Carob-Based Functional Beverages: Nutritional Value and Health Properties
Abstract
1. Introduction
2. Ceratonia siliqua L.: Botanical Characteristics
3. Health Benefits
3.1. Pod Pulp
3.2. Carob Seeds (Locust Bean Gum)
3.3. Whole Pod
| Health Benefits | References | |
|---|---|---|
| Pod Pulp | Antitumor | [20,21,22] |
| Antidiabetic | [20] | |
| Antioxidant | [20] | |
| Anti-aging | [20,21,22] | |
| Cardiovascular health | [8,21,22] | |
| Gastrointestinal health | [1] | |
| Carob seeds | Food additive | [2,23,24] |
| Antioxidant | [21] | |
| Gut health | [26,27] | |
| Whole pod | Food additive | [4,6,15,24,28] |
| Antioxidant | [22,31,32,35,36,37,39,41,48] | |
| Gut health | [34,35,41,48] | |
| Anti-inflammatory | [5,37] | |
| Antiradical | [38,39] | |
| Antibacterial | [38] | |
| Hypoglycemic | [22,38,39,41,43,44] | |
| Antiulcer | [38,48,49] | |
| Antidiarrhea | [38,48] | |
| Cardiovascular health | [41,44,45,46,47] |
| Bioactive Compounds | Effect on Health | References |
|---|---|---|
| Gallic acid, cinnamic acid, epigallocatechin, catechin, quercetin, myricetin, kaempferol, rutin | Antioxidant, anti-inflammatory, antibacterial, antitumoral, cardiovascular effects | [24,25,42,43] |
| Tannins | Antidiarrheal and cardiovascular effects | [1,24] |
| Galactomannan, cellulose, hemicellulose | Gastrointestinal effects, hypocholesterolemic, hypolipidemic, antidiabetic | [8,20,21,30] |
| D-pinitol | Antidiabetic, antitumoral, antioxidant, anti-aging, hypoglycemic | [22,23] |
| Flavonol glycosides | Hypocholesterolemic | [39] |
4. Carob’s Safety
5. Potential Use in Food Processing
6. Carob-Based Beverages
6.1. Production of Carob Beverages
6.2. Bioactive Compounds in Carob-Based Beverages
6.3. Sensory Evaluation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins-Loução, M.A.; Correia, P.J.; Romano, A. Carob: A Mediterranean Resource for the Future. Plants 2024, 13, 1188. [Google Scholar] [CrossRef]
- Tzatzani, T.-T.; Ouzounidou, G. Carob as an Agrifood Chain Product of Cultural, Agricultural and Economic Importance in the Mediterranean Region. J. Innov. Econ. Manag. 2023, 42, 127–147. [Google Scholar] [CrossRef]
- Basharat, Z.; Afzaal, M.; Saeed, F.; Islam, F.; Hussain, M.; Ikram, A.; Pervaiz, M.U.; Awuchi, C.G. Nutritional and Functional Profile of Carob Bean (Ceratonia siliqua): A Comprehensive Review. Int. J. Food Prop. 2023, 26, 389–413. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional by-Product Worldwide Spread in the Formulation of Different Food Products and Beverages. A Review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Brandão, T.R.; Silva, C.L.; Pintado, M. Carob Bean (Ceratonia siliqua L.): A New Perspective for Functional Food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Loullis, A.; Pinakoulaki, E. Carob as Cocoa Substitute: A Review on Composition, Health Benefits and Food Applications. Eur. Food Res. Technol. 2018, 244, 959–977. [Google Scholar] [CrossRef]
- Kaderi, M.; Ben Hamouda, G.; Zaeir, H.; Hanana, M.; Hamrouni, L. Ethnobotanical and Phytopharmacological Notes on Ceratonia siliqua (L.). Phytothérapie 2015, 13, 144–147. [Google Scholar] [CrossRef]
- Kahkahi, E.; Moustaine, M.; Zouhair, R. Botanical, Chemical, and Pharmacological Characteristics of Carob Tree (Cera-Tonia Siliqua L.). Med Discov. 2024, 3, 1168. [Google Scholar] [CrossRef] [PubMed]
- Batlle, I. Carob Tree: Ceratonia siliqua L.-Promoting the Conservation and Use of Underutilized and Neglected Crops. 17; Institute of Plant Genetics and Crop Plant Research: Gatersleben, Germany, 1997; Volume 17, ISBN 92-9043-328-0. [Google Scholar]
- El Hajaji, H.; Lachkar, N.; Alaoui, K.; Cherrah, Y.; Farah, A.; Ennabili, A.; El Bali, B.; Lachkar, M. Antioxidant Activity, Phytochemical Screening, and Total Phenolic Content of Extracts from Three Genders of Carob Tree Barks Growing in Morocco. Arab. J. Chem. 2011, 4, 321–324. [Google Scholar] [CrossRef]
- Iipumbu, L. Compositional Analysis of Locally Cultivated Carob (Ceratonia siliqua) Cultivars and Development of Nutritional Food Products for a Range of Market Sectors. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2008. [Google Scholar]
- Ramón-Laca, L.; Mabberley, D. The Ecological Status of the Carob-Tree (Ceratonia siliqua, Leguminosae) in the Mediterranean. Bot. J. Linn. Soc. 2004, 144, 431–436. [Google Scholar] [CrossRef]
- Boublenza, I.; Ghezlaoui, S.; Mahdad, M.; Vasaï, F.; Chemat, F. Algerian Carob (Ceratonia siliqua L.) Populations. Morphological and Chemical Variability of Their Fruits and Seeds. Sci. Hortic. 2019, 256, 108537. [Google Scholar] [CrossRef]
- Naghmouchi, S.; Khouja, M.; Romero, A.; Tous, J.; Boussaid, M. Tunisian Carob (Ceratonia siliqua L.) Populations: Morphological Variability of Pods and Kernel. Sci. Hortic. 2009, 121, 125–130. [Google Scholar] [CrossRef]
- Youssef, M.K.E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Phenolic Compounds of Carob (Ceratonia siliqua L.). Food Public Health 2013, 3, 304–308. [Google Scholar]
- Simsek, S.; Ozcan, M.M.; Al Juhaimi, F.; ElBabiker, E.; Ghafoor, K. Amino Acid and Sugar Contents of Wild and Cultivated Carob (Ceratonia siliqua) Pods Collected in Different Harvest Periods. Chem. Nat. Compd. 2017, 53, 1008–1009. [Google Scholar] [CrossRef]
- El Batal, H.; Hasib, A.; Ouatmane, A.; Dehbi, F.; Jaouad, A.; Boulli, A. Sugar Composition and Yield of Syrup Production from the Pulp of Moroccan Carob Pods (Ceratonia siliqua L.). Arab. J. Chem. 2016, 9, S955–S959. [Google Scholar] [CrossRef]
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Achchoub, M.; Azzouzi, H.; Elhajji, L.; Benbati, M.; Elfazazi, K.; Salmaoui, S. Evaluation of Physicochemical, Functional and Sensory Properties of Carob Pulp Beverage (Ceratonia siliqua L.). Biosci. Biotechnol. Res. Asia 2021, 18, 611. [Google Scholar] [CrossRef]
- Ruiz-Roso, B.; Quintela, J.C.; de la Fuente, E.; Haya, J.; Pérez-Olleros, L. Insoluble Carob Fiber Rich in Polyphenols Lowers Total and LDL Cholesterol in Hypercholesterolemic Sujects. Plant Foods Hum. Nutr. 2010, 65, 50–56. [Google Scholar] [CrossRef]
- Zunft, H.; Lüder, W.; Harde, A.; Haber, B.; Graubaum, H.; Koebnick, C.; Grünwald, J. Carob Pulp Preparation Rich in Insoluble Fibre Lowers Total and LDL Cholesterol in Hypercholesterolemic Patients. Eur. J. Nutr. 2003, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, J.; Moreno, D.A.; García-Viguer, C. D-Pinitol, a Highly Valuable Product from Carob Pods: Health-Promoting Effects and Metabolic Pathways of This Natural Super-Food Ingredient and Its Derivatives. AIMS Agric. Food 2018, 3, 41–63. [Google Scholar] [CrossRef]
- Navarro, J.A.; Decara, J.; Medina-Vera, D.; Tovar, R.; Suarez, J.; Pavón, J.; Serrano, A.; Vida, M.; Gutierrez-Adan, A.; Sanjuan, C. D-Pinitol from Ceratonia siliqua Is an Orally Active Natural Inositol That Reduces Pancreas Insulin Secretion and Increases Circulating Ghrelin Levels in Wistar Rats. Nutrients 2020, 12, 2030. [Google Scholar] [CrossRef]
- Fidan, H.; Stankov, S.; Petkova, N.; Petkova, Z.; Iliev, A.; Stoyanova, M.; Ivanova, T.; Zhelyazkov, N.; Ibrahim, S.; Stoyanova, A. Evaluation of Chemical Composition, Antioxidant Potential and Functional Properties of Carob (Ceratonia siliqua L.) Seeds. J. Food Sci. Technol. 2020, 57, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated Gastrointestinal Digestion and in Vitro Colonic Fermentation of Carob Polyphenols: Bioaccessibility and Bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U. Re-evaluation of Sodium Nitrate (E 251) and Potassium Nitrate (E 252) as Food Additives. EFSA J. 2017, 15, e04787. [Google Scholar] [PubMed]
- Barak, S.; Mudgil, D. Locust Bean Gum: Processing, Properties and Food Applications—A Review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional Characterisation and Bioactive Components of Commercial Carobs Flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J. Locust Bean Gum: A Versatile Biopolymer. Carbohydr. Polym. 2013, 94, 814–821. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional Characterization of Carobs and Traditional Carob Products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar]
- Oziyci, H.R.; Tetik, N.; Turhan, I.; Yatmaz, E.; Ucgun, K.; Akgul, H.; Gubbuk, H.; Karhan, M. Mineral Composition of Pods and Seeds of Wild and Grafted Carob (Ceratonia siliqua L.) Fruits. Sci. Hortic. 2014, 167, 149–152. [Google Scholar] [CrossRef]
- Biner, B.; Gubbuk, H.; Karhan, M.; Aksu, M.; Pekmezci, M. Sugar Profiles of the Pods of Cultivated and Wild Types of Carob Bean (Ceratonia siliqua L.) in Turkey. Food Chem. 2007, 100, 1453–1455. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Paraskevopoulou, A.; Pantazi, F.; Skendi, A. Cake Perception, Texture and Aroma Profile as Affected by Wheat Flour and Cocoa Replacement with Carob Flour. Foods 2020, 9, 1586. [Google Scholar] [CrossRef] [PubMed]
- Karababa, E.; Coşkuner, Y. Physical Properties of Carob Bean (Ceratonia siliqua L.): An Industrial Gum Yielding Crop. Ind. Crops Prod. 2013, 42, 440–446. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Arslan, D.; Gökçalik, H. Some Compositional Properties and Mineral Contents of Carob (Ceratonia siliqua) Fruit, Flour and Syrup. Int. J. Food Sci. Nutr. 2007, 58, 652–658. [Google Scholar] [CrossRef]
- Čepo, D.V.; Mornar, A.; Nigović, B.; Kremer, D.; Radanović, D.; Dragojević, I.V. Optimization of Roasting Conditions as an Useful Approach for Increasing Antioxidant Activity of Carob Powder. LWT-Food Sci. Technol. 2014, 58, 578–586. [Google Scholar] [CrossRef]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.-S.; Nakayama, T. Antioxidant Activity of Polyphenols in Carob Pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- Carbas, B.; Salinas, M.V.; Serrano, C.; Passarinho, J.A.; Puppo, M.C.; Ricardo, C.; Brites, C. Chemical Composition and Antioxidant Activity of Commercial Flours from Ceratonia siliqua and Prosopis Spp. J. Food Meas. Charact. 2019, 13, 305–311. [Google Scholar] [CrossRef]
- Gruendel, S.; Otto, B.; Garcia, A.L.; Wagner, K.; Mueller, C.; Weickert, M.O.; Heldwein, W.; Koebnick, C. Carob Pulp Preparation Rich in Insoluble Dietary Fibre and Polyphenols Increases Plasma Glucose and Serum Insulin Responses in Combination with a Glucose Load in Humans. Br. J. Nutr. 2007, 98, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Fidan, H.; Mihaylova, D.; Petkova, N.; Sapoundzhieva, T.; Slavov, A.; Krastev, L. Determination of Chemical Composition, Antibacterial and Antioxidant Properties of Products Obtained from Carob and Honey Locust. Turk. J. Biochem. 2019, 44, 316–322. [Google Scholar] [CrossRef]
- Benchikh, Y.; Louailèche, H. Effects of Extraction Conditions on the Recovery of Phenolic Compounds and in Vitro Antioxidant Activity of Carob (Ceratonia siliqua L.) Pulp. Acta Bot. Gall. 2014, 161, 175–181. [Google Scholar] [CrossRef]
- Saci, F.; Bachir bey, M.; Louaileche, H.; Gali, L.; Bensouici, C. Changes in Anticholinesterase, Antioxidant Activities and Related Bioactive Compounds of Carob Pulp (Ceratonia siliqua L.) during Ripening Stages. J. Food Meas. Charact. 2020, 14, 937–945. [Google Scholar] [CrossRef]
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Souli, A.; Sebai, H.; El-Benna, J.; Amri, M.; Marzouki, L. Gastroprotective Effect of Carob (Ceratonia siliqua L.) against Ethanol-Induced Oxidative Stress in Rat. BMC Complement. Altern. Med. 2015, 15, 292. [Google Scholar] [CrossRef]
- Qasem, M.A.; Noordin, M.I.; Arya, A.; Alsalahi, A.; Jayash, S.N. Evaluation of the Glycemic Effect of Ceratonia siliqua Pods (Carob) on a Streptozotocin-Nicotinamide Induced Diabetic Rat Model. PeerJ 2018, 6, e4788. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. In Vitro Antioxidant and Inhibitory Activity of Water Decoctions of Carob Tree (Ceratonia siliqua L.) on Cholinesterases, α-Amylase and α-Glucosidase. Nat. Prod. Res. 2015, 29, 2155–2159. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Saidani, K.; Sebai, H.; Amri, M.; Eto, B.; Marzouki, L. Ceratonia siliqua L.(Immature Carob Bean) Inhibits Intestinal Glucose Absorption, Improves Glucose Tolerance and Protects against Alloxan-induced Diabetes in Rat. J. Sci. Food Agric. 2017, 97, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.; Bertocco, G.; Naes, F.; Bastida, S.; Sánchez-Muniz, F.; Benedí, J. Fiber Purified Extracts of Carob Fruit Decrease Carbohydrate Absorption. Food Funct. 2017, 8, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.; Celada, P.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F. Carob-Fruit-Extract-Enriched Meat Modulates Lipoprotein Metabolism and Insulin Signaling in Diabetic Rats Induced by High-Saturated-Fat Diet. J. Funct. Foods 2020, 64, 103600. [Google Scholar] [CrossRef]
- Tetik, N.; Turhan, I.; Oziyci, H.R.; Karhan, M. Determination of D-Pinitol in Carob Syrup. Int. J. Food Sci. Nutr. 2011, 62, 572–576. [Google Scholar] [CrossRef] [PubMed]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Rodríguez de Fonseca, F. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Abu Hafsa, S.; Ibrahim, S.; Hassan, A. Carob Pods (Ceratonia siliqua L.) Improve Growth Performance, Antioxidant Status and Caecal Characteristics in Growing Rabbits. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Ruiz-Roso, B.; Martín de la Torre, I.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Can Carob-Fruit-Extract-Enriched Meat Improve the Lipoprotein Profile, VLDL-Oxidation, and LDL Receptor Levels Induced by an Atherogenic Diet in STZ-NAD-Diabetic Rats? Nutrients 2019, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Macho-González, A.; Garcimartín, A.; Naes, F.; López-Oliva, M.; Amores-Arrojo, A.; González-Muñoz, M.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Effects of Fiber Purified Extract of Carob Fruit on Fat Digestion and Postprandial Lipemia in Healthy Rats. J. Agric. Food Chem. 2018, 66, 6734–6741. [Google Scholar] [CrossRef] [PubMed]
- El Rabey, H.A.; Al-Seeni, M.N.; Al-Ghamdi, H.B. Comparison between the Hypolipidemic Activity of Parsley and Carob in Hypercholesterolemic Male Rats. BioMed Res. Int. 2017, 2017, 3098745. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, K.M.; Youssef, M.K.E.; Ali, H.M.; El-Manfaloty, M.M. The Influence of Carob Powder on Lipid Profile and Histopathology of Some Organs in Rats. Comp. Clin. Pathol. 2015, 24, 1509–1513. [Google Scholar] [CrossRef]
- Valero-Muñoz, M.; Ballesteros, S.; Ruiz-Roso, B.; Pérez-Olleros, L.; Martín-Fernández, B.; Lahera, V.; de Las Heras, N. Supplementation with an Insoluble Fiber Obtained from Carob Pod (Ceratonia siliqua L.) Rich in Polyphenols Prevents Dyslipidemia in Rabbits through SIRT1/PGC-1α Pathway. Eur. J. Nutr. 2019, 58, 357–366. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Jabri, M.-A.; Mamadou, G.; Limas-Nzouzi, N.; Sebai, H.; El-Benna, J.; Marzouki, L.; Eto, B.; Amri, M. Effects of Aqueous Extracts from Ceratonia siliqua L. Pods on Small Intestinal Motility in Rats and Jejunal Permeability in Mice. RSC Adv. 2016, 6, 44345–44353. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-Benna, J.; Sebai, H.; Marzouki, L. Chemical Constituents and Pharmacological Actions of Carob Pods and Leaves (Ceratonia siliqua L.) on the Gastrointestinal Tract: A Review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.A.; Salem, A.A.-E.; Fahmy, H.A.; Mouneir, S.M.; Soliman, A.S.; Abbas, M.S. Effect of Carob, Doum, and Cinnamon Powder on Blood Lipid Profile in Diabetic Rats. Egypt. J. Chem. 2022, 65, 317–328. [Google Scholar] [CrossRef]
- Aboura, I.; Nani, A.; Belarbi, M.; Murtaza, B.; Fluckiger, A.; Dumont, A.; Benammar, C.; Tounsi, M.S.; Ghiringhelli, F.; Rialland, M. Protective Effects of Polyphenol-Rich Infusions from Carob (Ceratonia siliqua) Leaves and Cladodes of Opuntia Ficus-Indica against Inflammation Associated with Diet-Induced Obesity and DSS-Induced Colitis in Swiss Mice. Biomed. Pharmacother. 2017, 96, 1022–1035. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Oqal, M.; Alqudah, M.; AbuDalo, R.; Nabil, A.-H. Ceratonia siliqua Leaves Ethanol Extracts Exert Anti-Nociceptive and Anti-Inflammatory Effects. Heliyon 2022, 8, e10400. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, S.S.; Shabnam, M.; Alireza, F.; Ghasem, S.; Alireza, E.; Ghayour, M.M.; Samaneh, B.-N. Hepatotoxicity and Nephrotoxicity Evaluation of Carob Extract (Ceratonia siliqua) in Balb/c Mice. J. Med. Plants 2019, 18, fa267–fa273. [Google Scholar]
- Ibrahim, R.M.; Abdel-Salam, F.F.; Farahat, E. Utilization of Carob (Ceratonia siliqua L.) Extract as Functional Ingredient in Some Confectionery Products. Food Nutr. Sci. 2020, 11, 757–772. [Google Scholar]
- Higazy, M.; ELDiffrawy, E.; Zeitoun, M.; Shaltout, O.; El-Yazeed, A. Nutrients of Carob and Seed Powders and Its Application in Some Food Products. J. Adv. Agric. Res. 2018, 23, 130–147. [Google Scholar]
- Goulas, V.; Georgiou, E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods 2019, 9, 20. [Google Scholar] [CrossRef]
- Ikram, A.; Khalid, W.; Wajeeha Zafar, K.; Ali, A.; Afzal, M.F.; Aziz, A.; Faiz ul Rasool, I.; Al-Farga, A.; Aqlan, F.; Koraqi, H. Nutritional, Biochemical, and Clinical Applications of Carob: A Review. Food Sci. Nutr. 2023, 11, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-J.; Zayed, M.Z.; Zhu, H.-X.; Zhao, J.; Li, S.-P. Functional Polysaccharides of Carob Fruit: A Review. Chin. Med. 2019, 14, 40. [Google Scholar] [CrossRef]
- Şanlı, T. Investigation of Utilizing Whey in Dairy-Based Dessert Formulations with Carob Powder. Gıda 2023, 48, 670–681. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padró, T.; Vilahur, G.; López-Bernal, S.; Rocha, M.; Hernández-Mijares, A.; Badimon, L. Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Casolani, N.; Ruggeri, M.; Spizzirri, U.G.; Aiello, F.; Chiodo, E.; Martuscelli, M.; Restuccia, D.; Mastrocola, D. Sensory Evaluation and Consumers’ Acceptance of a Low Glycemic and Gluten-Free Carob-Based Bakery Product. Foods 2024, 13, 2815. [Google Scholar] [CrossRef]
- Červenka, L.; Frühbauerová, M.; Velichová, H. Functional Properties of Muffin as Affected by Substituing Wheat Flour with Carob Powder. In Potravinarstvo, Slovak Journal of Food Sciences; HACCP Consulting: Fairfax, VA, USA, 2019; Volume 13. [Google Scholar]
- Pawłowska, K.; Kuligowski, M.; Jasińska-Kuligowska, I.; Kidoń, M.; Siger, A.; Rudzińska, M.; Nowak, J. Effect of Replacing Cocoa Powder by Carob Powder in the Muffins on Sensory and Physicochemical Properties. Plant Foods Hum. Nutr. 2018, 73, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Román, L.; González, A.; Espina, T.; Gómez, M. Degree of Roasting of Carob Flour Affecting the Properties of Gluten-Free Cakes and Cookies. J. Food Sci. Technol. 2017, 54, 2094–2103. [Google Scholar] [CrossRef]
- Restuccia, D.; Esposito, L.; Spizzirri, U.G.; Martuscelli, M.; Caputo, P.; Rossi, C.O.; Clodoveo, M.L.; Pujia, R.; Mazza, E.; Pujia, A. Formulation of a Gluten-Free Carob-Based Bakery Product: Evaluation of Glycemic Index, Antioxidant Activity, Rheological Properties, and Sensory Features. Fermentation 2023, 9, 748. [Google Scholar] [CrossRef]
- Tsatsaragkou, K.; Gounaropoulos, G.; Mandala, I. Development of Gluten Free Bread Containing Carob Flour and Resistant Starch. LWT-Food Sci. Technol. 2014, 58, 124–129. [Google Scholar] [CrossRef]
- Martin-Diana, A.B.; Izquierdo, N.; Albertos, I.; Sanchez, M.S.; Herrero, A.; Sanz, M.A.; Rico, D. Valorization of Carob’s Germ and Seed Peel as Natural Antioxidant Ingredients in Gluten-free Crackers. J. Food Process. Preserv. 2017, 41, e12770. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Ramakrishna, G.; Srivastava, H.; Gaikwad, K. A Comprehensive Review on Leguminous Galactomannans: Structural Analysis, Functional Properties, Biosynthesis Process and Industrial Applications. Crit. Rev. Food Sci. Nutr. 2021, 62, 443–465. [Google Scholar] [CrossRef]
- Singh, A.K.; Malviya, R.; Rao, G.S.N.K. Locust Bean Gum: Processing, Properties and Food Applications. Recent Adv. Food Nutr. Agric. 2022, 13, 93–102. [Google Scholar] [CrossRef]
- Nasser, S.A.A. Effect of Adding Carob Extract to Yogurt. J. Food Dairy Sci. 2020, 11, 195–198. [Google Scholar] [CrossRef]
- Moreira, T.C.; da Silva, Á.T.; Fagundes, C.; Ferreira, S.M.R.; Cândido, L.M.B.; Passos, M.; Krüger, C.C.H. Elaboration of Yogurt with Reduced Level of Lactose Added of Carob (Ceratonia siliqua L.). LWT-Food Sci. Technol. 2017, 76, 326–329. [Google Scholar] [CrossRef]
- Arab, R.; Hano, C.; Oomah, D.; Yous, F.; Ayouaz, S.; Madani, K.; Boulekbache-Makhlouf, L. Impact of Carob (Ceratonia Ciliqua L.) Pulp Flour Supplementation on Probiotic Viability, Milk Fermentation and Antioxidant Capacity during Yogurt Storage. North Afr. J. Food Nutr. Res. 2022, 6, 154–164. [Google Scholar] [CrossRef]
- Mahtout, R.; Zaidi, F.; Saadi, L.O.; Boudjou, S.; Oomah, B.D.; Hosseinian, F. Carob (Ceratonia siliqua L.) Supplementation Affects Kefir Quality and Antioxidant Capacity during Storage. Int. J. Eng. Tech. 2016, 2, 168. [Google Scholar]
- Melilli, M.G.; Buzzanca, C.; Di Stefano, V. Quality Characteristics of Cereal-Based Foods Enriched with Different Degree of Polymerization Inulin: A Review. Carbohydr. Polym. 2024, 332, 121918. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Delgado, A. Sustainability Opportunities for Mediterranean Food Products through New Formulations Based on Carob Flour (Ceratonia siliqua L.). Sustainability 2021, 13, 8026. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Recent Development in Formation, Toxic Effects, Human Health and Analytical Techniques of Food Contaminants. Food Rev. Int. 2023, 39, 1157–1183. [Google Scholar] [CrossRef]
- Gunel, Z.; Torun, M.; Sahin-Nadeem, H. Sugar, D-pinitol, Volatile Composition, and Antioxidant Activity of Carob Powder Roasted by Microwave, Hot Air, and Combined Microwave/Hot Air. J. Food Process. Preserv. 2020, 44, e14371. [Google Scholar] [CrossRef]
- Aydın, S.; Özdemir, Y. Development and Characterization of Carob Flour Based Functional Spread for Increasing Use as Nutritious Snack for Children. J. Food Qual. 2017, 2017, 5028150. [Google Scholar] [CrossRef]
- Sengül, M.; Fatih Ertugay, M.; Sengül, M.; Yüksel, Y. Rheological Characteristics of Carob Pekmez. Int. J. Food Prop. 2007, 10, 39–46. [Google Scholar] [CrossRef]
- Ibrahim, I.M.A.; Aal, H.Z.A.; Saleh, H.M. Utilization of Hibiscus, Tamarind and Carob in Production of Low Calories Healthy Soft Drinks. Eur. J. Nutr. Food Saf. 2024, 16, 160–173. [Google Scholar] [CrossRef]
- Elfazazi, K.; Harrak, H.; Achchoub, M.; Benbati, M. Physicochemical Criteria, Bioactive Compounds and Sensory Quality of Moroccan Traditional Carob Drink. Mater. Today: Proc. 2020, 27, 3249–3253. [Google Scholar] [CrossRef]
- Thallaj, N. Evaluation of Antimicrobial Activities and Bioactive Compounds of Different Extracts Related to Syrian Traditional Products of Damask Rose (Rosa Damascena). Open Access Libr. J. 2020, 7, 1. [Google Scholar] [CrossRef]
- Aboul-Enein, B.H. Total Dietary Fiber Content of Selected Traditional Beverages in Egypt: A Brief Profile. Beverages 2015, 1, 311–319. [Google Scholar] [CrossRef]
- Dahmani, W.; Elaouni, N.; Abousalim, A.; Akissi, Z.L.E.; Legssyer, A.; Ziyyat, A.; Sahpaz, S. Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities. Plants 2023, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.F.; Cattaneo, F.; Zech, X.V.; Svavh, E.; Pérez, M.J.; Zampini, I.C.; Isla, M.I. Aloja and Añapa, Two Traditional Beverages Obtained from Prosopis Alba Pods: Nutritional and Functional Characterization. Food Biosci. 2020, 35, 100546. [Google Scholar] [CrossRef]
- Yatmaz, E.; Turhan, I. Carob as a Carbon Source for Fermentation Technology. Biocatal. Agric. Biotechnol. 2018, 16, 200–208. [Google Scholar] [CrossRef]
- Gülhan, M.F.; Gülhan, A.; Düşgün, C. Physico-Chemical and Microbiological Properties of Water Kefir Produced from Carob (Ceratonia siliqua L.) Sherbet. Food Sci. Biotechnol. 2024, 1–12. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.G.; Bendali, F.B.; Hosseinian, F. Functional Fermented Carob Milk: Probiotic Variability and Polyphenolic Profile. J. Food Bioact. 2021, 14. [Google Scholar] [CrossRef]
- Azaizeh, H.; Abu Tayeh, H.N.; Schneider, R.; Venus, J. Pilot Scale for Production and Purification of Lactic Acid from Ceratonia siliqua L.(Carob) Bagasse. Fermentation 2022, 8, 424. [Google Scholar] [CrossRef]
- Bahry, H.; Abdallah, R.; Chezeau, B.; Pons, A.; Taha, S.; Vial, C. Biohydrogen Production from Carob Waste of the Lebanese Industry by Dark Fermentation. Biofuels 2022, 13, 219–229. [Google Scholar] [CrossRef]
- Polanowska, K.; Varghese, R.; Kuligowski, M.; Majcher, M. Carob Kibbles as an Alternative Raw Material for Production of Kvass with Probiotic Potential. J. Sci. Food Agric. 2021, 101, 5487–5497. [Google Scholar] [CrossRef]
- Macho-González, A.; Garcimartín, A.; Redondo, N.; Cofrades, S.; Bastida, S.; Nova, E.; Benedí, J.; Sánchez-Muniz, F.J.; Marcos, A.; López-Oliva, M.E. Carob Fruit Extract-Enriched Meat, as Preventive and Curative Treatments, Improves Gut Microbiota and Colonic Barrier Integrity in a Late-Stage T2DM Model. Food Res. Int. 2021, 141, 110124. [Google Scholar] [CrossRef]
- Vitali, M.; Gandía, M.; Garcia-Llatas, G.; Tamayo-Ramos, J.A.; Cilla, A.; Gamero, A. Exploring the Potential of Rice, Tiger Nut and Carob for the Development of Fermented Beverages in Spain: A Comprehensive Review on the Production Methodologies Worldwide. Beverages 2023, 9, 47. [Google Scholar] [CrossRef]
- Srour, N.; Daroub, H.; Toufeili, I.; Olabi, A. Developing a Carob-based Milk Beverage Using Different Varieties of Carob Pods and Two Roasting Treatments and Assessing Their Effect on Quality Characteristics. J. Sci. Food Agric. 2016, 96, 3047–3057. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.D.; Savva, I.K.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Phenolic Profile, Antioxidant Activity, and Chemometric Classification of Carob Pulp and Products. Molecules 2023, 28, 2269. [Google Scholar] [CrossRef] [PubMed]
- Benchikh, Y.; Paris, C.; Louaileche, H.; Charbonne, C.; Ghoul, M.; Chebil, L.; Desk, S. Comparative Characterization of Green and Ripe Carob (Ceratonia siliqua L.): Physicochemical Attributes and Phenolic Profile. SDRP J. Food Sci. Technol. 2016, 1, 85–91. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The Influence of in Vitro Gastrointestinal Digestion on the Chemical Composition and Antioxidant and Enzyme Inhibitory Capacities of Carob Liqueurs Obtained with Different Elaboration Techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Al-u’datt, M.; Ereifej, K.; Almajwal, A.; Al-Mahasneh, M.; Brewer, S.; Alsheyab, F.; Yang, W. Chemical, Functional and Sensory Properties of Carob Juice. J. Food Qual. 2013, 36, 238–244. [Google Scholar] [CrossRef]
- Boublenza, I.; Lazouni, H.A.; Ghaffari, L.; Ruiz, K.; Fabiano-Tixier, A.-S.; Chemat, F. Influence of Roasting on Sensory, Antioxidant, Aromas, and Physicochemical Properties of Carob Pod Powder (Ceratonia siliqua L.). J. Food Qual. 2017, 2017, 4193672. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass Spectrometry-Based Metabolomics of Volatiles as a New Tool for Understanding Aroma and Flavour Chemistry in Processed Food Products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Shimizu, T.; Fujii, Y.; Fushimi, T.; Calabrese, V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules 2024, 14, 234. [Google Scholar] [CrossRef]
- Morais, A.; Rodrigues, M. Optimization and Consumer Acceptability of Carob Powder as Cocoa Substitute in Lactose-Free Cashew Nut Almonds-Based Beverage. Int. Food Res. J. 2018, 25, 2268–2274. [Google Scholar]
- Krstonošić, V.; Jovičić-Bata, J.; Maravić, N.; Nikolić, I.; Dokić, L. Rheology, Structure, and Sensory Perception of Hydrocolloids. In Food Structure and Functionality; Elsevier: Amsterdam, The Netherlands, 2021; pp. 23–47. [Google Scholar]

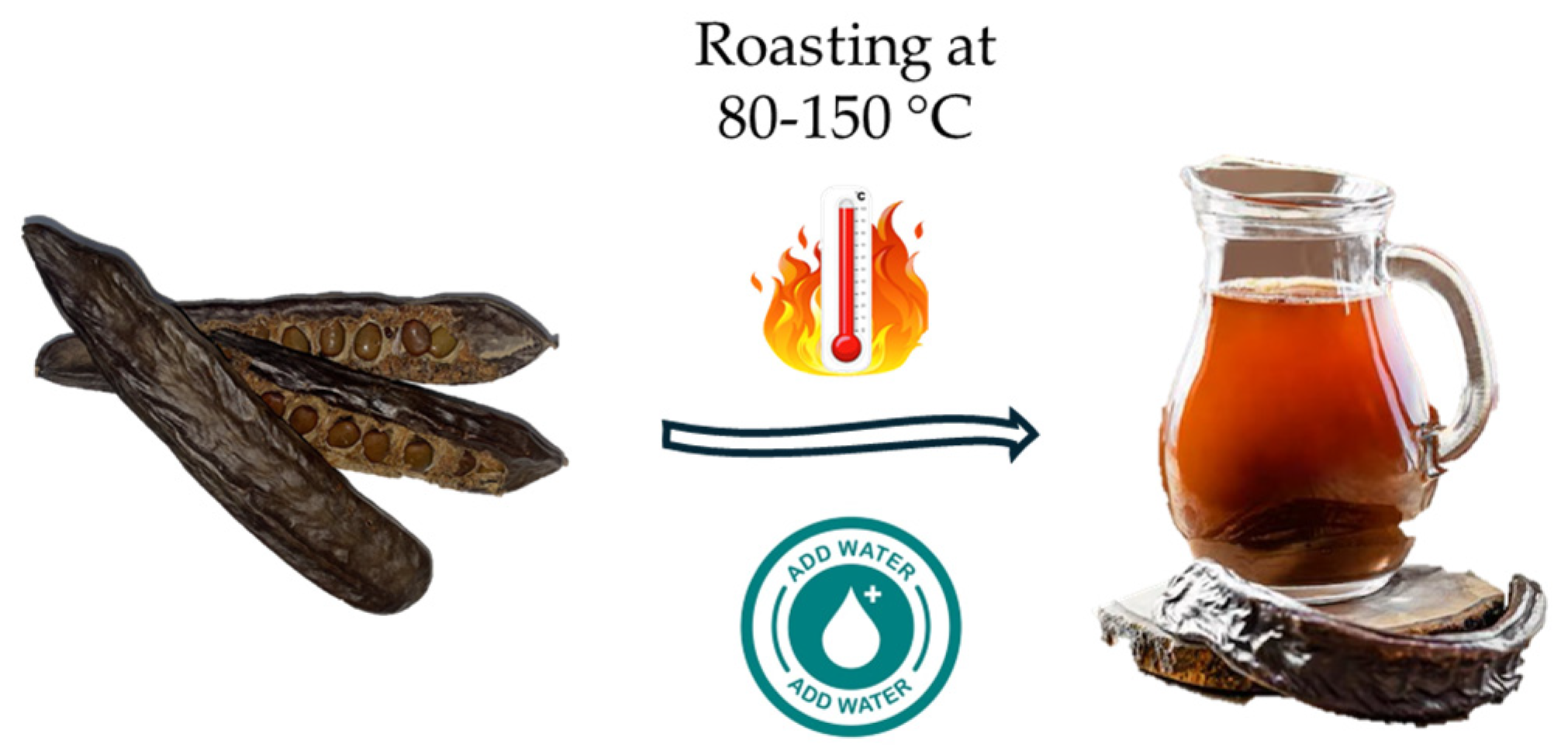
| Name | Origin | Production Method | References |
|---|---|---|---|
| Carob pulp beverage | Laboratory | Crush, clean, and dry carob pulp at 80 °C for 18 h, then mill. Combine 750 g pulp powder with 4 L water, heat to 80 °C for 60 min. Filter, sweeten, and pasteurize at 110 °C for 10 min. Store at 4 °C. | [19] |
| Kharrub | Morocco | Mix carob pulp with water. Heat to 80 °C while stirring for 45 min. Filter and cool. | [77] |
| Jallab | Syria | Boil the ingredients. Filter and serve with ice. | [78] |
| Aloja | Argentina | Crush pods with a pestle and soak in the dark for over 48 h. | [82] |
| Carob water kefir | Laboratory | Boil, filter, and pasteurize carob; add sucrose for sweetened version. Ferment water kefir (48 h). Add kefir to carob, ferment anaerobically (48 h). Store at 4 °C (up to 28 days). | [84] |
| Fermented carob milk | Laboratory | Reconstitute and sterilize skim milk. Inoculate with Lactococcus lactis C15 and Lactobacillus brevis. Add 4% carob. Incubate at 30 °C (16 h), store at 4 °C (28 days). | [85] |
| Carob-based fermented drink | Poland | Roast carob at 130 °C and mill. Mix with other ingredients, boil (5 min), cool, and filter. Inoculate and ferment at 27 °C (4 h), then 34 °C (4 h). Cool. | [89] |
| Carob-based dairy drink | Laboratory | Toast whole pods (150 °C, 60 min) and crush. Mix ingredients, heat to 75 °C (2 min). Filter and store at 4 °C. | [91] |
| Carob juice | Laboratory | Wash, drain, and air-dry carob fruits (2 h); separate seeds. Mix pulp with water (1:2), stir at 43 °C (160 min). Centrifuge to separate juice. Pasteurize juice (63 °C, 30 min). Store at 4 °C. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzzanca, C.; D’Amico, A.; Pistorio, E.; Di Stefano, V.; Melilli, M.G. Carob-Based Functional Beverages: Nutritional Value and Health Properties. Beverages 2025, 11, 1. https://doi.org/10.3390/beverages11010001
Buzzanca C, D’Amico A, Pistorio E, Di Stefano V, Melilli MG. Carob-Based Functional Beverages: Nutritional Value and Health Properties. Beverages. 2025; 11(1):1. https://doi.org/10.3390/beverages11010001
Chicago/Turabian StyleBuzzanca, Carla, Angela D’Amico, Enrica Pistorio, Vita Di Stefano, and Maria Grazia Melilli. 2025. "Carob-Based Functional Beverages: Nutritional Value and Health Properties" Beverages 11, no. 1: 1. https://doi.org/10.3390/beverages11010001
APA StyleBuzzanca, C., D’Amico, A., Pistorio, E., Di Stefano, V., & Melilli, M. G. (2025). Carob-Based Functional Beverages: Nutritional Value and Health Properties. Beverages, 11(1), 1. https://doi.org/10.3390/beverages11010001







