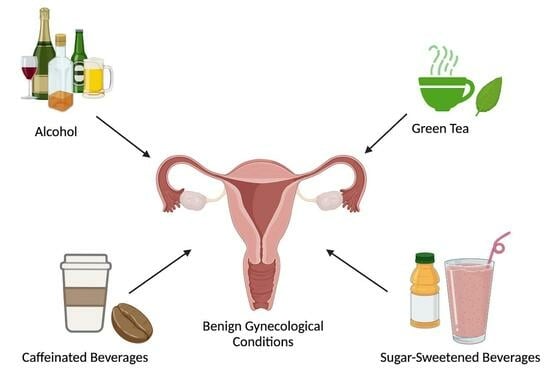
Common Beverage Consumption and Benign Gynecological Conditions
Abstract
1. Introduction
2. Uterine Fibroids
2.1. Uterine Fibroids and Sugar-Sweetened Beverages
2.2. Uterine Fibroids and Caffeinated Beverages
2.3. Uterine Fibroids and Green Tea
2.4. Uterine Fibroids and Alcohol
3. Endometriosis
3.1. Endometriosis and Sugar-Sweetened Beverages
3.2. Endometriosis and Caffeinated Beverages
3.3. Endometriosis and Green Tea
3.4. Endometriosis and Alcohol
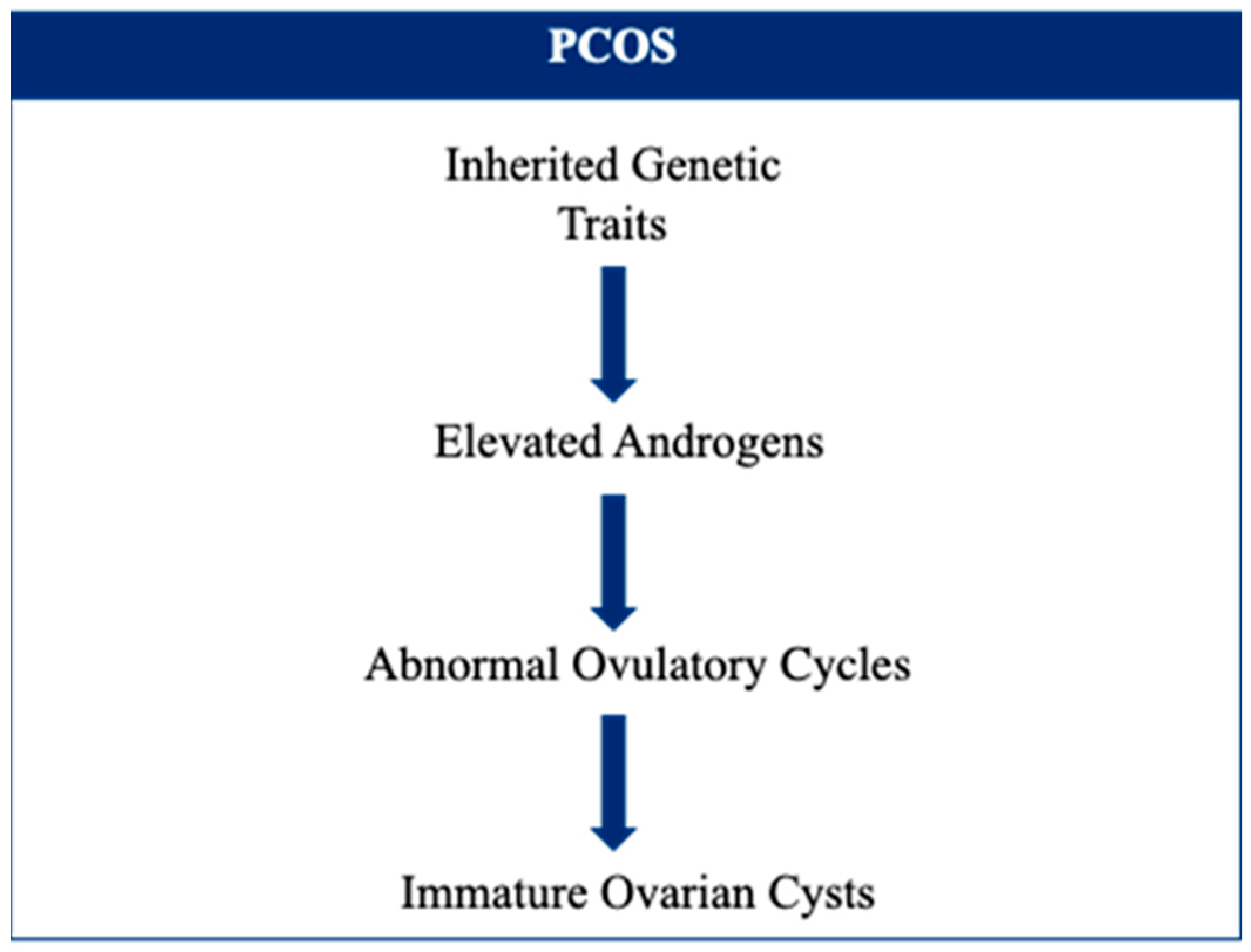
4. Polycystic Ovary Syndrome
4.1. PCOS and Sugar-Sweetened Beverages
4.2. PCOS and Caffeinated Beverages
4.3. PCOS and Green Tea
4.4. PCOS and Alcohol
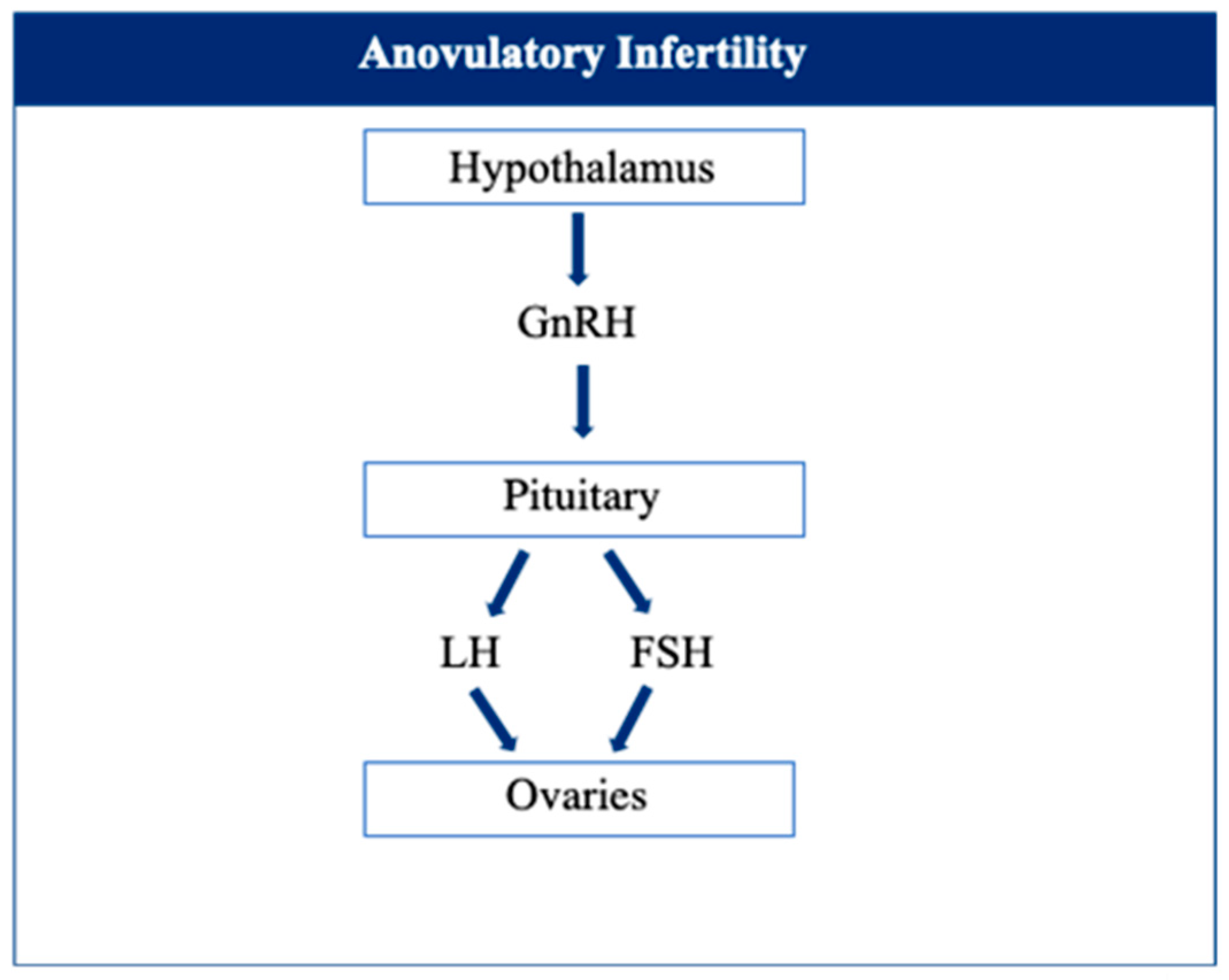
5. Anovulatory Infertility
5.1. Anovulatory Infertility and Sugar-Sweetened Beverages
5.2. Anovulatory Infertility and Caffeinated Beverages
5.3. Anovulatory Infertility and Green Tea
5.4. Anovulatory Infertility and Alcohol
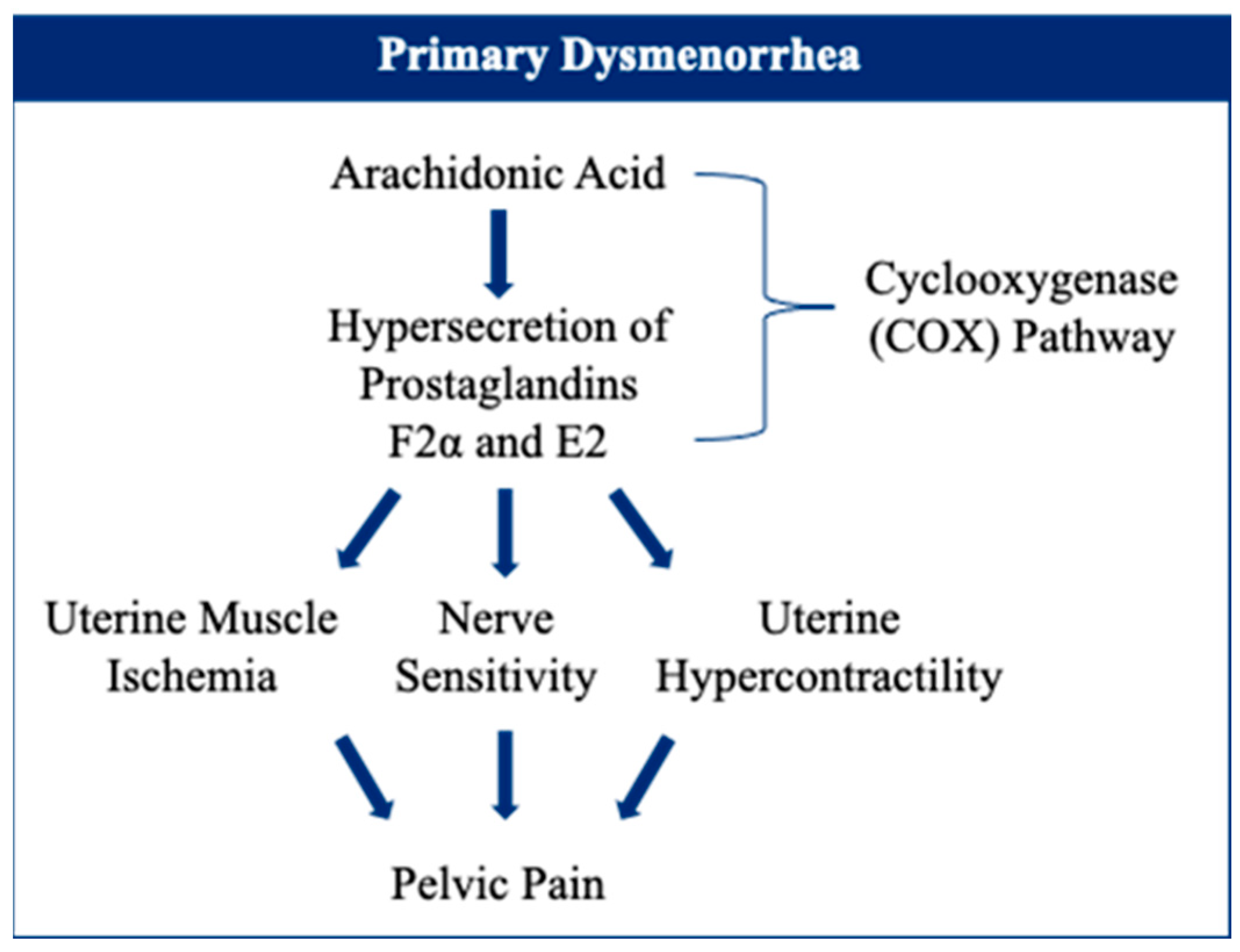
6. Primary Dysmenorrhea
6.1. Primary Dysmenorrhea and Sugar-Sweetened Beverages
6.2. Primary Dysmenorrhea and Caffeinated Beverages
6.3. Primary Dysmenorrhea and Green Tea
6.4. Primary Dysmenorrhea and Alcohol
7. Limitations
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wijeratne, D.; Fiander, A. Gynaecological Disease in the Developing World: A Silent Pandemic. Obstet. Gynaecol. 2018, 20, 237–244. [Google Scholar] [CrossRef]
- Lara-Castor, L.; Micha, R.; Cudhea, F.; Miller, V.; Shi, P.; Zhang, J.; Sharib, J.R.; Erndt-Marino, J.; Cash, S.B.; Mozaffarian, D.; et al. Sugar-Sweetened Beverage Intakes among Adults between 1990 and 2018 in 185 Countries. Nat. Commun. 2023, 14, 5957. [Google Scholar] [CrossRef]
- Meredith, S.E.; Juliano, L.M.; Hughes, J.R.; Griffiths, R.R. Caffeine Use Disorder: A Comprehensive Review and Research Agenda. J. Caffeine Res. 2013, 3, 114–130. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic Changes in Amino Acids, Catechins, Caffeine and Gallic Acid in Green Tea during Withering. J. Food Compos. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of Tea Components with Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef]
- Chen, D.; Dou, Q. Tea Polyphenols and Their Roles in Cancer Prevention and Chemotherapy. Int. J. Mol. Sci. 2008, 9, 1196–1206. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Salamt, N.; Zaid, S.S.M.; Mokhtar, M.H. Beneficial Effects of Green Tea Catechins on Female Reproductive Disorders: A Review. Molecules 2021, 26, 2675. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH Assay Comparison to Assess Antioxidant Capacity of Tea Infusions: Relationship between Total Polyphenol and Individual Catechin Content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols for Health Promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. de Kombuchas from Green and Black Teas Have Different Phenolic Profile, Which Impacts Their Antioxidant Capacities, Antibacterial and Antiproliferative Activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green Tea Preparation and Its Influence on the Content of Bioactive Compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.-G.; Shibamoto, T. Antioxidative Activities of Volatile Extracts from Green Tea, Oolong Tea, and Black Tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Information System on Alcohol and Health; World Health Organization: Geneva, Switzerland, 2018.
- Holdsworth-Carson, S.J.; Zaitseva, M.; Vollenhoven, B.J.; Rogers, P.A.W. Clonality of Smooth Muscle and Fibroblast Cell Populations Isolated from Human Fibroid and Myometrial Tissues. MHR Basic Sci. Reprod. Med. 2014, 20, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Day Baird, D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High Cumulative Incidence of Uterine Leiomyoma in Black and White Women: Ultrasound Evidence. Am. J. Obs. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Laughlin-Tommaso, S.K.; Jacoby, V.L.; Myers, E.R. Disparities in Fibroid Incidence, Prognosis, and Management. Obs. Gynecol. Clin. N. Am. 2017, 44, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and Management of Uterine Fibroids. Int. J. Gynecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.; Ezzati, M.; Segars, J.H.; McCarthy, K. The Impact of Uterine Leiomyomas on Reproductive Outcomes. Minerva Ginecol. 2010, 62, 225–236. [Google Scholar] [PubMed]
- Ezzati, M.; Norian, J.M.; Segars, J.H. Management of Uterine Fibroids in the Patient Pursuing Assisted Reproductive Technologies. Women’s Health 2009, 5, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Fritton, K.; Borahay, M. New and Emerging Therapies for Uterine Fibroids. Semin. Reprod. Med. 2017, 35, 549–559. [Google Scholar] [CrossRef]
- Radin, R.G.; Palmer, J.R.; Rosenberg, L.; Kumanyika, S.K.; Wise, L.A. Dietary Glycemic Index and Load in Relation to Risk of Uterine Leiomyomata in the Black Women’s Health Study. Am. J. Clin. Nutr. 2010, 91, 1281–1288. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Added Sugars Drive Insulin Resistance, Hyperinsulinemia, Hypertension, Type 2 Diabetes and Coronary Heart Disease. Mo. Med. 2022, 119, 519–523. [Google Scholar] [PubMed]
- Baird, D.D.; Travlos, G.; Wilson, R.; Dunson, D.B.; Hill, M.C.; D’Aloisio, A.A.; London, S.J.; Schectman, J.M. Uterine Leiomyomata in Relation to Insulin-like Growth Factor-I, Insulin, and Diabetes. Epidemiology 2009, 20, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.D.; Hopkins, B.D.; Cantley, L.C. Dietary Fat and Sugar in Promoting Cancer Development and Progression. Annu. Rev. Cancer Biol. 2019, 3, 255–273. [Google Scholar] [CrossRef]
- AlAshqar, A.; Reschke, L.; Kirschen, G.W.; Borahay, M.A. Role of Inflammation in Benign Gynecologic Disorders: From Pathogenesis to Novel Therapies. Biol. Reprod. 2021, 105, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Reschke, L.; Afrin, S.; El Sabah, M.; Charewycz, N.; Miyashita-Ishiwata, M.; Borahay, M.A. Leptin Induces Leiomyoma Cell Proliferation and Extracellular Matrix Deposition via JAK2/STAT3 and MAPK/ERK Pathways. F S Sci. 2022, 3, 383–391. [Google Scholar] [CrossRef]
- ALASHQAR, A.; EL OUWEINI, H.; GORNET, M.; YENOKYAN, G.; BORAHAY, M.A. Cardiometabolic Profile of Women with Uterine Leiomyoma: A Cross-Sectional Study. Minerva Obstet. Gynecol. 2023, 75, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Ramaiyer, M.; Begum, U.A.M.; Borahay, M.A. Adipocyte and Adipokines Promote a Uterine Leiomyoma Friendly Microenvironment. Nutrients 2023, 15, 715. [Google Scholar] [CrossRef]
- Afrin, S.; El Sabah, M.; Manzoor, A.; Miyashita-Ishiwata, M.; Reschke, L.; Borahay, M.A. Adipocyte Coculture Induces a Pro-Inflammatory, Fibrotic, Angiogenic, and Proliferative Microenvironment in Uterine Leiomyoma Cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166564. [Google Scholar] [CrossRef]
- Afrin, S.; Kirschen, G.W.; Borahay, M.A. Obesity Contributes to Transformation of Myometrial Stem-Cell Niche to Leiomyoma via Inducing Oxidative Stress, DNA Damage, Proliferation, and Extracellular Matrix Deposition. Genes 2023, 14, 1625. [Google Scholar] [CrossRef]
- AlAshqar, A.; Patzkowsky, K.; Afrin, S.; Wild, R.; Taylor, H.S.; Borahay, M.A. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obs. Gynecol. Surv. 2019, 74, 661–673. [Google Scholar] [CrossRef]
- Wise, L.A. Risk of Uterine Leiomyomata in Relation to Tobacco, Alcohol and Caffeine Consumption in the Black Women’s Health Study. Hum. Reprod. 2004, 19, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.; Samuels, S.; Young, J.; Gerthoux, P.M.; Needham, L.; Patterson, D.; Olive, D.; Gavoni, N.; Vercellini, P.; et al. Serum Dioxin Concentrations and Risk of Uterine Leiomyoma in the Seveso Women’s Health Study. Am. J. Epidemiol. 2007, 166, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F. Diet and Uterine Myomas. Obstet. Gynecol. 1999, 94, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Lucero, J.; Harlow, B.L.; Barbieri, R.L.; Sluss, P.; Cramer, D.W. Early Follicular Phase Hormone Levels in Relation to Patterns of Alcohol, Tobacco, and Coffee Use. Fertil. Steril. 2001, 76, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Cai, M.X.; Thomas, P.E.; Conney, A.H.; Zhu, B.T. Characterization of the Oxidative Metabolites of 17β-Estradiol and Estrone Formed by 15 Selectively Expressed Human Cytochrome P450 Isoforms. Endocrinology 2003, 144, 3382–3398. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.A.; Iwasaki, M.; Guengerich, F.P.; Kadlubar, F.F. Human Cytochrome P-450PA (P-450IA2), the Phenacetin O-Deethylase, Is Primarily Responsible for the Hepatic 3-Demethylation of Caffeine and N-Oxidation of Carcinogenic Arylamines. Proc. Natl. Acad. Sci. USA 1989, 86, 7696–7700. [Google Scholar] [CrossRef] [PubMed]
- Leonard, T.K.; Watson, R.R.; Mohs, M.E. The Effects of Caffeine on Various Body Systems: A Review. J. Am. Diet. Assoc. 1987, 87, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Sisti, J.S.; Hankinson, S.E.; Caporaso, N.E.; Gu, F.; Tamimi, R.M.; Rosner, B.; Xu, X.; Ziegler, R.; Eliassen, A.H. Caffeine, Coffee, and Tea Intake and Urinary Estrogens and Estrogen Metabolites in Premenopausal Women. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Parish, M.; Massoud, G.; Hazimeh, D.; Segars, J.; Islam, M.S. Green Tea in Reproductive Cancers: Could Treatment Be as Simple? Cancers 2023, 15, 862. [Google Scholar] [CrossRef]
- Hazimeh, D.; Massoud, G.; Parish, M.; Singh, B.; Segars, J.; Islam, M.S. Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage? Nutrients 2023, 15, 1439. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green Tea Extract Inhibits Proliferation of Uterine Leiomyoma Cells In Vitro and in Nude Mice. Am. J. Obs. Gynecol. 2010, 202, 289.e1–289.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Rajaratnam, V.; Al-Hendy, A. Antiproliferative and Proapoptotic Effects of Epigallocatechin Gallate on Human Leiomyoma Cells. Fertil. Steril. 2010, 94, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Rajaratnam, V.; Al-Hendy, O.; Halder, S.; Al-Hendy, A. Green Tea Extract Inhibition of Human Leiomyoma Cell Proliferation Is Mediated via Catechol-O-Methyltransferase. Gynecol. Obs. Investig. 2014, 78, 109–118. [Google Scholar] [CrossRef]
- Ozercan, I.H.; Sahin, N.; Akdemir, F.; Onderci, M.; Seren, S.; Sahin, K.; Kucuk, O. Chemoprevention of Fibroid Tumors by [−]-Epigallocatechin-3-Gallate in Quail. Nutr. Res. 2008, 28, 92–97. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Roshdy; Rajaratnam; Maitra; Sabry, M. Ait Allah Treatment of Symptomatic Uterine Fibroids with Green Tea Extract: A Pilot Randomized Controlled Clinical Study. Int. J. Women’s Health 2013, 5, 477–486. [Google Scholar] [CrossRef]
- Porcaro, G.; Santamaria, A.; Giordano, D.; Angelozzi, P. Vitamin D plus Epigallocatechin Gallate: A Novel Promising Approach for Uterine Myomas. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3344–3351. [Google Scholar] [CrossRef] [PubMed]
- Miriello, D.; Galanti, F.; Cignini, P.; Antonaci, D.; Schiavi, M.C.; Rago, R. Uterine Fibroids Treatment: Do We Have New Valid Alternative? Experiencing the Combination of Vitamin D plus Epigallocatechin Gallate in Childbearing Age Affected Women. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2843–2851. [Google Scholar] [CrossRef]
- Salama, S.A.; Ho, S.-L.; Wang, H.-Q.; Tenhunen, J.; Tilgmann, C.; Al-Hendy, A. Hormonal Regulation of Catechol-O-Methyl Transferase Activity in Women with Uterine Leiomyomas. Fertil. Steril. 2006, 86, 259–262. [Google Scholar] [CrossRef]
- Salama, S.A.; Ben Nasr, A.; Dubey, R.K.; Al-Hendy, A. Estrogen Metabolite 2-Methoxyestradiol Induces Apoptosis and Inhibits Cell Proliferation and Collagen Production in Rat and Human Leiomyoma Cells: A Potential Medicinal Treatment for Uterine Fibroids. J. Soc. Gynecol. Investig. 2006, 13, 542–550. [Google Scholar] [CrossRef]
- Navarro, A.; Bariani, M.V.; Yang, Q.; Al-Hendy, A. Understanding the Impact of Uterine Fibroids on Human Endometrium Function. Front. Cell Dev. Biol. 2021, 9, 633180. [Google Scholar] [CrossRef]
- Islam, M.S.; Parish, M.; Brennan, J.T.; Winer, B.L.; Segars, J.H. Targeting Fibrotic Signaling Pathways by EGCG as a Therapeutic Strategy for Uterine Fibroids. Sci. Rep. 2023, 13, 8492. [Google Scholar] [CrossRef] [PubMed]
- Templeman, C.; Marshall, S.F.; Clarke, C.A.; DeLellis Henderson, K.; Largent, J.; Neuhausen, S.; Reynolds, P.; Ursin, G.; Bernstein, L. Risk Factors for Surgically Removed Fibroids in a Large Cohort of Teachers. Fertil. Steril. 2009, 92, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Nakamura, K.; Oba, S.; Hayashi, M.; Takeda, N.; Yasuda, K. Association of Intakes of Fat, Dietary Fibre, Soya Isoflavones and Alcohol with Uterine Fibroids in Japanese Women. Br. J. Nutr. 2009, 101, 1427. [Google Scholar] [CrossRef]
- Kim, S.; Han, K.; Choi, S.-Y.; Yang, S.Y.; Choi, S.H.; Yim, J.Y.; Kim, J.J.; Kim, M.-J. Alcohol Consumption and the Risk of New-Onset Uterine Leiomyomas: A Nationwide Population-Based Study in 2.5 Million Korean Women Aged 20 to 39 Years. Am. J. Obs. Gynecol. 2023, 229, 45.e1–45.e18. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, S.E.; Willett, W.C.; Manson, J.E.; Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Longcope, C.; Speizer, F.E. Alcohol, Height, and Adiposity in Relation to Estrogen and Prolactin Levels in Postmenopausal Women. JNCI J. Natl. Cancer Inst. 1995, 87, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Muti, P.; Trevisan, M.; Micheli, A.; Krogh, V.; Bolelli, G.; Sciajno, R.; Schünemann, H.J.; Berrino, F. Alcohol Consumption and Total Estradiol in Premenopausal Women. Cancer Epidemiol. Biomark. Prev. 1998, 7, 189–193. [Google Scholar]
- Reichman, M.E.; Judd, J.T.; Longcope, C.; Schatzkin, A.; Clevidence, B.A.; Nair, P.P.; Campbell, W.S.; Taylor, P.R. Effects of Alcohol Consumption on Plasma and Urinary Hormone Concentrations in Premenopausal Women. JNCI J. Natl. Cancer Inst. 1993, 85, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, E.S. Effects of Alcohol Ingestion on Estrogens in Postmenopausal Women. JAMA J. Am. Med. Assoc. 1996, 276, 1747. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Imir, G.; Utsunomiya, H.; Thung, S.; Gurates, B.; Tamura, M.; Lin, Z. Aromatase in Endometriosis and Uterine Leiomyomata. J. Steroid Biochem. Mol. Biol. 2005, 95, 57–62. [Google Scholar] [CrossRef]
- Wise, L.A.; Laughlin-Tommaso, S.K. Epidemiology of Uterine Fibroids. Clin. Obs. Gynecol. 2016, 59, 2–24. [Google Scholar] [CrossRef]
- Bulun, S.E. Uterine Fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.V. Estrogen, Alcohol Consumption, and Breast Cancer. Alcohol. Clin. Exp. Res. 2011, 35, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Oyesanmi, O.; Snyder, D.; Sullivan, N.; Reston, J.; Treadwell, J.; Schoelles, K.M. Alcohol Consumption and Cancer Risk: Understanding Possible Causal Mechanisms for Breast and Colorectal Cancers; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2010; pp. 1–151.
- Englund, K.; Blanck, A.; Gustavsson, I.; Lundkvist, U.; Sjöblom, P.; Norgren, A.; Lindblom, B. Sex Steroid Receptors in Human Myometrium and Fibroids: Changes during the Menstrual Cycle and Gonadotropin-Releasing Hormone Treatment. J. Clin. Endocrinol. Metab. 1998, 83, 4092–4096. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone Is Essential for Maintenance and Growth of Uterine Leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef]
- Wise, L.A. Reproductive Factors, Hormonal Contraception, and Risk of Uterine Leiomyomata in African-American Women: A Prospective Study. Am. J. Epidemiol. 2004, 159, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Decherney, A.H. Medical Management of Endometriosis. Clin. Obs. Gynecol. 2017, 60, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Missmer, S.A. Pathophysiology, Diagnosis, and Management of Endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and Treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef]
- Zhou, J.; Chern, B.S.M.; Barton-Smith, P.; Phoon, J.W.L.; Tan, T.Y.; Viardot-Foucault, V.; Ku, C.W.; Tan, H.H.; Chan, J.K.Y.; Lee, Y.H. Peritoneal Fluid Cytokines Reveal New Insights of Endometriosis Subphenotypes. Int. J. Mol. Sci. 2020, 21, 3515. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Alashqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients 2021, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive Intake of Sugar: An Accomplice of Inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Holtz, D.N.; Schmidt, N.; Kolipaka, S.; Hata, E.; Sutton, M.; Znayenko-Miller, T.; Hazen, N.D.; Cobb, C.; Kahleova, H. Nutrition in the Prevention and Treatment of Endometriosis: A Review. Front. Nutr. 2023, 10, 1089891. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Troiano, E.; Mazza, S.; Ferro, Y.; Abbinante, A.; Agneta, M.T.; Montalcini, T.; Pujia, A. The Impact of Endometriosis on Dietary Choices and Activities of Everyday Life: A Cross-Sectional Study. Front. Nutr. 2023, 10, 1273976. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-P.; Zhang, Y.-Y.; Jin, J.-N.; Ying, Y.; Song, Z.-M.; Xu, Q.-Q.; Tu, M.-X.; Ye, X.-H.; Tang, H.-N.; Ni, F.-D.; et al. Effects of Dysregulated Glucose Metabolism on the Occurrence and ART Outcome of Endometriosis. Eur. J. Med. Res. 2023, 28, 305. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Viganò, P.; Candiani, M.; Fedele, L. Diet and Endometriosis Risk: A Literature Review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Kuja-Halkola, R.; Tornvall, P.; Marions, L. Reproductive and Lifestyle Factors Associated with Endometriosis in a Large Cross-Sectional Population Sample. J. Women’s Health 2017, 26, 152–158. [Google Scholar] [CrossRef]
- Hemmert, R.; Schliep, K.C.; Willis, S.; Peterson, C.M.; Louis, G.B.; Allen-Brady, K.; Simonsen, S.E.; Stanford, J.B.; Byun, J.; Smith, K.R. Modifiable Life Style Factors and Risk for Incident Endometriosis. Paediatr. Perinat. Epidemiol. 2019, 33, 19–25. [Google Scholar] [CrossRef]
- Parazzini, F.; Chiaffarino, F.; Surace, M.; Chatenoud, L.; Cipriani, S.; Chiantera, V.; Benzi, G.; Fedele, L. Selected Food Intake and Risk of Endometriosis. Hum. Reprod. 2004, 19, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Marshall, L.M.; Hunter, D.J. Incidence of Laparoscopically Confirmed Endometriosis by Demographic, Anthropometric, and Lifestyle Factors. Am. J. Epidemiol. 2004, 160, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Bravi, F.; Cipriani, S.; Parazzini, F.; Ricci, E.; Viganò, P.; La Vecchia, C. Coffee and Caffeine Intake and Risk of Endometriosis: A Meta-Analysis. Eur. J. Nutr. 2014, 53, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.S.; Triantafyllidis, K.K.; Kyriakidou, M.; Giannos, P.; Kalliala, I.; Veroniki, A.A.; Paraskevaidi, M.; Kyrgiou, M. The Relation between Caffeine Consumption and Endometriosis: An Updated Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3457. [Google Scholar] [CrossRef] [PubMed]
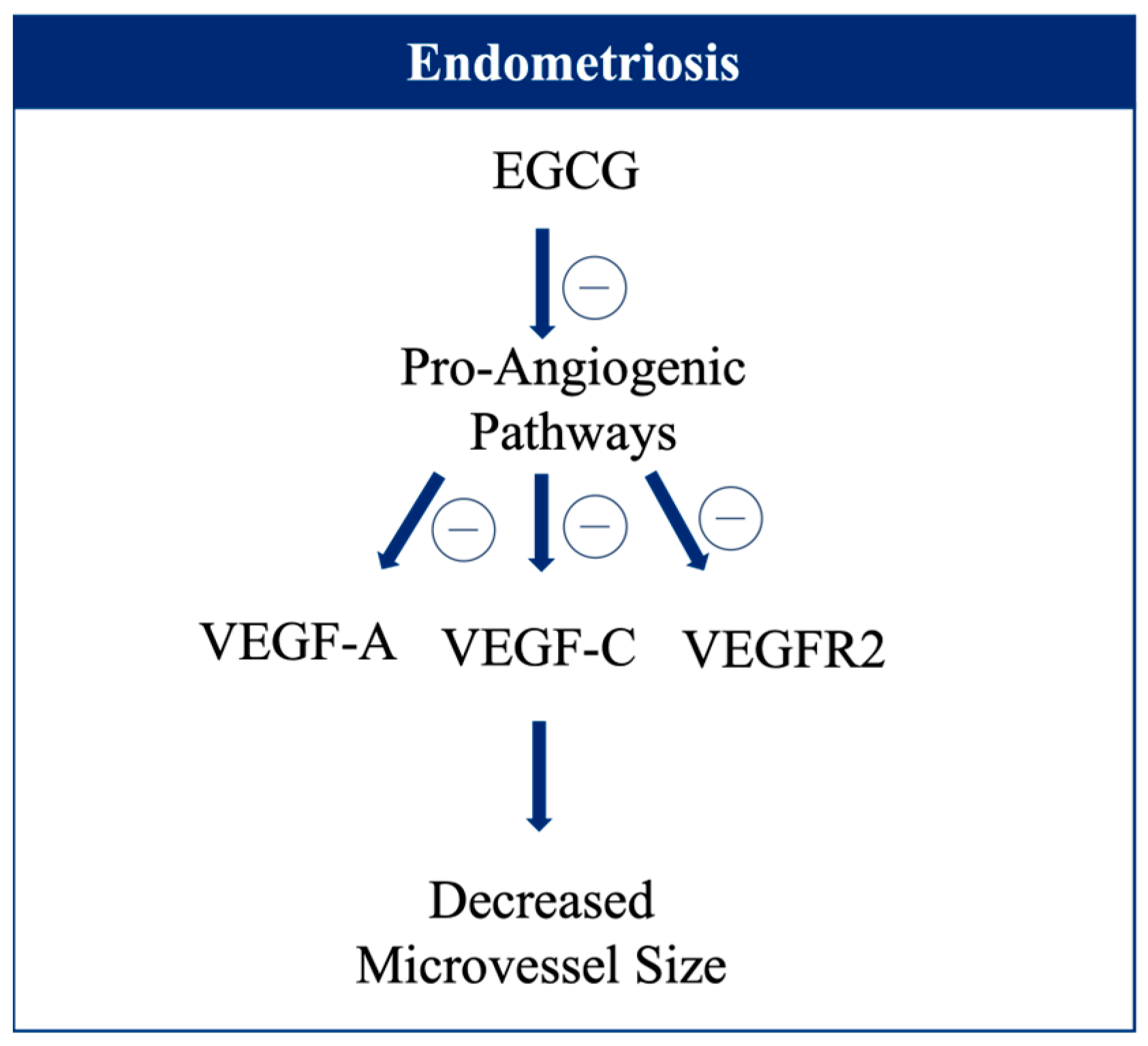
- Chen, X.; Man, G.C.W.; Hung, S.W.; Zhang, T.; Fung, L.W.Y.; Cheung, C.W.; Chung, J.P.W.; Li, T.C.; Wang, C.C. Therapeutic Effects of Green Tea on Endometriosis. Crit. Rev. Food Sci. Nutr. 2023, 63, 3222–3235. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Xu, H.; Man, G.C.W.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.Y.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of Green Tea Epigallocatechin-3-Gallate (Pro-EGCG) as a Potent Anti-Angiogenesis Agent for Endometriosis in Mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lui, W.T.; Chu, C.Y.; Ng, P.S.; Wang, C.C.; Rogers, M.S. Anti-Angiogenic Effects of Green Tea Catechin on an Experimental Endometriosis Mouse Model. Hum. Reprod. 2009, 24, 608–618. [Google Scholar] [CrossRef]
- Laschke, M.W.; Schwender, C.; Scheuer, C.; Vollmar, B.; Menger, M.D. Epigallocatechin-3-Gallate Inhibits Estrogen-Induced Activation of Endometrial Cells in Vitro and Causes Regression of Endometriotic Lesions In Vivo. Hum. Reprod. 2008, 23, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wai Man, G.C.; Wang, C.C. Green Tea Epigallocatechin-3-Gallate Inhibits Angiogenesis and Suppresses Vascular Endothelial Growth Factor C/Vascular Endothelial Growth Factor Receptor 2 Expression and Signaling in Experimental Endometriosis In Vivo. Fertil. Steril. 2011, 96, 1021–1028.e1. [Google Scholar] [CrossRef]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural Therapies Assessment for the Treatment of Endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef]
- Guan, Q.H.; Shi, W.J.; Zhou, L.S.; Tao, A.L.; Li, L. Effect of Epigallocatechin-3-Gallate on the Status of DNA Methylation of E-Cadherin Promoter Region on Endometriosis Mouse. J. Obstet. Gynaecol. Res. 2020, 46, 2076–2083. [Google Scholar] [CrossRef]
- Matalliotakis, I.M.; Cakmak, H.; Fragouli, Y.G.; Goumenou, A.G.; Mahutte, N.G.; Arici, A. Epidemiological Characteristics in Women with and without Endometriosis in the Yale Series. Arch. Gynecol. Obs. 2008, 277, 389–393. [Google Scholar] [CrossRef]
- Ek, M.; Roth, B.; Nilsson, P.M.; Ohlsson, B. Characteristics of Endometriosis: A Case-Cohort Study Showing Elevated IgG Titers against the TSH Receptor (TRAb) and Mental Comorbidity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 8–14. [Google Scholar] [CrossRef]
- Parazzini, F.; Cipriani, S.; Bravi, F.; Pelucchi, C.; Chiaffarino, F.; Ricci, E.; Viganò, P. A Metaanalysis on Alcohol Consumption and Risk of Endometriosis. Am. J. Obs. Gynecol. 2013, 209, 106.e1–106.e10. [Google Scholar] [CrossRef]
- Li Piani, L.; Chiaffarino, F.; Cipriani, S.; Viganò, P.; Somigliana, E.; Parazzini, F. A Systematic Review and Meta-Analysis on Alcohol Consumption and Risk of Endometriosis: An Update from 2012. Sci. Rep. 2022, 12, 19122. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 131, e157–e171. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, Prevalence, and Phenotypes of Polycystic Ovary Syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Riestenberg, C.; Jagasia, A.; Markovic, D.; Buyalos, R.P.; Azziz, R. Health Care-Related Economic Burden of Polycystic Ovary Syndrome in the United States: Pregnancy-Related and Long-Term Health Consequences. J. Clin. Endocrinol. Metab. 2022, 107, 575–585. [Google Scholar] [CrossRef]
- Walker, M.H.; Tobler, K.J. Female Infertility; StatPearls: Treasure Island, CA, USA, 2019. [Google Scholar]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of Polycystic Ovary Syndrome in a Dutch Twin-Family Study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef]
- Kosova, G.; Urbanek, M. Genetics of the Polycystic Ovary Syndrome. Mol. Cell. Endocrinol. 2013, 373, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with Polycystic Ovary Syndrome Have Intrinsic Insulin Resistance on Euglycaemic-Hyperinsulaemic Clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Misso, M.L.; Wild, R.A.; Norman, R.J. Impaired Glucose Tolerance, Type 2 Diabetes and Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2010, 16, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Brower, M.A.; Hai, Y.; Jones, M.R.; Guo, X.; Chen, Y.-D.I.; Rotter, J.I.; Krauss, R.M.; Legro, R.S.; Azziz, R.; Goodarzi, M.O. Bidirectional Mendelian Randomization to Explore the Causal Relationships between Body Mass Index and Polycystic Ovary Syndrome. Hum. Reprod. 2019, 34, 127–136. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 3346–3360. [Google Scholar] [CrossRef]
- Roberts, J.S.; Perets, R.A.; Sarfert, K.S.; Bowman, J.J.; Ozark, P.A.; Whitworth, G.B.; Blythe, S.N.; Toporikova, N. High-Fat High-Sugar Diet Induces Polycystic Ovary Syndrome in a Rodent Model. Biol. Reprod. 2017, 96, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Volk, K.M.; Pogrebna, V.V.; Roberts, J.A.; Zachry, J.E.; Blythe, S.N.; Toporikova, N. High-Fat, High-Sugar Diet Disrupts the Preovulatory Hormone Surge and Induces Cystic Ovaries in Cycling Female Rats. J. Endocr. Soc. 2017, 1, 1488–1505. [Google Scholar] [CrossRef]
- de Melo, G.B.; Soares, J.F.; Costa, T.C.L.; Benevides, R.O.A.; Vale, C.C.; Paes, A.M.D.A.; Gaspar, R.S. Early Exposure to High-Sucrose Diet Leads to Deteriorated Ovarian Health. Front. Endocrinol. 2021, 12, 656831. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-Rutkowska, J.; Ziętek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome—Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef]
- MacKenzie, T.; Comi, R.; Sluss, P.; Keisari, R.; Manwar, S.; Kim, J.; Larson, R.; Baron, J.A. Metabolic and Hormonal Effects of Caffeine: Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Metabolism 2007, 56, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Raoofi, A.; Rezaie, M.J.; Delbari, A.; Ghoreishi, S.A.-H.; Sichani, P.H.; Maleki, S.; Nasiry, D.; Akhlaghi, M.; Ebrahimi, V.; Mousavi Khaneghah, A. Therapeutic Potentials of the Caffeine in Polycystic Ovary Syndrome in a Rat Model. Allergol. Immunopathol. 2022, 50, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Deng, H.; Bu, X.-Q.; Li, T.; Zhong, Z.-H.; Tang, X.-J.; Feng, Q.; Fu, L.-J. Coffee Consumption and the Risk of Polycystic Ovary Syndrome: Evidence from a Case-Control Study. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Mombaini, E.; Jafarirad, S.; Husain, D.; Haghighizadeh, M.H.; Padfar, P. The Impact of Green Tea Supplementation on Anthropometric Indices and Inflammatory Cytokines in Women with Polycystic Ovary Syndrome. Phytother. Res. 2017, 31, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, H.; Allahdadian, M.; Zarre, F.; Ranjbar, H.; Allahdadian, F. Effect of Green Tea on Metabolic and Hormonal Aspect of Polycystic Ovarian Syndrome in Overweight and Obese Women Suffering from Polycystic Ovarian Syndrome: A Clinical Trial. J. Educ. Health Promot. 2017, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, M.; Barati, S.; Mahmoodi, M.; Barati Mosleh, A.; Yavangui, M. Comparison of Green Tea and Metformin Effects on Anthropometric Indicators in Women with Polycystic Ovarian Syndrome: A Clinical Trial Study. J. Rep. Pharm. Sci. 2020, 9, 97. [Google Scholar] [CrossRef]
- Chan, C.C.W.; Koo, M.W.L.; Ng, E.H.Y.; Tang, O.-S.; Yeung, W.S.B.; Ho, P.-C. Effects of Chinese Green Tea on Weight, and Hormonal and Biochemical Profiles in Obese Patients with Polycystic Ovary Syndrome—A Randomized Placebo-Controlled Trial. J. Soc. Gynecol. Investig. 2006, 13, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Colonetti, L.; Grande, A.J.; Toreti, I.R.; Ceretta, L.B.; da Rosa, M.I.; Colonetti, T. Green Tea Promotes Weight Loss in Women with Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Nutr. Res. 2022, 104, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.T.; Loxton, D.; Bahri Khomami, M.; Teede, H.; Harrison, C.L.; Joham, A.E. High Prevalence of Medical Conditions and Unhealthy Lifestyle Behaviours in Women with PCOS during Preconception: Findings from the Australian Longitudinal Study on Women’s Health. Hum. Reprod. 2023, 38, 2267–2276. [Google Scholar] [CrossRef]
- Kazemi, M.; Kim, J.Y.; Wan, C.; Xiong, J.D.; Michalak, J.; Xavier, I.B.; Ganga, K.; Tay, C.T.; Grieger, J.A.; Parry, S.A.; et al. Comparison of Dietary and Physical Activity Behaviors in Women with and without Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of 39,471 Women. Hum. Reprod. Update 2022, 28, 910–955. [Google Scholar] [CrossRef]
- Hamilton-Fairley, D. Anovulation. BMJ 2003, 327, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Chera-aree, P.; Tanpong, S.; Thanaboonyawat, I.; Laokirkkiat, P. Clomiphene Citrate plus Letrozole versus Clomiphene Citrate Alone for Ovulation Induction in Infertile Women with Ovulatory Dysfunction: A Randomized Controlled Trial. BMC Women’s Health 2023, 23, 602. [Google Scholar] [CrossRef] [PubMed]
- Jurczewska, J.; Szostak-Węgierek, D. The Influence of Diet on Ovulation Disorders in Women—A Narrative Review. Nutrients 2022, 14, 1556. [Google Scholar] [CrossRef]
- Hatch, E.E.; Wesselink, A.K.; Hahn, K.A.; Michiel, J.J.; Mikkelsen, E.M.; Sorensen, H.T.; Rothman, K.J.; Wise, L.A. Intake of Sugar-Sweetened Beverages and Fecundability in a North American Preconception Cohort. Epidemiology 2018, 29, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. A Prospective Study of Dietary Carbohydrate Quantity and Quality in Relation to Risk of Ovulatory Infertility. Eur. J. Clin. Nutr. 2009, 63, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Gaskins, A.J.; Mansur, A.; Adir, M.; Racowsky, C.; Baccarelli, A.A.; Hauser, R.; Chavarro, J.E. Association between Preconception Maternal Beverage Intake and In Vitro Fertilization Outcomes. Fertil. Steril. 2017, 108, 1026–1033. [Google Scholar] [CrossRef]
- Hatch, E.E.; Wise, L.A.; Mikkelsen, E.M.; Christensen, T.; Riis, A.H.; Sørensen, H.T.; Rothman, K.J. Caffeinated Beverage and Soda Consumption and Time to Pregnancy. Epidemiology 2012, 23, 393–401. [Google Scholar] [CrossRef]
- Lyngsø, J.; Ramlau-Hansen, C.H.; Bay, B.; Ingerslev, H.J.; Hulman, A.; Kesmodel, U.S. Association between Coffee or Caffeine Consumption and Fecundity and Fertility: A Systematic Review and Dose-Response Meta-Analysis. Clin. Epidemiol. 2017, 9, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Caffeinated and Alcoholic Beverage Intake in Relation to Ovulatory Disorder Infertility. Epidemiology 2009, 20, 374–381. [Google Scholar] [CrossRef]
- Bu, F.L.; Feng, X.; Yang, X.Y.; Ren, J.; Cao, H.J. Relationship between Caffeine Intake and Infertility: A Systematic Review of Controlled Clinical Studies. BMC Women’s Health 2020, 20, 125. [Google Scholar] [CrossRef]
- Rahman, S.; Huang, Y.; Zhu, L.; Feng, S.; Khan, I.; Wu, J.; Li, Y.; Wang, X. Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review. Nutrients 2018, 10, 834. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.L. Potential Role of Green Tea Catechins in the Management of Oxidative Stress-Associated Infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.Y.; Marcus, M.; Taylor, K.C. The Association between Alcohol Intake and Fecundability during Menstrual Cycle Phases. Hum. Reprod. 2021, 36, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, J.S.; Kjær, S.K.; Holst, C.; Sharif, H.; Munk, C.; Osler, M.; Schmidt, L.; Andersen, A.M.N.; Grønbæk, M. Alcohol Use as Predictor for Infertility in a Representative Population of Danish Women. Acta Obs. Gynecol. Scand. 2003, 82, 744–749. [Google Scholar] [CrossRef]
- Eggert, J.; Theobald, H.; Engfeldt, P. Effects of Alcohol Consumption on Female Fertility during an 18-Year Period. Fertil. Steril. 2004, 81, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M.J. Primary Dysmenorrhea: Pathophysiology, Diagnosis, and Treatment Updates. Korean J. Fam. Med. 2022, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.A.; Fogleman, C.D. Dysmenorrhea. Am. Fam. Phys. 2021, 104, 164–170. [Google Scholar]
- Steege, O.F. The Prevalence of Dysmenorrhea, Dyspareunia, Pelvic Pain, and Irritable Bowel Syndrome in Primary Care Practices. Obstet. Gynecol. 1996, 87, 55–58. [Google Scholar]
- Harel, Z. Dysmenorrhea in Adolescents. Ann. N. Y. Acad. Sci. 2008, 1135, 185–195. [Google Scholar] [CrossRef]
- Ju, H.; Jones, M.; Mishra, G. The Prevalence and Risk Factors of Dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef]
- Armour, M.; Parry, K.; Manohar, N.; Holmes, K.; Ferfolja, T.; Curry, C.; MacMillan, F.; Smith, C.A. The Prevalence and Academic Impact of Dysmenorrhea in 21,573 Young Women: A Systematic Review and Meta-Analysis. J. Women’s Health 2019, 28, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M. Dysmenorrhea and Related Disorders. F1000Research 2017, 6, 1645. [Google Scholar]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Harada, T. Dysmenorrhea and Endometriosis in Young Women. Yonago Acta Medica 2013, 56, 81. [Google Scholar] [PubMed]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory Markers in Dysmenorrhea and Therapeutic Options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef] [PubMed]
- Sitter, T.; Haslinger, B.; Mandl, S.; Fricke, H.; Held, E.; Sellmayert, A. High Glucose Increases Prostaglandin E2 Synthesis in Human Peritoneal Mesothelial Cells: Role of Hyperosmolarity. J. Am. Soc. Nephrol. 1998, 9, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Gagua, T.; Tkeshelashvili, B.; Gagua, D. Primary Dysmenorrhea: Prevalence in Adolescent Population of Tbilisi, Georgia and Risk Factors. J. Turk. Ger. Gynecol. Assoc. 2012, 13, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ozerdogan, N.; Sayiner, D.; Ayranci, U.; Unsal, A.; Giray, S. Prevalence and Predictors of Dysmenorrhea among Students at a University in Turkey. Int. J. Gynecol. Obstet. 2009, 107, 39–43. [Google Scholar] [CrossRef]
- Muluneh, A.A.; Nigussie, T.S.; Gebreslasie, K.Z.; Anteneh, K.T.; Kassa, Z.Y. Prevalence and Associated Factors of Dysmenorrhea among Secondary and Preparatory School Students in Debremarkos Town, North-West Ethiopia. BMC Women’s Health 2018, 18, 57. [Google Scholar] [CrossRef]
- Najafi, N.; Khalkhali, H.; Moghaddam Tabrizi, F.; Zarrin, R. Major Dietary Patterns in Relation to Menstrual Pain: A Nested Case Control Study. BMC Women’s Health 2018, 18, 69. [Google Scholar] [CrossRef]
- Monday, I.; Anthony, P.; Olunu, E.; Otohinoyi, D.; Abiodun, S.; Owolabi, A.; Mobolaji, B.; Fakoya, A.O.J. Prevalence and Correlation between Diet and Dysmenorrhea among High School and College Students in Saint Vincent and Grenadines. Open Access Maced. J. Med. Sci. 2019, 7, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Ferrari, S.; Xholli, A.; Cannoletta, M.; Palma, F.; Romani, C.; Volpe, A.; Cagnacci, A. Prevalence of Menstrual Pain in Young Women: What Is Dysmenorrhea? J. Pain Res. 2012, 5, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tavallaee, M.; Joffres, M.R.; Corber, S.J.; Bayanzadeh, M.; Rad, M.M. The Prevalence of Menstrual Pain and Associated Risk Factors among Iranian Women. J. Obstet. Gynaecol. Res. 2011, 37, 442–451. [Google Scholar] [CrossRef]
- Duman, N.B.; Yıldırım, F.; Vural, G. Risk Factors for Primary Dysmenorrhea and the Effect of Complementary and Alternative Treatment Methods: Sample from Corum, Turkey. Int. J. Health Sci. 2022, 16, 35. [Google Scholar]
- Naraoka, Y.; Hosokawa, M.; Minato-Inokawa, S.; Sato, Y. Severity of Menstrual Pain Is Associated with Nutritional Intake and Lifestyle Habits. Healthcare 2023, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Molema, M.M.; Dekker, M.C.; Voermans, N.C.; van Engelen, B.G.; Aarnoutse, R.E. Caffeine and Muscle Cramps: A Stimulating Connection. Am. J. Med. 2007, 120, e1–e2. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial Effects of Green Tea: A Literature Review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-Gallate (EGCG): Chemical and Biomedical Perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Chen, D.; Huang, R.; Tian, Y.; Zhang, P.; Zhang, J. Association of Tea Drinking and Dysmenorrhoea among Reproductive-Age Women in Shanghai, China (2013–2015): A Cross-Sectional Study. BMJ Open 2019, 9, e026643. [Google Scholar] [CrossRef]
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, Inflammation, and Gut-Liver-Brain Interactions in Tissue Damage and Disease Development. World J. Gastroenterol. 2010, 16, 1304. [Google Scholar] [CrossRef]
- Nyirenda, T.; Nyagumbo, E.; Murewanhema, G.; Mukonowenzou, N.; Kagodora, S.B.; Mapfumo, C.; Bhebhe, M.; Mufunda, J. Prevalence of Dysmenorrhea and Associated Risk Factors among University Students in Zimbabwe. Women’s Health 2023, 19, 17455057231189549. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Tozzi, L.; Mezzopane, R.; Luchini, L.; Marchini, M.; Fedele, L. Cigarette_smoking,_alcohol_consumption,_and_risk.16 (1). Epidemiology 1994, 5, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, J. The Association Between Alcohol Consumption and Dysmenorrhea in University Students in North China. 2020. [Google Scholar] [CrossRef]
- Cedars Sinai Dysmenorrhea. Cedars Sinai 2024. Available online: https://www.cedars-sinai.org/health-library/diseases-and-conditions/d/dysmenorrhea.html#:~:text=Any%20woman%20can%20have%20painful,drink%20alcohol%20during%20their%20period (accessed on 12 February 2024).
- Boston Children’s Hospital Dysmenorrhea. Available online: https://www.childrenshospital.org/conditions/dysmenorrhea#:~:text=Any%20teen%20girl%20or%20woman,being%20overweight (accessed on 12 February 2024).
- Johns Hopkins Medicine Dysmenorrhea. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/dysmenorrhea#:~:text=While%20any%20woman%20can%20develop,tends%20to%20prolong%20menstrual%20pain) (accessed on 12 February 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, R.; Hazimeh, D.; Saad, E.E.; Olson, S.L.; Musselman, K.; Elgindy, E.; Borahay, M.A. Common Beverage Consumption and Benign Gynecological Conditions. Beverages 2024, 10, 33. https://doi.org/10.3390/beverages10020033
Michel R, Hazimeh D, Saad EE, Olson SL, Musselman K, Elgindy E, Borahay MA. Common Beverage Consumption and Benign Gynecological Conditions. Beverages. 2024; 10(2):33. https://doi.org/10.3390/beverages10020033
Chicago/Turabian StyleMichel, Rachel, Dana Hazimeh, Eslam E. Saad, Sydney L. Olson, Kelsey Musselman, Eman Elgindy, and Mostafa A. Borahay. 2024. "Common Beverage Consumption and Benign Gynecological Conditions" Beverages 10, no. 2: 33. https://doi.org/10.3390/beverages10020033
APA StyleMichel, R., Hazimeh, D., Saad, E. E., Olson, S. L., Musselman, K., Elgindy, E., & Borahay, M. A. (2024). Common Beverage Consumption and Benign Gynecological Conditions. Beverages, 10(2), 33. https://doi.org/10.3390/beverages10020033