertensive effect in conscious rats produced by some medicinal plants used in the state of Sao Paulo. J. Ethnopharmacol. 1986, 15, 261–269. [Google Scholar] [CrossRef]
- Zarzuelo, A.; Duarte, J.; Jimenez, M.; Utrilla, P. Vasodilator effect of olive leaf. Planta Med. 1991, 57, 417–419. [Google Scholar] [CrossRef]
- Fehri, B.; Aiache, J.M.; Memmi, A.; Korbi, S.; Yacoubi, M.T.; Mrad, S.; Lamaison, J.L. Hypotension, hypoglycemia and hypouricemia recorded after repeated administration of aqueous leaf extract of O. europaea L. J. Pharm. Belg. 1994, 49, 101–108. [Google Scholar]
- Huang, Y.; Guan, Q.; Zhang, Z.; Wang, P.; Li, C. Oleacein: A comprehensive review of its extraction, purification, absorption, metabolism, and health effects. Food Chem. 2024, 433, 137334. [Google Scholar] [CrossRef]
- Cherif, S.; Rahal, N.; Haouala, M.; Hizaoui, B.; Dargouth, F.; Gueddiche, M.; Kallel, Z.; Balansard, G.; Boukef, K. A clinical trial of a titrated Olea extract in the treatment of essential arterial hypertension. J. Pharm. Belg. 1996, 51, 69–71. [Google Scholar] [PubMed]
- Somova, L.I.; Shode, F.O.; Ramnanan, P.; Nadar, A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Franco-Ávila, T.; Moreno-González, R.; Juan, M.E.; Planas, J.M. Table olive elicits antihypertensive activity in spontaneously hypertensive rats. J. Sci. Food Agric. 2023, 103, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef]
- Ruano, J.; Lopez-Miranda, J.; Fuentes, F.; Moreno, J.A.; Bellido, C.; Perez-Martinez, P.; Lozano, A.; Gómez, P.; Jiménez, Y.; Pérez Jiménez, F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 2005, 46, 1864–1868. [Google Scholar] [CrossRef]
- Valls, R.M.; Farràs, M.; Suárez, M.; Fernández-Castillejo, S.; Fitó, M.; Konstantinidou, V.; Fuentes, F.; López-Miranda, J.; Giralt, M.; Covas, M.I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.; Villar, J.; Stiefel, P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef]
- Fitó, M.; Cladellas, M.; De la Torre, R.; Marti, J.; Alcantara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; Vila, J.; et al. The members of the SOLOS Investigators. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef]
- Hohmann, C.D.; Cramer, H.; Michalsen, A.; Kessler, C.; Steckhan, N.; Choi, K.; Dobos, G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine 2015, 22, 631–640. [Google Scholar] [CrossRef]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Valero-Muñoz, M.; Martín-Fernández, B.; Ballesteros, S.; de la Fuente, E.; Quintela, J.C.; Lahera, V.; de las Heras, N. Protective effect of a pomace olive oil concentrated in triterpenic acids in alterations related to hypertension in rats: Mechanisms involved. Mol. Nutr. Food Res. 2014, 58, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Lima-Cabello, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Roca, M.; Espejo-Calvo, J.A.; Gil-Extremera, B.; Soria-Florido, M.; de la Torre, R.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Metabolic Syndrome and Endothelial Functional Risk Biomarkers in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2018, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; El-Ghazaly, M.A.; Abdallah, D.M.; Nassar, N.N.; Okpanyi, S.N.; Kreuter, M.H. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in l-name induced hypertension in rats. Arzneim. Forsch./Drug Res. 2002, 52, 797–802. [Google Scholar] [CrossRef]
- Scheffler, A.; Rauwald, H.W.; Kampa, B.; Mann, U.; Mohr, F.W.; Dhein, S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. J. Ethnopharmacol. 2008, 120, 233–240. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Miloradović, Z.; Gvozdenov, M.; Jovović, Đ.; Mihailović-Stanojević, N.; Ivanov, M.; Vajić, U.; Karanović, D.; Milanović, S.D.; Grujić-Milanović, J. Uticaj ekstrakta lista masline (Olea europea L.) na hemodinamski status i nivo lipidne peroksidacije kod pacova sa urođenom hipertenzijom/Effect of Olea europea L. leaf extract on haemodynamic status and lipid peroxidation in spontaneously hypertensive rats. Vet. Glas. 2013, 67, 303–315. [Google Scholar] [CrossRef]
- Perrinjaquet-Moccetti, T.; Busjahn, A.; Schmidlin, C.; Schmidt, A.; Bradl, B.; Aydogan, C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother. Res. 2008, 22, 1239–1242. [Google Scholar] [CrossRef]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef]
- Javadi, H.; Yaghoobzadeh, H.; Esfahani, Z.; Memarzadeh, M.R.; Mirhashemi, S.M. Effects of olive leaf extract on metabolic response, liver and kidney functions and inflammatory biomarkers in hypertensive patients. Pak. J. Biol. Sci. 2019, 22, 342–348. [Google Scholar] [CrossRef]
- Nekooeian, A.; Khalili, A.; Khosravi, M. Oleuropein offers cardioprotection in rats with simultaneous type 2 diabetes and renal hypertension. Indian J. Pharmacol. 2014, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; De Sotomayor, M.A.; Ruiz-Gutierrez, V. Effects of pomace olive oil-enriched diets on endothelial function of small mesenteric arteries from spontaneously hypertensive rats. Br. J. Nutr. 2009, 102, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Nekooeian, A.A.; Khalili, A.; Khosravi, M.B. Effects of oleuropein in rats with simultaneous type 2 diabetes and renal hypertension: A study of antihypertensive mechanisms. J. Asian Nat. Prod. Res. 2014, 16, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.L.; Carraro, C.C.; Belló-Klein, A.; Kalil, A.N.; Aerts, N. Oxidative stress in carotid arteries of patients submitted to carotid endarterectomy. The role of aging process. Acta Cir. Bras. 2016, 31, 564–568. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Liang, K.; Pandian, R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension 2002, 39, 135–141. [Google Scholar] [CrossRef]
- Mihailovic-Stanojevic, N.; Miloradovic, Z.; Ivanov, M.; Bugarski, B.; Jovovic, D.; Karanovic, D.; Vajić, U.J.; Komes, D.; Grujić-Milanović, J. Upregulation of heme oxygenase-1 in response to wild thyme treatment protects against hypertension and oxidative stress. Oxid. Med. Cell Longev. 2016, 2016, 1458793. [Google Scholar] [CrossRef]
- Mihailovic-Stanojevic, N.; Savikin, K.; Zivkovic, J.; Zdunic, G.; Miloradovic, Z.; Ivanov, M.; Karanovic, D.; Vajic, U.J.; Jovovic, D.; Grujic-Milanovic, J. Moderate consumption of alcohol-free red wine provide more beneficial effects on systemic haemodynamics, lipid profile and oxidative stress in spontaneously hypertensive rats than red wine. J. Funct. Foods 2016, 26, 719–730. [Google Scholar] [CrossRef]
- Bouallagui, Z.M.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor–negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G.; Vanella, L. Antiproliferative effect of oleuropein in prostate cell lines. Int. J. Oncol. 2012, 41, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Fabiani, R. Oleuropein Prevents Azoxymethane-Induced Colon Crypt Dysplasia and Leukocytes DNA Damage in A/J Mice. J. Med. Food 2016, 19, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; de la Lastra, C.A. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell Through Downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the in Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef]
- Samara, P.; Christoforidou, N.; Lemus, C.; Argyropoulou, A.; Ioannou, K.; Vougogiannopoulou, K.; Aligiannis, N.; Paronis, E.; Gaboriaud-Kolar, N.; Tsitsilonis, O.; et al. New semi-synthetic analogs of oleuropein show improved anticancer activity in vitro and in vivo. Eur. J. Med. Chem. 2017, 137, 11–29. [Google Scholar] [CrossRef]
- Yao, J.; Wu, J.; Yang, X.; Yang, J.; Zhang, Y.; Du, L. Oleuropein induced apoptosis in HeLa cells via a mitochondrial apoptotic cascade associated with activation of the c-Jun NH2-terminal kinase. J. Pharmacol. Sci. 2014, 125, 300–311. [Google Scholar] [CrossRef]
- Yan, C.M.; Chai, E.Q.; Cai, H.Y.; Miao, G.Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef]
- Seçme, M.; Eroğlu, C.; Dodurga, Y.; Bağcı, G. Investigation of anticancer mechanism of oleuropein via cell cycle and apoptotic pathways in SH-SY5Y neuroblastoma cells. Gene 2016, 585, 93–99. [Google Scholar] [CrossRef]
- Zeriouh, W.; Nani, A.; Belarbi, M.; Dumont, A.; de Rosny, C.; Aboura, I.; Ghanemi, F.Z.; Murtaza, B.; Patoli, D.; Thomas, C.; et al. Correction: Phenolic extract from oleaster (Olea europaea var. Sylvestris) leaves reduces colon cancer growth and induces caspase-dependent apoptosis in colon cancer cells via the mitochondrial apoptotic pathway. PLoS ONE 2017, 12, e0176574. [Google Scholar] [CrossRef]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millán, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A.; Luque de Castro, M.D. A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 723, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Niaz, K.; Maqbool, F.; Abdollahi, M.; Rastrelli, L.; Nabavi, S.M. STAT3 targeting by polyphenols: Novel therapeutic strategy for melanoma: STAT3 targeting by polyphenols. BioFactors 2017, 43, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Clericuzio, M.; Borghesi, B.; Cornara, L.; Ribulla, S.; Gosetti, F.; Marengo, E.; Burlando, B. Oleuropein-Enriched Olive Leaf Extract Affects Calcium Dynamics and Impairs Viability of Malignant Mesothelioma Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 908493. [Google Scholar] [CrossRef] [PubMed]
- Abtin, M.; Alivand, M.R.; Khaniani, M.S.; Bastami, M.; Zaeifizadeh, M.; Derakhshan, S.M. Simultaneous downregulation of miR-21 and miR-155 through oleuropein for breast cancer prevention and therapy. J. Cell. Biochem. 2018, 119, 7151–7165. [Google Scholar] [CrossRef]
- Tezcan, G.; Tunca, B.; Bekar, A.; Budak, F.; Sahin, S.; Cecener, G.; Egeli, U.; Taskapılıoglu, M.O.; Kocaeli, H.; Tolunay, S.; et al. Olea europaea leaf extract improves the treatment response of GBM stem cells by modulating miRNA expression. Am. J. Cancer Res. 2014, 4, 572–590. [Google Scholar]
- Bayat, S.; Shekari Khaniani, M.; Choupani, J.; Alivand, M.R.; Mansoori Derakhshan, S. HDACis (class I), cancer stem cell, and phytochemicals: Cancer therapy and prevention implications. Biomed. Pharmacother. 2018, 97, 1445–1453. [Google Scholar] [CrossRef]
- Song, H.; Lim, D.Y.; Jung, J.I.; Cho, H.J.; Park, S.Y.; Kwon, G.T.; Kang, Y.H.; Lee, K.W.; Choi, M.S.; Park, J.H.Y. Dietary oleuropein inhibits tumor angiogenesis and lymphangiogenesis in the B16F10 melanoma allograft model: A mechanism for the suppression of high-fat diet-induced solid tumor growth and lymph node metastasis. Oncotarget 2017, 8, 32027–32042. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Huang, B.; Chen, A.; Li, X. Oleuropein inhibits the proliferation and invasion of glioma cells via suppression of the AKT signaling pathway. Oncol. Rep. 2016, 36, 2009–2016. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Al-Olayan, E.M.; Elobeid, M.A.; Virk, P.; Mohammed, O.B. Oleuropein induces anti-metastatic effects in breast cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4555–4559. [Google Scholar] [CrossRef]
- Papachristodoulou, A.; Tsoukala, M.; Benaki, D.; Kostidis, S.; Gioti, K.; Aligiannis, N.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.-L.; Mikros, E.; et al. Oleuropein is a Powerful Sensitizer of Doxorubicin-mediated Killing of Prostate Cancer Cells and Exerts Its Action via Induction of Autophagy. J. Cancer Res. Treat. 2018, 4, 61–68. [Google Scholar] [CrossRef]
- Andreadou, I.; Mikros, E.; Ioannidis, K.; Sigala, F.; Naka, K.; Kostidis, S.; Farmakis, D.; Tenta, R.; Kavantzas, N.; Bibli, S.I.; et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J. Mol. Cell. Cardiol. 2014, 69, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, G.; Taskapilioglu, M.O.; Tunca, B.; Bekar, A.; Demirci, H.; Kocaeli, H.; Aksoy, S.A.; Egeli, U.; Cecener, G.; Tolunay, S. Olea europaea leaf extract and bevacizumab synergistically exhibit beneficial efficacy upon human glioblastoma cancer stem cells through reducing angiogenesis and invasion in vitro. Biomed. Pharmacother. 2017, 90, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Gaglione, A.; Limanaqi, F.; Biagioni, F.; Familiari, P.; Frati, A.; Esposito, V.; Fornai, F. The Autophagy Status of Cancer Stem Cells in Gliobastoma Multiforme: From Cancer Promotion to Therapeutic Strategies. IJMS 2019, 20, 3824. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; la Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Carrasco-Pancorbo, A.; Garcia-Villalba, R.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Olive oil’s bitter principle reverses acquired autoresistance to trastuzumab (HerceptinTM) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80. [Google Scholar] [CrossRef]
- Sherif, I.O.; Al-Gayyar, M.M.H. Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sci. 2018, 198, 87–93. [Google Scholar] [CrossRef]
- Xu, T.; Xiao, D. Oleuropein enhances radiation sensitivity of nasopharyngeal carcinoma by downregulating PDRG1 through HIF1α-repressed microRNA-519d. J. Exp. Clin. Cancer Res. 2017, 36, 3. [Google Scholar] [CrossRef]
- Fredrickson, W.R. Method and Composition for Antiviral Therapy with Olive Leaves. U.S. Patent No. 6,117,844, 2000. [Google Scholar]
- Micol, V.; Caturla, N.; Pe’rez-Fons, L.; Ma’s, V.; Pe’rez, L.; Estepa, A. The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV). Antivir. Res. 2005, 66, 129–136. [Google Scholar] [CrossRef]
- Motamedifar, M.; Nekooeian, A.A.; Moatari, A. The Effect of Hydroalcoholic Extract of Olive Leaves against Herpes Simplex Virus Type 1. Iran. J. Med. Sci. 2007, 32, 222–227. [Google Scholar]
- Ben-Amor, I.; Musarra-Pizzo, M.; Smeriglio, A.; D’Arrigo, M.; Pennisi, R.; Attia, H.; Gargouri, B.; Trombetta, D.; Mandalari, G.; Sciortino, M.T. Phytochemical Characterization of Olea europea Leaf Extracts and Assessment of Their Anti-Microbial and Anti-HSV-1 Activity. Viruses 2021, 13, 1085. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Zhang, L.; Huang, P.L.; Chang, Y.T.; Huang, P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, R.; Ben Amor, I.; Gargouri, B.; Attia, H.; Zaabi, R.; Chira, A.B.; Saoudi, M.; Piperno, A.; Trischitta, P.; Tamburello, M.P.; et al. Analysis of Antioxidant and Antiviral Effects of Olive (Olea europaea L.) Leaf Extracts and Pure Compound Using Cancer Cell Model. Biomolecules 2023, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yin, Z.; Dong, J. Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L. var. grandiflorum. J. Ethnopharmacol. 2009, 125, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Majrashi, T.A.; El Hassab, M.A.; Mahmoud, S.H.; Mostafa, A.; Wahsh, E.A.; Elkaeed, E.B.; Hassan, F.E.; EldehnaID, W.M.; Abdelgawad, S.M. In vitro biological evaluation and in silico insights into the antiviral activity of standardized olive leaves extract against SARS-CoV-2. PLoS ONE 2024, 19, e0301086. [Google Scholar] [CrossRef]
- Ma, S.C.; He, Z.D.; Deng, X.L.; But, P.P.H.; Ooi, V.E.C.; Xu, H.X.; Lee, S.H.S.; Lee, S.F. In vitro evaluation of secoiridoid glucosides from the fruits of Ligustrum lucidum as antiviral agents. Chem. Pharm. Bull. 2001, 49, 1471–1473. [Google Scholar] [CrossRef]
- Abdelgawad, S.M.; El Hassab, M.A.; Abourehab, M.A.; Elkaeed, E.B.; Eldehna, W.M. Olive Leaves as a Potential Phytotherapy in the Treatment of COVID-19 Disease; A Mini-Review. Front. Pharmacol. 2022, 13, 879118. [Google Scholar] [CrossRef]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. PPR 2020, 2020030226. [Google Scholar]
- Vijayan, R.; Gourinath, S. Structure-based inhibitor screening of natural products against NSP15 of SARS-CoV-2 revealed Thymopentin and Oleuropein as potent inhibitors. Protein J. 2021, 12, 71–80. [Google Scholar] [CrossRef]
- Sampangi-Ramaiah, M.H.; Vishwakarma, R.; Shaanker, R.U. Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Curr. Sci. 2020, 118, 1087–1092. [Google Scholar] [CrossRef]
- Vardhan, S.; Sahoo, S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef]
- Yu, R.; Chen, L.; Lan, R.; Shen, R.; Li, P. Computational screening of antagonists against the SARS-CoV-2 (COVID-19) coronavirus by molecular docking. Int. J. Antimicrob. Agents 2020, 56, 106012. [Google Scholar] [CrossRef] [PubMed]
- Shawky, E.; Nada, A.A.; Ibrahim, R.S. Potential role of medicinal plants and their constituents in the mitigation of SARS-CoV-2: Identifying related therapeutic targets using network pharmacology and molecular docking analyses. RSC Adv. 2020, 10, 27961–27983. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Heng, W.; Wang, Y.; Qiu, J.; Wei, X.; Peng, S.; Saleem, S.; Khan, M.; Ali, S.S.; Wei, D.Q. In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro). Phytother. Res. 2021, 35, 2841–2845. [Google Scholar] [CrossRef] [PubMed]
- Topuz, S.; Bayram, M. Oleuropein extraction from leaves of three olive varieties (Olea europaea L.): Antioxidant and antimicrobial properties of purified oleuropein and oleuropein extracts. J. Food Process. Preserv. 2021, 46, e15697. [Google Scholar] [CrossRef]
- Takeda, Y.; Jamsransuren, D.; Matsuda, S.; Crea, R.; Ogawa, H. The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and its Use as a Virucidal Cream for Topical Application. Viruses 2021, 13, 232. [Google Scholar] [CrossRef]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Zhang, H.; Luo, H.; Zhu, L.; Jiang, P.; et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. Virol. J. 2004, 78, 11334–11339. [Google Scholar] [CrossRef]
- Borgio, J.F.; Alsuwat, H.S.; Al Otaibi, W.M.; Ibrahim, A.M.; Almandil, N.; Al Asoom, L.I.; Salahuddin, M.; Kamaraj, B.; AbdulAzeez, S. State-of-the-art tools unveil potent drug targets amongst clinically approved drugs to inhibit helicase in SARS-CoV-2. Arch. Med. Sci. 2020, 16, 508–518. [Google Scholar] [CrossRef]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nature reviews. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef]
- Jeong, G.U.; Song, H.; Yoon, G.Y.; Kim, D.; Kwon, Y.C. Therapeutic strategies against COVID-19 and structural characterization of SARS-CoV-2: A review. Front. Microbiol. 2020, 11, 1723. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Roero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef] [PubMed]
- Laincer, F.; Laribi, R.; Tamendjari, A.; Arrar, L.; Rovellini, P.; Venturini, S. Olive oils from Algeria: Phenolic compounds, antioxidant and antibacterial activities. Grasas Aceites 2014, 65, e001. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Cascio, R.L.; Crisafi, G.; Uccella, N.; Saija, A. On the in vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.; Mandrell, R. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Dominciano, L.C.C.; Lee, S.H.I.; Corassin, C.H.; Martinis, E.C.P.; Oliveira, C.A.F. Effects of oleuropein and peracetic acid as sanitizing agents for inactivation of biofilms. Open Conf. Proc. J. 2016, 7, 1–6. [Google Scholar] [CrossRef][Green Version]
- Dominciano, L.C.C.; Oliveira, C.A.F.; Lee, S.H.; Corassin, C.H. Individual and Combined Antimicrobial Activity of Oleuropein and Chemical Sanitizers. J. Food Chem. Nanotechnol. 2016, 2, 124–127. [Google Scholar] [CrossRef]
- Caturla, N.; Pérez-Fons, L.; Estepa, A.; Micol, V. Differential effects of oleuropein, a biophenol from Olea europaea, on anionic and zwiterionic phospholipid model membranes. Chem. Phys. Lipids 2005, 137, 2–17. [Google Scholar] [CrossRef]
- Casas-Sanchez, J.; Alsina, A.M.; Herrlein, M.K.; Mestres, C. Interaction between the antibacterial compound, oleuropein, and model membranes. Colloid Polym. Sci. 2007, 285, 1351–1360. [Google Scholar] [CrossRef]
| Herb Species and Herbal Drinks | Chemical Composition | References |
|---|---|---|
| Green tea | Flavonoids, terpenoids, cardiac glycosides saponins and tannins | [14] |
| Green tea infusions containing broccoli by-products | Catechins, hydroxycinnamic acids, flavonols and glucosinolates | [14] |
| Tea from Artemisia annua L. | Coumarins, terpenes, flavonoids, acetylenes and phenols | [14] |
| Loloh cemcem, Bali traditional drink | Tannins, terpenoids, flavonoids, alkaloids, and phenols | [15] |
| Olive leaf extracts | Flavonoids (luteolin, apigenin, luteolin-7-O-glucoside, etc.), ferulic acid, caffeic acid, tyrosol and hydroxytyrosol and secoiridoids (ligstroside, oleuropein dimethyloleuropein), | [16] |
| Parameter | Control | Diabetic | Diabetic + Oleuropein |
|---|---|---|---|
| TG (mg/dL) | 82.33 ± 14.75 | 126.57 ± 18.59 | 97.69 ± 13.91 |
| TC (mg/dL) | 72.01 ± 16.35 | 110.88 ± 28.48 | 83.38 ± 20.75 |
| HDL (mg/dL) | 39.00 ± 13.29 | 25.01 ± 9.19 | 38.13 ± 10.67 |
| LDL (mg/dL) | 26.52 ± 21.58 | 60.57 ± 28.18 | 25.73 ± 21.06 |
| VLDL (mg/dL) | 16.47 ± 2.49 | 25.30 ± 3.44 | 19.55 ± 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panou, A.A.; Karabagias, I.K. Olive Leaf Extracts as a Medicinal Beverage: Origin, Physico-Chemical Properties, and Bio-Functionality. Beverages 2025, 11, 66. https://doi.org/10.3390/beverages11030066
Panou AA, Karabagias IK. Olive Leaf Extracts as a Medicinal Beverage: Origin, Physico-Chemical Properties, and Bio-Functionality. Beverages. 2025; 11(3):66. https://doi.org/10.3390/beverages11030066
Chicago/Turabian StylePanou, Andreas Alexandros, and Ioannis Konstantinos Karabagias. 2025. "Olive Leaf Extracts as a Medicinal Beverage: Origin, Physico-Chemical Properties, and Bio-Functionality" Beverages 11, no. 3: 66. https://doi.org/10.3390/beverages11030066
APA StylePanou, A. A., & Karabagias, I. K. (2025). Olive Leaf Extracts as a Medicinal Beverage: Origin, Physico-Chemical Properties, and Bio-Functionality. Beverages, 11(3), 66. https://doi.org/10.3390/beverages11030066








