Abstract
Herbal teas made from agricultural waste or by-products are gaining attention as eco-friendly alternatives to support circular economy practices. Fig (Ficus carica L.) leaves are well known for their biological activities. The research aims to investigate the possibility of using fig waste leaves to produce healthy and sustainable herbal teas. Different drying technologies have been used, including air drying (AD) and microwave drying (MWD), and consumer acceptability was tested and related to the sensory features and volatile odor compounds. Sensory descriptive analysis and hedonic consumer tests were carried out. Odor volatiles were analyzed by headspace–solid-phase microextraction–mass spectrometry–gas chromatography (HS-SPME-GC-MS). The teas were also evaluated for their phenolic content and antioxidant capacity. Results indicate that MWD increases the total phenolic compound amount by 20%, reduces C6 alcohols and aldehydes responsible for green and herbaceous sensory notes, and increases pentanal, octanal, nonanal, ketones (especially 6 methyl-5-hepten-2-one) and terpenes, such as β-cyclocitral, which are related to the fruity and honey odors; this leads to a more appreciated color and taste. This study demonstrated that dehydrated fig waste leaves, especially those processed through the eco-friendly microwave drying method, can be utilized to produce herbal teas with favorable sensory and nutritional properties. This approach aligns with sustainability objectives and presents a promising strategy for diversifying the herbal tea market while promoting the valorization of agricultural wastes.
1. Introduction
The European herbal tea market has experienced significant growth in recent years, mainly driven by an increasing consumer interest in healthy and natural products and the growing preference for organic and sustainable ingredients [1]. The herbal tea market is projected to grow to USD 12.47 billion by 2032, with a compound annual growth rate (CAGR) of 5.49% during the period 2024–2032 [2]. The rise of functional teas, which are formulated to support specific health goals, and the growing interest in personalized wellness solutions are also shaping the future of the herbal tea market [3]. In this context, herbal teas produced from plant by-products or agricultural waste have been paid more attention, as they represent a sustainable alternative that can reduce the environmental impact associated with the disposal of these materials, in alignment with the circular economy principles. Recent studies have demonstrated the potential of using leaves, stems, and other plant residues from different crops to create functional herbal teas that are rich in bioactive compounds [4,5,6]. As an example, olive leaves have been proposed for herbal tea production, showing interesting activity for lipids and glycemic control [7].
The fig (Ficus carica L.) is the third classical fruit crop associated with the beginning of horticulture in the Mediterranean basin and south-west Asia, and it is an important crop worldwide, consumed as fresh and dried fruit or used to produce jam [8]. Fig production is increasing in the Mediterranean area (making up 70% of global production), with a considerable quantity of waste leaves that have to be disposed of.
Fig fruits have traditionally been appreciated for their taste and nutritional value, hiding other potential uses of the leaves. Fig leaves are a sustainable and valuable source of bioactive compounds, including antioxidants like tocopherols, polyphenols, and flavonoids, with strong antioxidants and antimicrobial properties. In particular, it has been demonstrated that the amount of phenolic compounds in figs is higher than in red wine and tea [9]. Moreover, a range of health-promoting effects, such as anti-tumor, hypolipidemic, antioxidant, antibacterial, and hypoglycemic properties have been demonstrated [10,11]. Recently, different authors also evidenced that fig leaves could have potential applications in the food industry as natural alternatives to synthetic additives, enhancing foods’ physical, sensory, and health benefits [12].
Despite this, limited information is present in the literature about fig leaf herbal tea. The only topics include its health-promoting properties against mild atopic dermatitis and in reducing blood glucose levels [13,14,15], as well as its tannin content, color, potential antioxidant activity, and polyphenol content in formulation with other herbs [16,17]. However, no study has taken into consideration the consumer acceptability, odor volatiles, and sensory features of fig leaf herbal teas.
The recovery of fig leaves in agreement with the Global Sustainability Goals for 2030 could support the sustainability of their production, reducing their environmental impact and aligning with the circular economy, thus ensuring all parts of the fig tree are used efficiently. They could become a source of profit for farmers and manufacturers, particularly in regions where fig cultivation is widely spread.
In herbal tea manufacturing, the quality of the dried leaves is defined by their sensory features and the amount of bioactive compounds. Different drying techniques have be used in recent years and, among these, microwave drying, which is a faster and green technology, has attracted attention; usually, in comparison with many drying methods, it leads to a better color, aroma, and rehydration capacity, but in some cases, such as rosemary and marjoram, a major reduction in volatile aromatic compounds, has been demonstrated [18].
Considering this, the present study aims to investigate the possibility of using fig leaves to produce healthy and sustainable herbal teas. In this context, different drying technologies will be tested, including air drying (AD) and microwave drying (MWD), and consumers’ acceptability will be tested and related to the sensory features and volatile odor compounds of the herbal teas.
2. Materials and Methods
2.1. Sample Collection and Drying Processes
Fresh fig leaves (Ficus carica L. cv. Dottato) were collected after harvesting and before the natural fall in September 2024 in an open field site in Sicily, Italy. Once collected, the leaves were transported to the laboratory, where they were immediately processed. After a preliminary cleaning process with cold water, they were divided into two aliquots and subjected to air drying (AD) and microwave drying (MWD) processes, respectively. The drying procedures were chosen and performed according to those reported by Cincotta et al. [13]. Briefly, ten leaves were placed in a tray dryer (Armfield Ltd., Model UOP8, Hampshire, UK) at 50 °C for 3 h at a constant air velocity (1.5 m/s) for AD and on a ceramic plate in a microwave oven at 400 W power for 4 min for MWD. The drying time–temperature/power combinations were set according to the previous test [19] and considering the best sensory features evaluated by the sensory panel in preliminary trials; the leaves had a starting moisture of 73%, and the time was set until the products reached a moisture loss of 98%, determined by periodically weighting the samples in an analytical balance (Sartorius mod. QUINTIX 65-1S, Gottingen, Germany) with an accuracy of 0.0001 g. The drying procedures were repeated three times under the same conditions.
2.2. Fig Tea Preparation
The fig leaf herbal teas were prepared according to the protocol proposed by Cincotta et al. [19]. In detail, 3 g of chopped fig leaves were placed in 120 mL of boiled mineral water at ~95 °C for 10 min. Subsequently, the infusion was filtered through a filter paper (Munktell & Filtrak, Barenstein, Germany), cooled to room temperature, and used for the successive analyses. Each sample was analyzed in triplicate.
2.3. Total Phenolic Content and Antioxidant Capacity
The total polyphenol content (TPC) and antioxidant capacity (AC) of fig leaf tea were evaluated by spectrophotometric analysis using the Folin–Ciocalteu method and DPPH assays, respectively, as described by Vinci et al. [20].
2.4. Volatile Aroma Compound Analysis
The volatile compounds of the fig leaf tea were determined by headspace–solid-phase microextraction–gas chromatography–mass spectrometry (HS-SPME-GC–MS). Eighteen mL of fig herbal tea and 4 g of NaCl were introduced into a 40 mL glass vial and equilibrated for 30 min at 30 °C. A DVB/CARB/PDMS fibre was introduced in the headspace of the vial for 30 min for the volatile compounds’ extraction and then in the injector of the GC for 3 min at 260 °C.
For GC–MS analysis, a Shimadzu GC 2010 Plus gas chromatograph coupled to a TQMS 8040 triple-quadrupole mass spectrometer (Shimadzu, Milan, Italy) and fitted with a Vf-Wax-ms capillary column (60 m × 0.25 mm i.d.; coating thickness 0.25 nm) was used.
The applied conditions were as follows: an injector temperature set at 260 °C; splitless injection mode; oven temperature programmed at 45 °C for 5 min, then ramped to 110 °C at 5 °C/min and further to 260 °C at 20 °C/min; helium used as the carrier gas with a constant flow rate of 1 mL/min; a transfer line temperature of 250 °C; an acquisition range of 40–400 m/z; and a scan speed of 1250 amu/s.
Compound identification was assessed by comparing the mass spectra with the NIST’20 library (NIST/EPA/NIH Mass Spectra Library, Wiley, Gaithersburg, MD, USA) and the FFNSC 3.0 database, the injection of reference standards, calculation of linear retention indices (LRIs) using the Van den Dool and Kratz equation, and the relevant literature. The results were expressed as peak area percentage.
2.5. Qualitative Descriptive Analysis
The qualitative descriptive analysis (QDA) of the fig herbal teas was carried out according to the method reported by Cincotta et al. [19]. All the participants involved in the study signed an informed consent form according to the principles of the Declaration of Helsinki.
The sensory panel underwent a four-week training in compliance with ISO 8586:2023. During the preliminary sessions, several sensory descriptors were generated, and seventeen were selected based on their citation frequency (Figure 1). Each descriptor was then thoroughly defined and explained to eliminate any ambiguity about its meaning. Sensory descriptors were then validated by using reference standards, helping panelists to calibrate their sensory perceptions and ensure consistent use of descriptors across different sessions.
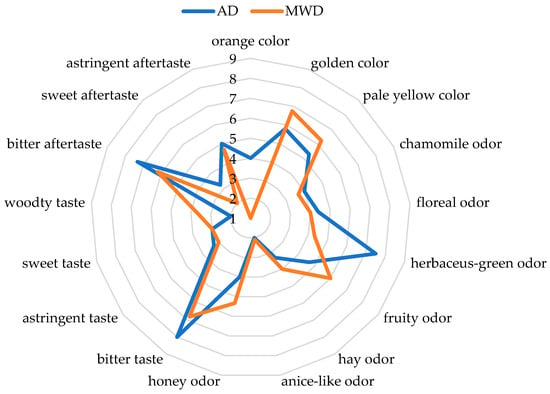
Figure 1.
QDA spider plot of significant descriptors of fig leaf herbal teas. AD: air-dried fig leaf herbal tea. MWD: microwave-dried fig leaf herbal tea. Numbers from 1 to 9 refers to sensory scores where 1 (absence of sensation) and 9 (extremely intense).
The judges assessed the intensity of each descriptor by assigning a score ranging from 1 (absence of sensation) to 9 (extremely intense). Each sample was evaluated by the judges across four sessions. Evaluations took place between 10:00 and 12:00 a.m. in individual booths illuminated with white light. The order of sample presentation was randomized across judges and sessions. Water and unsalted crackers were provided between samples to cleanse the palate.
All data were recorded using a computerized registration system (FIZZ Byosistemes ver. 2.00 M, Couternon, France).
2.6. Consumers’ Acceptability Test
The acceptability test included 80 participants (41 males and 39 females) aged between 24 and 60 years. They were randomly selected through convenience sampling among students and staff of the University of Messina who regularly consume herbal teas. Participation in the survey was completely voluntary. The evaluation of the samples was conducted based on four attributes—color, appearance, odor, taste, and overall acceptability—using an hedonic scale ranging from 1 (extreme dislike) to 9 (extreme appreciation).
2.7. Statistical Analysis
XLStat software, version 2024.1 (Addinsoft, New York, NY, USA), including XLStat sensory, was used for the statistical analysis of data. Grubbs’s test was used to detect and remove the outliers. The Kolmogorov–Smirnov test was applied to verify if the data followed a normal distribution. A two-way ANOVA and Duncan’s multiple-range test, conducted at a 95% confidence level, were used on chemical and sensory data to identify significant differences among the samples.
3. Results and Discussions
3.1. Total Phenolic Content and Antioxidant Activity
Figure 2 reports the total phenolic concentration and antioxidant capacity of fig herbal teas prepared by using AD and MWD leaves.
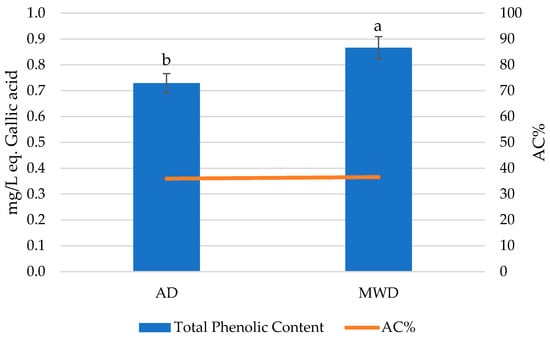
Figure 2.
Total phenolic content and antioxidant capacity of fig leaf herbal teas. AD: air-dried fig leaf herbal tea. MWD: microwave-dried fig leaf herbal tea. Different letters indicate statistically significant differences at p < 0.05 by Duncan’s test.
The total phenolic content was higher in MWD samples than in AD ones (p < 0.05), while the antioxidant capacity showed similar values with no statistically significant differences (p > 0.05).
The drying method usually influences the content of phenolic compounds. In this context, different authors have observed a higher total phenolic content in MWD-dried vegetables and fruits than those processed using conventional drying methods such as sun drying or hot air drying [19,21,22,23,24]. This behavior could be due to the rapid evaporation of water from food, thus providing relatively shorter drying times and limiting the phenolic losses during the heating process [18,25].
3.2. Volatile Aroma Compounds
Table 1 reports the volatile composition of AD and MWD fig leaf herbal teas as classified by chemical classes, their LRI, odor descriptors, and the quantitative statistical significance as determined through the ANOVA. The identified volatile compounds belong to different chemical classes, including alcohols, aldehydes, esters, furans, ketones, and terpenes, each one contributing to the overall aroma profile of the teas. Most of these compounds exhibited statistically significant differences in relation to the utilized drying method, highlighting its impact on the volatile composition and thus on the sensory properties of the fig leaf herbal teas.

Table 1.
Volatile percentage composition of the fig leaf herbal teas.
Globally, aldehydes were the most represented chemical class, representing more than 50% of the volatile fraction, followed by alcohols, terpenes, ketones, esters, and furans (Figure 3).
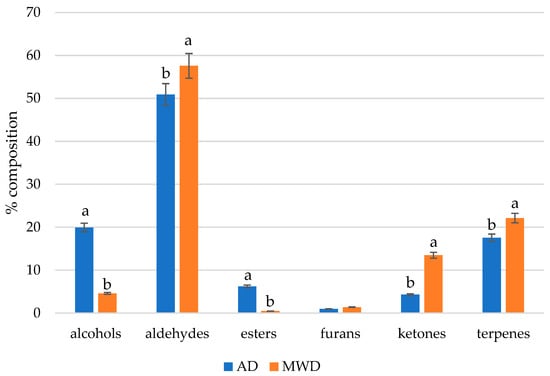
Figure 3.
Volatile percentage composition as classes of substances of fig leaf herbal teas. AD: air-dried fig leaf herbal tea. MWD: microwave-dried fig leaf herbal tea. Different letters in the same class of substances indicate statistically significant differences at p < 0.05 by Duncan’s test.
These compounds arise from fatty acid oxidation processes. Lipid oxidation plays a crucial role in odor compounds’ development and involves a series of radical reactions. During this process, unsaturated fatty acids, in the presence of radicals and reactive oxygen species, form hydroperoxides, which subsequently decompose into alkyl radicals and hydroperoxyl radicals. As these reactions progress, various radicals interact, producing more stable compounds such as aldehydes, alcohols, ketones, and other volatile substances that contribute to odor [26]. Notably, (E)-2-hexenal, well known as leaf aldehyde, exhibited the highest content in AD samples (25.27%) and the lowest content in MWD samples (0.82%); conversely, saturated aldehydes such as pentanal, octanal, nonanal, and benzaldehyde were most represented in MWD fig herbal tea. Aldehydes like decanal and nonanal contribute to citrus, fruity, and floral notes, while benzaldehyde contributes to cinnamon and almond notes [27].
The total alcohol content exhibited significant differences (p < 0.001) between the two drying methods, with MWD significantly reducing the overall alcohol concentration compared to AD, especially for (Z)-3-hexen-1-ol, the leaf alcohol, which shows a high concentration in the AD samples (14.73%).
Esters were more present in AD fig leaf herbal tea, particularly (3Z)-hexenyl butyrate (4.01%), which was almost absent in MWD samples. Moreover, (3Z)-hexenyl butyrate, which is responsible for green, fruity, apple and brandy notes, is widely present in vegetables, and it has been demonstrated that it can induce stomatal closure in grapevine and tomato plants [28].
The total ketone content showed a significant difference (p < 0.001) between drying methods, with a total higher content in MWD fig herbal teas, mainly due to the content of 6-methyl-5-hepten-2-one; this compound arises from the carotenoid degradation and is responsible for citrus and fruity odors [29].
Terpenes were the second class of volatile compounds most represented in MWD fig herbal tea. A different ratio among terpenes and alcohols resulted from the different drying methods, as shown in Figure 2. The most represented were limonene, β-cyclocitral, and estragole. Some terpenes, such as β-cyclocitral, were significantly higher (p < 0.01) in MWD fig herbal tea (13.37% vs. 6.89% in AD), while others like limonene did not show statistically significant differences (p > 0.05).
β-cyclocitral is a volatile short-chain apocarotenoid generated by enzymatic or non-enzymatic oxidation of the carotenoid β-carotene, with a characteristic hay-like, mild floral flavor [30]. 6-Methyl-5-hepten-2-one and β-cyclocitral both arise from carotenoid degradation; in this regard, it has been demonstrated that the MWD process can reduce the total carotenoid content in relation to the microwave power [31]. In our sample, microwaves also act on the carbon double bonds of (E)-2-Hexenal and (Z)-3-Hexen-1-ol, drastically reducing the amount of these substances which are responsible for green and herbal notes. Otherwise, it is well known that microwaves have the potential to modify food flavor, as happened in our samples [32].
3.3. Sensory Analysis
3.3.1. Qualitative Descriptive Analysis
Figure 1 shows the graphical representation of the QDA data of the sensory profiles of fig leaf herbal teas prepared using the two different drying methods, AD and MWD. The evaluation includes multiple sensory attributes for color, odor, and aftertaste.
The QDA was based on the evaluation of seventeen descriptors, three for the color, seven for odor, four for taste, and three for aftertaste.
Concerning color, the AD fig herbal tea showed a more intense orange color, whereas the MWD tea had a paler yellow color. Regarding odor, the AD fig herbal tea had a stronger green odor. In contrast, the scores of the trained assessors indicated that MWD enhances fruity and honey odors.
The AD fig herbal tea also had a more intense bitter and astringent taste and aftertaste, while the MWD fig herbal tea showed higher scores for sweet taste. The different scores of the panel for the odor and color sensory descriptors resulted in agreement with the effects of the drying technology on the volatile profile.
More precisely, a decrease in volatile compounds such as the 2-hexen derivatives in MDW samples could be related to the lowest score for green and herbaceous notes; the highest score for fruity notes for MWD samples could be related to the higher content of aliphatic aldehydes and ketones, whereas the lowest score for the orange color could be relate to the breakdown of carotenoids whose products also enhance the fruity notes.
3.3.2. Consumers’ Acceptability
The acceptability results from the consumers were statistically elaborated and the data are reported in Figure 4. Color, odor, flavor, and taste acceptability were considered. The MWD fig leaf herbal teas showed the highest overall acceptability. At the same time, the acceptability scores for color, odor, flavor, and taste were higher in the MDW samples than in the AD samples. The consumers preferred MDW samples, mainly distinguished by a lower intensity of green herbaceous notes, which act as drivers of “dislike” for the consumers. The higher intensity of golden and pale yellow color and fruity and honey odors could be considered drivers of “liking” for the consumers.
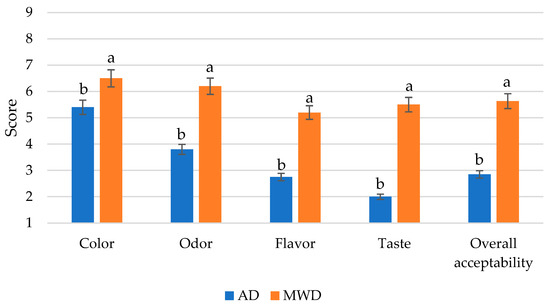
Figure 4.
Consumer acceptability of fig leaf herbal teas. 1: extreme dislike, 9: extreme appreciation. AD: air-dried fig leaf herbal tea. MWD: microwave-dried fig leaf herbal tea. Different letters for the same attribute indicate statistically significant differences at p < 0.05 by Duncan’s test.
4. Conclusions
Our research demonstrates the possibility of using dehydrated fig waste leaves to produce herbal teas that are appreciated by the consumers both for their nutritional and sensory features. Fig leaves represent an opportunity for the herbal tea market, whose growth requires diversification and agricultural waste valorization strategies. Fig leaf teas obtained by microwave drying process have distinct sensory features, with mild, sweet, and fruity flavors which are more broadly accepted by the consumers, especially in the Mediterranean area. The microwave drying applied to the fig leaves resulted the most suitable methods, since it reduces all the volatile compounds responsible for green and herbaceous notes which are disliked by consumers; it also led to a lighter color and a less bitter taste, with well-appreciated stronger fruity and honey odors. Our results demonstrated that in product development, great attention has to be paid to the drying process, which significantly affects the sensory characteristics of the leaves. Moreover, it highlights the possibility of using microwaves as a scalable and eco-friendly drying method in the herbal tea sector, thus saving energy and time. The use of fig leaves to obtain high-value products which respond to growing consumer demand for health foods is in agreement with the Sustainable Development Goals and circular economy principles.
Author Contributions
Conceptualization, A.V. and F.C.; methodology, M.M. and C.C.; software, F.C.; validation, A.V., C.C. and F.C.; formal analysis, M.T. and M.B.; investigation, M.M.; resources, F.C.; data curation, F.C.; writing—original draft preparation, F.C.; writing—review and editing, A.V.; visualization, F.C.; supervision, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Ethical review and approval for sensory analysis were waived for this study because the University of Messina does not have an Institutional Review Board (or Ethics Committee) regarding sensory analysis evaluation. All participants involved in the sensory analysis signed an informed consent form according to the principles of the Declaration of Helsinki before the beginning of the study. Participation in the consumer survey was on a voluntary basis.
Data Availability Statement
All relevant data will be shared upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Euromonitor International, 2023. Tea in Western Europe, February 2023. Available online: https://www.euromonitor.com/tea-in-western-europe/report (accessed on 8 November 2024).
- Global Herbal Tea Market Overview. Available online: https://www.marketresearchfuture.com/reports/herbal-tea-market-5420 (accessed on 9 January 2025).
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal teas and their health benefits: A scoping review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Huda, H.S.A.; Majid, N.B.A.; Chen, Y.; Adnan, M.; Ashraf, S.A.; Roszko, M.; Sasidharan, S. Exploring the ancient roots and modern global brews of tea and herbal beverages: A comprehensive review of origins, types, health benefits, market dynamics, and future trends. Food Sci. Nut 2024, 12, 6938–6955. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Pavlić, B.; Aćimović, M.; Sknepnek, A.; Miletić, D.; Mrkonjić, Ž.; Kljakić, A.C.; Teslić, N. Sustainable raw materials for efficient valorization and recovery of bioactive compounds. Ind. Crop Prod. 2023, 193, 116167. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, M.; Zhou, P.; Tian, M.; Zhou, J.; Zhang, L. Analysis of chemical composition in Chinese olive leaf tea by UHPLC-DAD-Q-TOF-MS/MS and GC–MS and its lipid-lowering effects on the obese mice induced by high-fat diet. Food Res. Int. 2020, 128, 108785. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Mikulic-Petkovsek, M. Phytochemical composition of common fig (Ficus carica L.) cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: London, UK, 2016; pp. 235–255. [Google Scholar] [CrossRef]
- Li, C.; Yu, M.; Li, S.; Yang, X.; Qiao, B.; Shi, S.; Zhao, C.; Fu, Y. Valorization of fig (Ficus carica L.) waste leaves: HPLC-QTOF-MS/MS-DPPH system for online screening and identification of antioxidant compounds. Plants 2021, 10, 2532. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Khali, M.; Benkhaled, A.; Benamirouche, K.; Baiti, I. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac. J. Trop. Med. 2016, 6, 239–245. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell B 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, C.S.H.; Zbiss, Y.; Roriz, C.L.; Dias, M.I.; Prieto, M.A.; Calhelha, R.C.; Alves, M.J.; Heleno, S.A.; da Cunha Mendes, V.; Carocho, M.; et al. Fig leaves (Ficus carica L.): Source of bioactive ingredients for industrial valorization. Processes 2023, 11, 1179. [Google Scholar] [CrossRef]
- Abe, T. Fig (Ficus carica L.) leaf tea suppresses allergy by acceleration disassembly of IgE-receptor complexes. Biosci. Biotech. Bioch 2020, 84, 1013–1022. [Google Scholar] [CrossRef]
- Abe, T.; Koyama, Y.; Nishimura, K.; Okiura, A.; Takahashi, T. Efficacy and safety of fig (Ficus carica L.) leaf tea in adults with mild atopic dermatitis: A double-blind, randomized, placebo-controlled preliminary trial. Nutrients 2022, 14, 4470. [Google Scholar] [CrossRef]
- Br Bangun, A.G.; Pardede, A.S.; Siagian, M. The Effect of Administration Fig Leaf Tea to Reduce Blood Glucose Levels in Diabetes Mellitus Patients. In Proceedings of the IEEE International Conference on Health, Instrumentation & Measurement, and Natural Sciences (InHeNce), Medan, Indonesia, 14–16 July 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Amanto, B.S.; Laily, F.N.; Nursiwi, A. Influence of withering time and leaf condition on physical and chemical characteristics of fig leaf tea. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 10–12 October 2019; IOP Publishing: Bristol, UK, 2019; Volume 633, p. 012042. [Google Scholar] [CrossRef]
- Iranza, T.A.; Suhaidi, I.; Nainggolan, R.J. The effect of the comparison of fig leaves with stevia leaves and drying time on the quality of fig leaf teabags. In Proceedings of the E3S Web of Conferences, Sanya, China, 28–29 August 2021; EDP Sciences: Les Ulis, France, 2021; Volume 332, p. 08003. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Cincotta, F.; Merlino, M.; Condurso, C.; Miller, A.; Torre, M.; Verzera, A. Avocado leaf-waste management: Drying technology and quality of leaf herbal teas of different varieties cultivated in the Mediterranean area. Int. J. Food Sci. Tech. 2024, 59, 2516–2523. [Google Scholar] [CrossRef]
- Vinci, G.; D’Ascenzo, F.; Maddaloni, L.; Prencipe, S.A.; Tiradritti, M. The influence of green and black tea infusion parameters on total polyphenol content and antioxidant activity by ABTS and DPPH assays. Beverages 2022, 8, 18. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Van Vuong, Q. Effect of drying techniques and operating conditions on the retention of color, phenolics, and antioxidant properties in dried lemon scented tea tree (Leptospermum petersonii) leaves. J. Food Proc. Pres. 2021, 45, e15257. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Al Juhaimi, F.; Ahmed, I.A.M.; Uslu, N.; Babiker, E.E.; Ghafoor, K. Effect of microwave and oven drying processes on antioxidant activity, total phenol and phenolic compounds of kiwi and pepino fruits. J. Food Scie Tech. 2020, 57, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Rawat, R.; Singh, B.; Ravindranath, S.D. Application of microwave energy in the manufacture of enhanced-quality green tea. J. Agric. Food Chem. 2003, 51, 4764–4768. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X.; Tian, Y.; Wang, Q.; Li, X.; An, F.; Luo, Z.; Shang, P.; Liu, Z.; Huang, Q. Mechanisms of cooking methods on flavor formation of Tibetan pork. Food Chem. X 2023, 19, 100873. [Google Scholar] [CrossRef]
- Catalano, A.; Mariconda, A.; D’Amato, A.; Iacopetta, D.; Ceramella, J.; Marra, M.; Longo, P. Aldehydes: What We Should Know About Them. Organics 2024, 5, 395–428. [Google Scholar] [CrossRef]
- Payá, C.; López-Gresa, M.P.; Intrigliolo, D.S.; Rodrigo, I.; Bellés, J.M.; Lisón, P. (Z)-3-hexenyl butyrate induces stomata closure and ripening in vitis vinifera. Agronomy 2020, 10, 1122. [Google Scholar] [CrossRef]
- Tian, Z.; Dong, T.; Wang, S.; Sun, J.; Chen, H.; Zhang, N.; Wang, S. A comprehensive review on chemical composition, flavors, and the impacts of heat processing on the aroma formation of fresh carrot. Food Chem. X 2024, 22, 101201. [Google Scholar] [CrossRef]
- Havaux, M. β-Cyclocitral and derivatives: Emerging molecular signals serving multiple biological functions. Plant Physiol. Bioch 2020, 155, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, X.; Li, D.; Meng, L.; Liu, C. Degradation of carotenoids in pumpkin (Cucurbita maxima L.) slices as influenced by microwave vacuum drying. Int. J. Food Prop. 2017, 20, 1479–1487. [Google Scholar] [CrossRef]
- Deng, X.; Huang, H.; Huang, S.; Yang, M.; Wu, J.; Ci, Z.; Zhang, D. Insight into the incredible effects of microwave heating: Driving changes in the structure, properties and functions of macromolecular nutrients in novel food. Front. Nutr. 2022, 9, 941527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).