Revealing the Unique Characteristics of Greek White Wine Made from Indigenous Varieties Through Volatile Composition and Sensory Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Reagents and Standards
2.3. Quantification of Volatile Compounds
2.3.1. Extraction Methods
2.3.2. GC-MS Analysis
2.4. Odor Activity Values
2.5. Sensory Analysis
2.6. Statistical Analysis
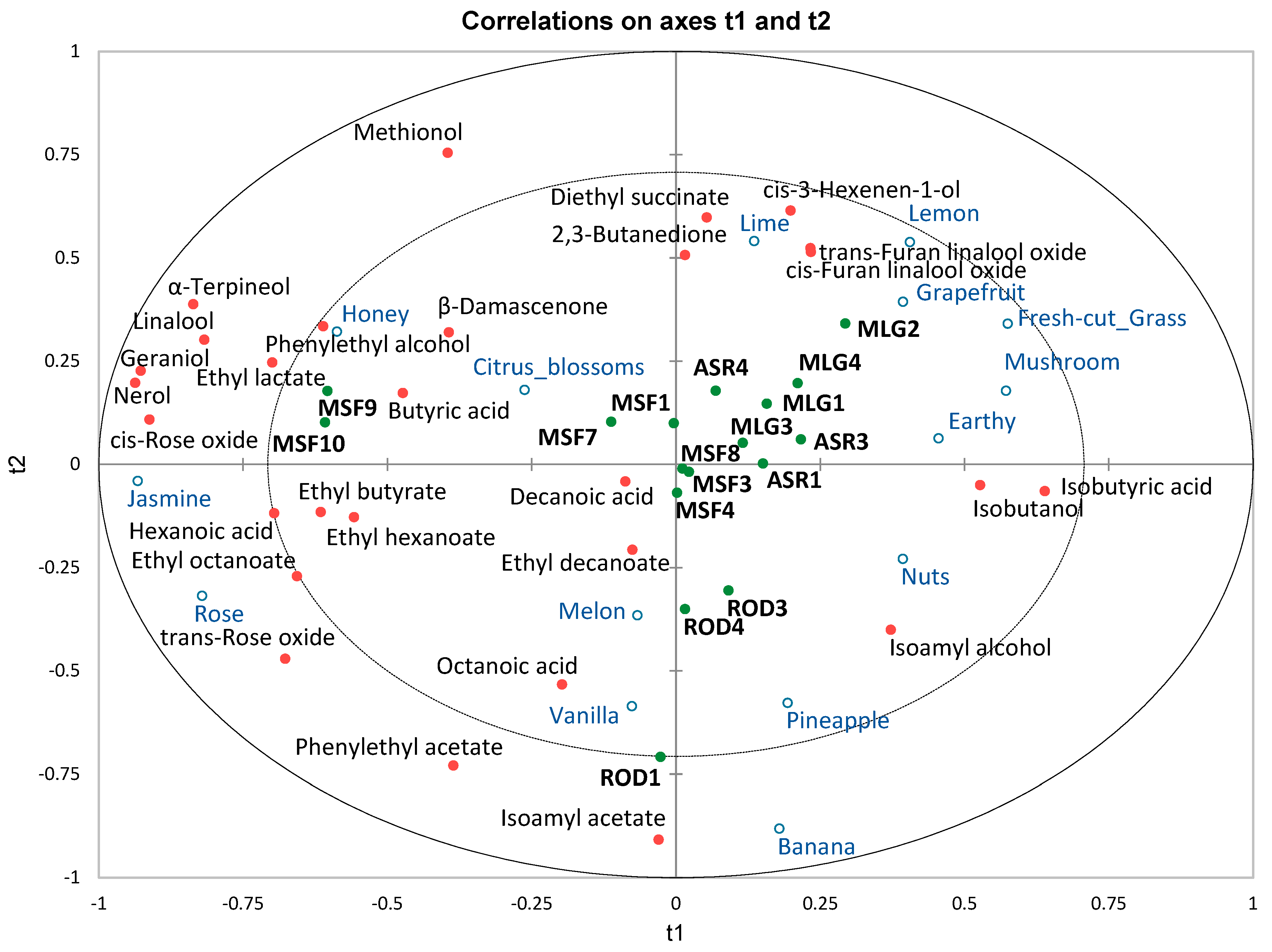
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vlachos, V.A. A macroeconomic estimation of wine production in Greece. Wine Econ. Policy 2017, 6, 3–13. [Google Scholar] [CrossRef]
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, Including Their Origins and Flavours; Penguin: London, UK, 2012; ISBN 978-0-06-220636-7. [Google Scholar]
- Tzamourani, A.; Paramithiotis, S.; Favier, M.; Coulon, J.; Moine, V.; Paraskevopoulos, I.; Dimopoulou, M. New Insights into the Production of Assyrtiko Wines from the Volcanic Terroir of Santorini Island Using Lachancea thermotolerans. Microorganisms 2024, 12, 786. [Google Scholar] [CrossRef]
- Nanou, E.; Mavridou, E.; Milienos, F.S.; Papadopoulos, G.; Tempère, S.; Kotseridis, Y. Odor characterization of white wines produced from indigenous Greek grape varieties using the frequency of attribute citation method with trained assessors. Foods 2020, 9, 1396. [Google Scholar] [CrossRef] [PubMed]
- Karampatea, A.; Vrhovšek, U.; Tsakiris, A.; Dimopoulou, M.; Kourkoutas, Y.; Skavdis, G. Organoleptic and Quality Characteristics of Malagousia Variety Grapes Fermented with Selected Indigenous Yeast Strains. S. Afr. J. Enol. Vitic. 2022, 43, 133–145. [Google Scholar] [CrossRef]
- Lazarakis, K. The Wines of Greece, 2nd ed.; Infinite Ideas Limited: Durrington, UK, 2018; ISBN 9781910902691. [Google Scholar]
- King, E.S.; Kievit, R.L.; Curtin, C.; Swiegers, J.H.; Pretorius, I.S.; Bastian, S.E.P.; Leigh Francis, I. The effect of multiple yeasts co-inoculations on Sauvignon Blanc wine aroma composition, sensory properties and consumer preference. Food Chem. 2010, 122, 618–626. [Google Scholar] [CrossRef]
- Soufleros, E. Factors affecting maturation and quality of grapes. In Oenology; Soufleros, E., Ed.; Evangelos Soufleros: Thessaloniki, Greece, 2015; pp. 119–124. ISBN 978-960-90699-6-0. [Google Scholar]
- Pozo-Bayón, M.Á.; Muñoz-González, C.; Esteban-Fernández, A. Wine Preference and Wine Aroma Perception. In Wine Safety, Consumer Preference, and Human Health; Springer International Publishing: Cham, Switzerland, 2016; pp. 139–162. [Google Scholar]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Rodríguez-Bencomo, J.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Beyond the characterization of wine aroma compounds: Looking for analytical approaches in trying to understand aroma perception during wine consumption. Anal. Bioanal. Chem. 2011, 401, 1497–1512. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of Grape and Wine Aroma. Part 1. Chemical Components and Viticultural Impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.-C. Study of Sensory Interactions among Red Wine Fruity Esters in a Model Solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef]
- Lopez, R.; Wen, Y.; Ferreira, V. The remarkable effects of the non-volatile matrix of wine on the release of volatile compounds evaluated by analysing their release to the headspaces. OENO One 2024, 58, 1–14. [Google Scholar] [CrossRef]
- Cameleyre, M.; Monsant, C.; Tempere, S.; Lytra, G.; Barbe, J.-C. Toward a Better Understanding of Perceptive Interactions between Volatile and Nonvolatile Compounds: The Case of Proanthocyanidic Tannins and Red Wine Fruity Esters—Methodological, Sensory, and Physicochemical Approaches. J. Agric. Food Chem. 2021, 69, 9895–9904. [Google Scholar] [CrossRef] [PubMed]
- Villamor, R.R.; Ross, C.F. Wine Matrix Compounds Affect Perception of Wine Aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of Grape and Wine Aroma. Part 2. Chemical and Sensory Analysis. Am. J. Enol. Vitic. 2014, 65, 25–42. [Google Scholar] [CrossRef]
- Marinaki, M.; Sampsonidis, I.; Lioupi, A.; Arapitsas, P.; Thomaidis, N.; Zinoviadou, K.; Theodoridis, G. Development of two-level Design of Experiments for the optimization of a HS-SPME-GC-MS method to study Greek monovarietal PDO and PGI wines. Talanta 2023, 253, 123987. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Badeka, A.V. Volatilome of white wines as an indicator of authenticity and adulteration control using statistical analysis. Aust. J. Grape Wine Res. 2021, 27, 269–279. [Google Scholar] [CrossRef]
- Kontogiannatos, D.; Troianou, V.; Dimopoulou, M.; Hatzopoulos, P.; Kotseridis, Y. Oenological potential of autochthonous Saccharomyces cerevisiae yeast strains from the greek varieties of agiorgitiko and moschofilero. Beverages 2021, 7, 27. [Google Scholar] [CrossRef]
- Nisiotou, A.; Mallouchos, A.; Tassou, C.; Banilas, G. Indigenous Yeast Interactions in Dual-Starter Fermentations May Improve the Varietal Expression of Moschofilero Wine. Front. Microbiol. 2019, 10, 1712:1–1712:14. [Google Scholar] [CrossRef] [PubMed]
- Tzamourani, A.; Evangelou, A.; Ntourtoglou, G.; Lytra, G.; Paraskevopoulos, I.; Dimopoulou, M. Effect of Non-Saccharomyces Species Monocultures on Alcoholic Fermentation Behavior and Aromatic Profile of Assyrtiko Wine. Appl. Sci. 2024, 14, 1522. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Troianou, V.; Paramithiotis, S.; Proksenia, N.; Kotseridis, Y. Evaluation of Malolactic Starters in White and Rosé Winemaking of Moschofilero Wines. Appl. Sci. 2022, 12, 5722. [Google Scholar] [CrossRef]
- Kechagia, D.; Paraskevopoulos, Y.; Symeou, E.; Galiotou-Panayotou, M.; Kotseridis, Y. Influence of prefermentative treatments to the major volatile compounds of Assyrtiko wines. J. Agric. Food Chem. 2008, 56, 4555–4563. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Bíró, I.; Bufa, A.; Kilár, F. Validation of a Method for Analysis of Aroma Compounds in Red Wine using Liquid–Liquid Extraction and GC–MS. Food Anal. Methods 2012, 5, 1427–1434. [Google Scholar] [CrossRef]
- Metafa, M.; Economou, A. Comparison of solid-phase extraction sorbents for the fractionation and determination of important free and glycosidically-bound varietal aroma compounds in wines by gas chromatography-mass spectrometry. Cent. Eur. J. Chem. 2013, 11, 228–247. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas Chromatography-Olfactometry and Chemical Quantitative Study of the Aroma of Six Premium Quality Spanish Aged Red Wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Etievant, P.X. Wine. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Escudero, A.; Gogorza, B.; Melús, M.A.; Ortín, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Ribreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Varietal Aroma. In Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons, Ltd.: West Sussex, UK, 2006; pp. 205–230. [Google Scholar]
- Bojanowski, V.; Hummel, T. Retronasal perception of odors. Physiol. Behav. 2012, 107, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Do, B.V.; Ferreira, V.; Valentin, D. Aroma properties of young Spanish monovarietal white wines: A study using sorting task, list of terms and frequency of citation. Aust. J. Grape Wine Res. 2008, 14, 104–115. [Google Scholar] [CrossRef]
- Campo, E.; Ballester, J.; Langlois, J.; Dacremont, C.; Valentin, D. Comparison of conventional descriptive analysis and a citation frequency-based descriptive method for odor profiling: An application to Burgundy Pinot noir wines. Food Qual. Prefer. 2010, 21, 44–55. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.-C. Olfactory Impact of Higher Alcohols on Red Wine Fruity Ester Aroma Expression in Model Solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: https://www.flavornet.org/ (accessed on 10 September 2024).
- Buettner, A. Investigation of Potent Odorants and Afterodor Development in Two Chardonnay Wines Using the Buccal Odor Screening System (BOSS). J. Agric. Food Chem. 2004, 52, 2339–2346. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Isoprenoids. In Understanding Wine Chemistry; John Wiley & Sons, Ltd.: West Sussex, UK, 2016; pp. 68–78. [Google Scholar]
- Ruiz-García, L.; Hellín, P.; Flores, P.; Fenoll, J. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.K.C.; Acree, T.E. Similarities in the Aroma Chemistry of Gewürztraminer Variety Wines and Lychee (Litchi chinesis Sonn.) Fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fuentes, C.; Loos, A.; Tomasino, E. Free Monoterpene Isomer Profiles of Vitis vinifera L. cv. White Wines. Foods 2018, 7, 27. [Google Scholar] [CrossRef]
- Mateo, J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Dziadas, M.; Jeleń, H.H. Analysis of terpenes in white wines using SPE–SPME–GC/MS approach. Anal. Chim. Acta 2010, 677, 43–49. [Google Scholar] [CrossRef]
- Guth, H. Identification of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Chigo-Hernandez, M.M.; DuBois, A.; Tomasino, E. Aroma Perception of Rose Oxide, Linalool and α-Terpineol Combinations in Gewürztraminer Wine. Fermentation 2022, 8, 30. [Google Scholar] [CrossRef]
- Vilanova, M.; Campo, E.; Escudero, A.; Graña, M.; Masa, A.; Cacho, J. Volatile composition and sensory properties of Vitis vinifera red cultivars from North West Spain: Correlation between sensory and instrumental analysis. Anal. Chim. Acta 2012, 720, 104–111. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Which Impact for β-Damascenone on Red Wines Aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Xue, J.; Cai, X.D.; Guo, J.; Li, B.; Wu, S. Assessment of the key aroma compounds in rose-based products. J. Food Drug Anal. 2016, 24, 471–476. [Google Scholar] [CrossRef] [PubMed]

| ASR (n = 3) | MLG (n = 4) | MSF (n = 7) | ROD (n = 3) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Alcohols (mg/L) | |||||||||
| Isobutanol | 57 | 6.8 | 83 | 24 | 60 | 17 | 64 | 20 | ns |
| Isoamyl alcohol | 340 a | 64 | 283 ab | 26 | 252 b | 44 | 324 ab | 34 | 0.038 |
| 1-Hexanol | 1.5 | 0.7 | 1.4 | 0.5 | 1.2 | 0.5 | 1.1 | 0.2 | ns |
| Phenylethyl alcohol | 38 | 14 | 32 | 8 | 36 | 14 | 26 | 8 | ns |
| Methionol | 0.94 ab | 0.12 | 1.2 a | 0.1 | 1.1 a | 0.4 | 0.41 b | 0.01 | 0.017 |
| trans-3-Hexen-1-ol | 0.03 a | 0.01 | 0.01 b | 0.01 | ND | 0.02 ab | 0.02 | 0.005 | |
| cis-3-Hexen-1-ol | 0.02 | 0.02 | 0.04 | 0.03 | 0.02 | 0.01 | 0.01 | 0.00 | ns |
| Total alcohols | 437 | 400 | 350 | 416 | |||||
| Carbonyl compounds (mg/L) | |||||||||
| 2,3-Butanedione | 4.5 | 1.5 | 3.1 | 1.1 | 2.6 | 1.7 | 1.9 | 0.7 | ns |
| γ-Butyrolactone | 5.9 | 1.5 | 6.1 | 1.7 | 5.3 | 1.7 | 3.6 | 0.7 | ns |
| Total carbonyl compounds | 10.4 | 9.2 | 7.9 | 5.5 | |||||
| Esters (mg/L) | |||||||||
| Ethyl butyrate | 0.31 | 0.04 | 0.22 | 0.07 | 0.37 | 0.25 | 0.36 | 0.07 | ns |
| Isoamyl acetate | 0.07 a | 0.05 | 0.14 a | 0.05 | 0.16 a | 0.04 | 0.83 b | 0.44 | <0.001 |
| Ethyl hexanoate | 0.74 a | 0.18 | 0.38 b | 0.02 | 0.70 a | 0.12 | 0.62 ab | 0.06 | 0.002 |
| Ethyl lactate | 14 | 2 | 11 | 2 | 17 | 17 | 11 | 2 | ns |
| Ethyl octanoate | 0.68 a | 0.09 | 0.44 b | 0.07 | 0.73 a | 0.12 | 0.70 a | 0.05 | 0.003 |
| Ethyl decanoate | 0.11 | 0.06 | 0.05 | 0.02 | 0.11 | 0.08 | 0.14 | 0.06 | ns |
| Diethyl succinate | 9.6 a | 1.9 | 5.7 b | 0.2 | 4.9 bc | 1.4 | 2.5 c | 0.8 | <0.001 |
| Phenylethyl acetate | 0.14 a | 0.04 | 0.20 a | 0.10 | 0.23 a | 0.11 | 0.49 b | 0.22 | 0.020 |
| Ethyl 3-hydroxy-butyrate | 0.14 | 0.04 | 0.14 | 0.02 | 0.10 | 0.05 | 0.13 | 0.06 | ns |
| Total esters | 26.0 | 18.7 | 24.9 | 17.0 | |||||
| Fatty Acids (mg/L) | |||||||||
| Isobutyric acid | 1.0 | 0.3 | 1.2 | 0.4 | 0.6 | 0.4 | 0.98 | 0.27 | ns |
| Butyric acid | 3.0 | 0.2 | 2.3 | 0.3 | 2.9 | 0.6 | 2.5 | 0.3 | ns |
| Hexanoic acid | 11 a | 1 | 7.6 b | 0.4 | 12 a | 1.5 | 10 ab | 0.1 | 0.002 |
| Octanoic acid | 10 ab | 2 | 7.0 a | 1.8 | 9.0 ab | 2.0 | 12 b | 0.7 | 0.028 |
| Decanoic acid | 1.5 | 0.9 | 1.1 | 0.3 | 1.7 | 1.1 | 1.6 | 0.4 | ns |
| Total fatty acids | 26.5 | 19.2 | 26.2 | 27.0 | |||||
| Terpenes/terpenoids/ norisoprenoids (μg/L) | |||||||||
| cis-Rose oxide | ND | 0.25 a | 0.17 | 0.90 b | 0.47 | 0.30 ab | 0.20 | 0.006 | |
| trans-Rose oxide | ND | 0.08 | 0.05 | 0.17 | 0.10 | 0.20 | 0.20 | ns | |
| trans-Furan linalool oxide | 5.9 a | 2.5 | 51 b | 32 | 18 a | 4 | 8.9 a | 12 | 0.011 |
| cis-Furan linalool oxide | 3.5 a | 1.4 | 24 b | 15 | 9.1 a | 2.2 | 4.9 a | 6.1 | 0.014 |
| Linalool | 1.9 a | 0.8 | 43 ab | 23 | 100 b | 48 | 14 a | 12 | 0.004 |
| α-Terpineol | 9.1 a | 0.9 | 122 ab | 31 | 212 b | 147 | 24 a | 30 | 0.033 |
| Citronellol | ND | 0.33 | 0.22 | 0.39 | 0.40 | 0.07 | 0.12 | ns | |
| Nerol | 0.07 | 0.12 | 0.60 | 0.50 | 18 | 22 | 1.5 | 0.3 | ns |
| β-Damascenone | 7.2 | 1.4 | 8.2 | 5.5 | 11 | 4 | 4.5 | 0.2 | ns |
| Geraniol | 2.4 | 0.3 | 10 | 4. | 133 | 171 | 4.3 | 2.6 | ns |
| Total terpenes/terpenoids/ norisoprenoids | 30.1 | 260 | 503 | 63.4 | |||||
| Volatile phenols (μg/L) | |||||||||
| Vanillin | 3.5 | 2.4 | 1.8 | 1.6 | 1.1 | 0.3 | 1.2 | 0.3 | ns |
| Total volatile phenols | 3.5 | 1.8 | 1.1 | 1.2 | |||||
| Odor Threshold | Reference | Odor Descriptors * | ASR | MLG | MSF | ROD | OAV Category ** | |
|---|---|---|---|---|---|---|---|---|
| Alcohols (mg/L) | ||||||||
| Isobutanol | 40 | [28] | wine, solvent, bitter | 1.43 | 2.06 | 1.50 | 1.61 | >1 |
| Isoamyl alcohol | 30 | [28] | whiskey, malt, burnt | 11.3 | 9.45 | 8.39 | 10.7 | >10 |
| 1-Hexanol | 8 | [28] | resin, flower, green | 0.18 | 0.18 | 0.14 | 0.14 | <0.5 |
| Phenylethyl alcohol | 14 | [29] | honey, spice, rose, lilac | 2.70 | 2.26 | 2.59 | 1.86 | >1 |
| Methionol | 1 | [29] | sweet, potato | 0.94 | 1.19 | 1.06 | 0.41 | >1 |
| trans-3-Hexen-1-ol | unknown | moss, fresh | ||||||
| cis-3-Hexen-1-ol | 0.4 | [28] | grass | 0.06 | 0.09 | 0.06 | 0.03 | <0.5 |
| Carbonyl compounds (mg/L) | ||||||||
| 2,3-Butanedione | 0.1 | [28] | butter | 44.8 | 31.4 | 25.5 | 18.7 | >10 |
| γ-Butyrolactone | unknown | caramel, sweet | ||||||
| Esters (mg/L) | ||||||||
| Ethyl butyrate | 0.02 | [28] | apple | 15.4 | 11.0 | 18.6 | 18.0 | >10 |
| Isoamyl acetate | 0.03 | [28] | banana | 2.47 | 4.70 | 5.20 | 27.5 | >10 |
| Ethyl hexanoate | 0.014 | [29] | apple peel, fruit | 52.8 | 27.3 | 50.1 | 44.3 | >10 |
| Ethyl lactate | 154 | [31] | fruit | 0.09 | 0.07 | 0.11 | 0.07 | <0.5 |
| Ethyl octanoate | 0.005 | [29] | fruit, fat | 135 | 88 | 146 | 139 | >10 |
| Ethyl decanoate | 0.2 | [29] | grape | 0.55 | 0.23 | 0.55 | 0.69 | >0.5 |
| Diethyl succinate | 200 | [31] | wine, fruit | 0.05 | 0.03 | 0.02 | 0.01 | <0.5 |
| Phenylethyl acetate | 0.25 | [28] | rose, honey, tobacco | 0.54 | 0.80 | 0.93 | 1.96 | >1 |
| Ethyl 3-hydroxy-butyrate | 20 | [30] | 0.01 | 0.01 | 0.01 | 0.01 | <0.5 | |
| Fatty Acids (mg/L) | ||||||||
| Isobutyric acid | 2.3 | [29] | rancid, butter, cheese | 0.49 | 0.53 | 0.27 | 0.42 | >0.5 |
| Butyric acid | 0.173 | [29] | rancid, cheese, sweat | 17.3 | 13.1 | 16.8 | 14.4 | >10 |
| Hexanoic acid | 0.42 | [29] | fat, cheese, barnyard | 25.2 | 18.0 | 27.6 | 24.4 | >10 |
| Octanoic acid | 0.5 | [29] | sweat, cheese | 20.4 | 14.0 | 18.7 | 23.9 | >10 |
| Decanoic acid | 1 | [29] | rancid, fat | 1.51 | 1.13 | 1.74 | 1.65 | >1 |
| Terpenes (μg/L) | ||||||||
| cis-Rose oxide | 0.2 | [28] | sweet, rose, green, flower | 0.00 | 1.25 | 4.50 | 1.50 | >1 |
| trans-Rose oxide | unknown | flower | ||||||
| trans-Furan linalool oxide | unknown | |||||||
| cis-Furan linalool oxide | unknown | |||||||
| Linalool | 25 | [29] | flower, lavender | 0.08 | 1.73 | 3.99 | 0.58 | >1 |
| α-Terpineol | 250 | [29] | oil, anise, mint | 0.04 | 0.49 | 0.85 | 0.10 | <0.5 |
| Citronellol | 100 | [28] | rose | 0.00 | 0.00 | 0.00 | 0.00 | <0.5 |
| Nerol | 400 | [33] | sweet | 0.00 | 0.00 | 0.05 | 0.00 | <0.5 |
| β-Damascenone | 0.05 | [28] | apple, rose, honey | 144 | 165 | 225 | 90 | >10 |
| Geraniol | 36 | [32] | rose, geranium | 0.07 | 0.29 | 3.69 | 0.12 | >1 |
| Volatile phenols (μg/L) | ||||||||
| Vanillin | 60 | [30] | vanilla | 0.06 | 0.03 | 0.02 | 0.02 | <0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanou, E.; Metafa, M.; Bastian, S.E.P.; Kotseridis, Y. Revealing the Unique Characteristics of Greek White Wine Made from Indigenous Varieties Through Volatile Composition and Sensory Properties. Beverages 2025, 11, 33. https://doi.org/10.3390/beverages11020033
Nanou E, Metafa M, Bastian SEP, Kotseridis Y. Revealing the Unique Characteristics of Greek White Wine Made from Indigenous Varieties Through Volatile Composition and Sensory Properties. Beverages. 2025; 11(2):33. https://doi.org/10.3390/beverages11020033
Chicago/Turabian StyleNanou, Evangelia, Maria Metafa, Susan E. P. Bastian, and Yorgos Kotseridis. 2025. "Revealing the Unique Characteristics of Greek White Wine Made from Indigenous Varieties Through Volatile Composition and Sensory Properties" Beverages 11, no. 2: 33. https://doi.org/10.3390/beverages11020033
APA StyleNanou, E., Metafa, M., Bastian, S. E. P., & Kotseridis, Y. (2025). Revealing the Unique Characteristics of Greek White Wine Made from Indigenous Varieties Through Volatile Composition and Sensory Properties. Beverages, 11(2), 33. https://doi.org/10.3390/beverages11020033






