Surgical Classification for Preclinical Rat Femoral Bone Defect Model: Standardization Based on Systematic Review, Anatomical Analysis and Virtual Surgery
Abstract
1. Introduction
2. Materials and Methods
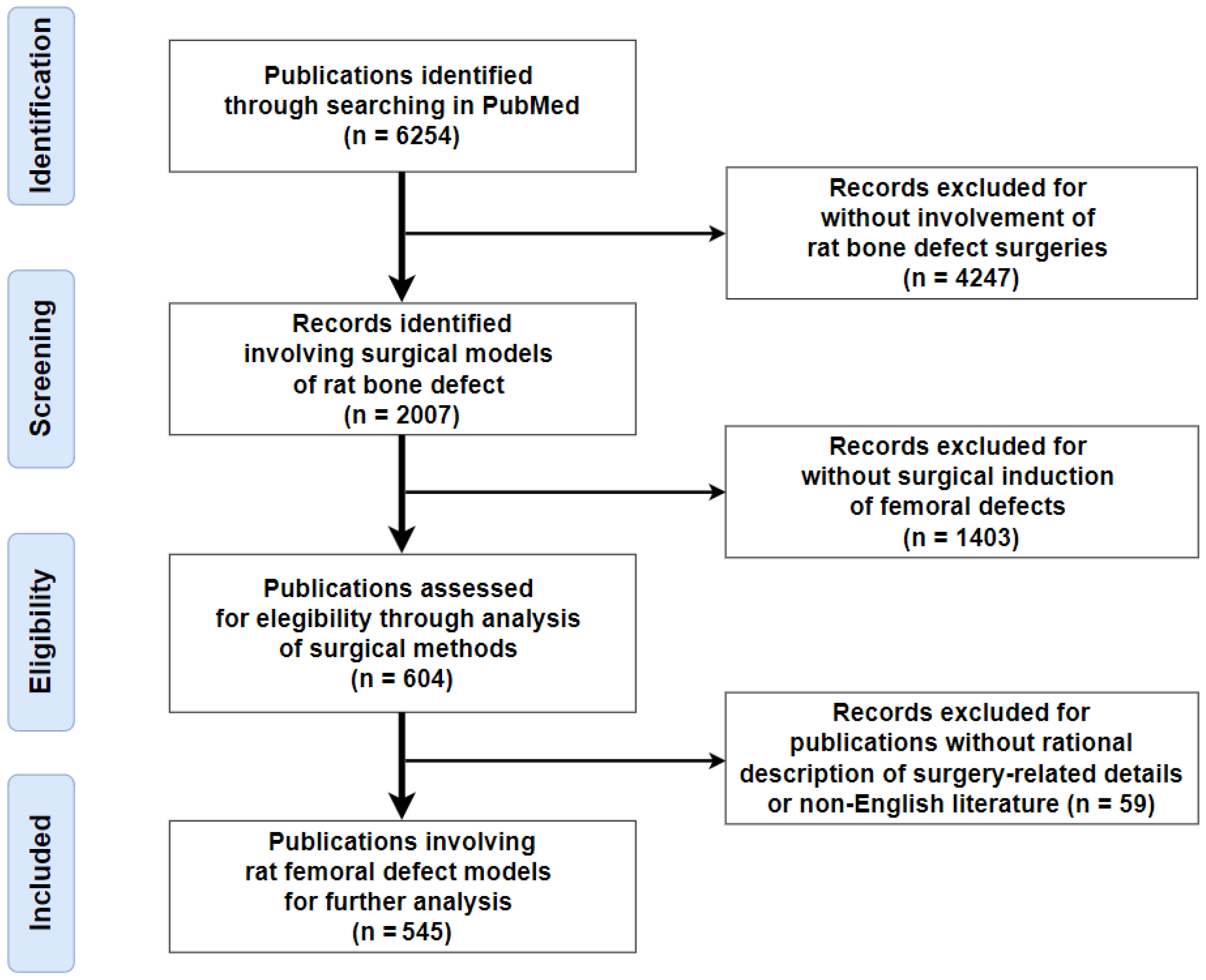
2.1. Literature Search Strategy, Criteria, and Study Selection
2.2. Micro-CT Data and Image Processing
3. Results
3.1. Systematic Review of the Literature Search Results
3.2. Establishment of the Surgical Classification System with Exemplar Illustrations
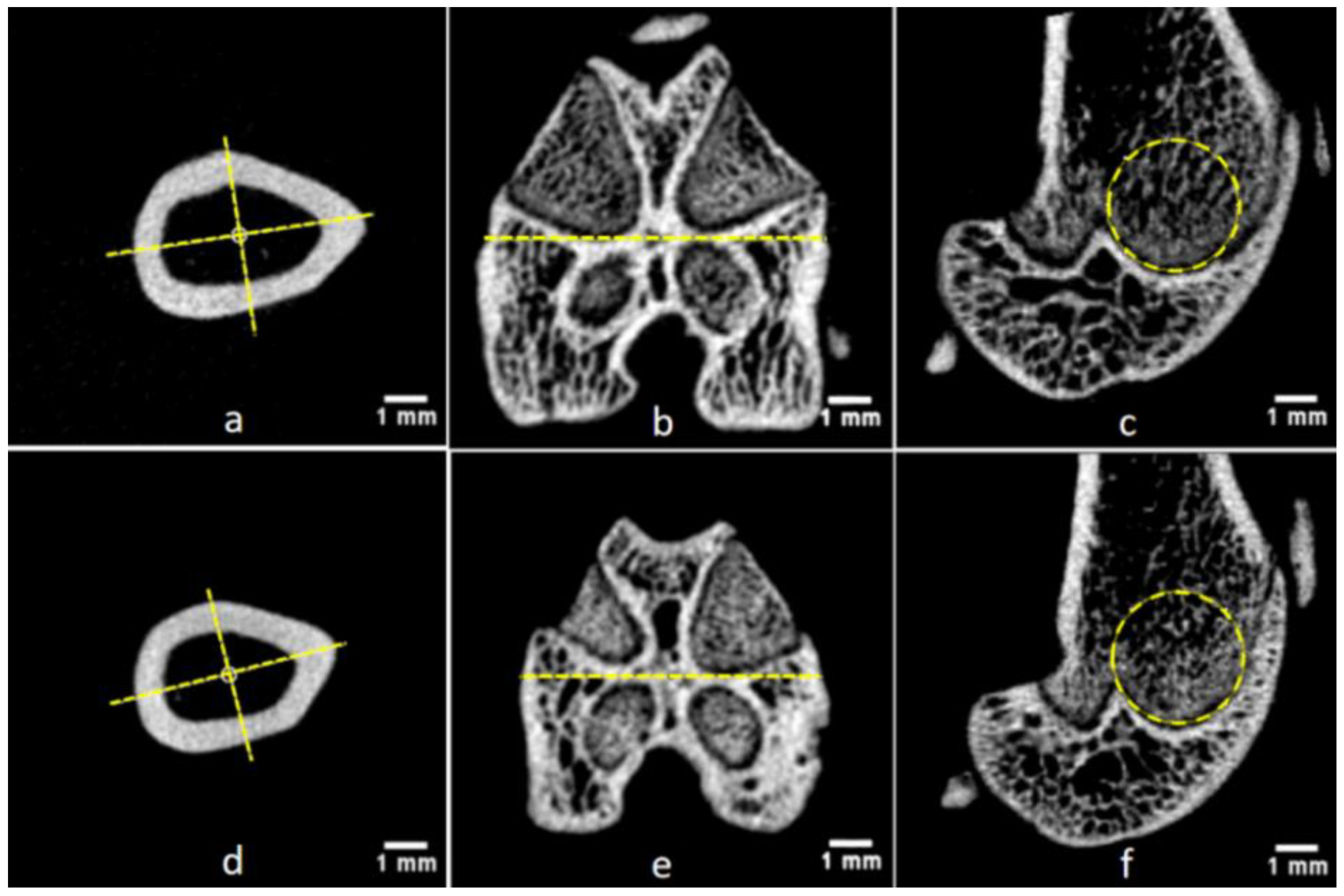
3.2.1. Identification of Anatomical Landmarks with Micro-CT Reconstruction
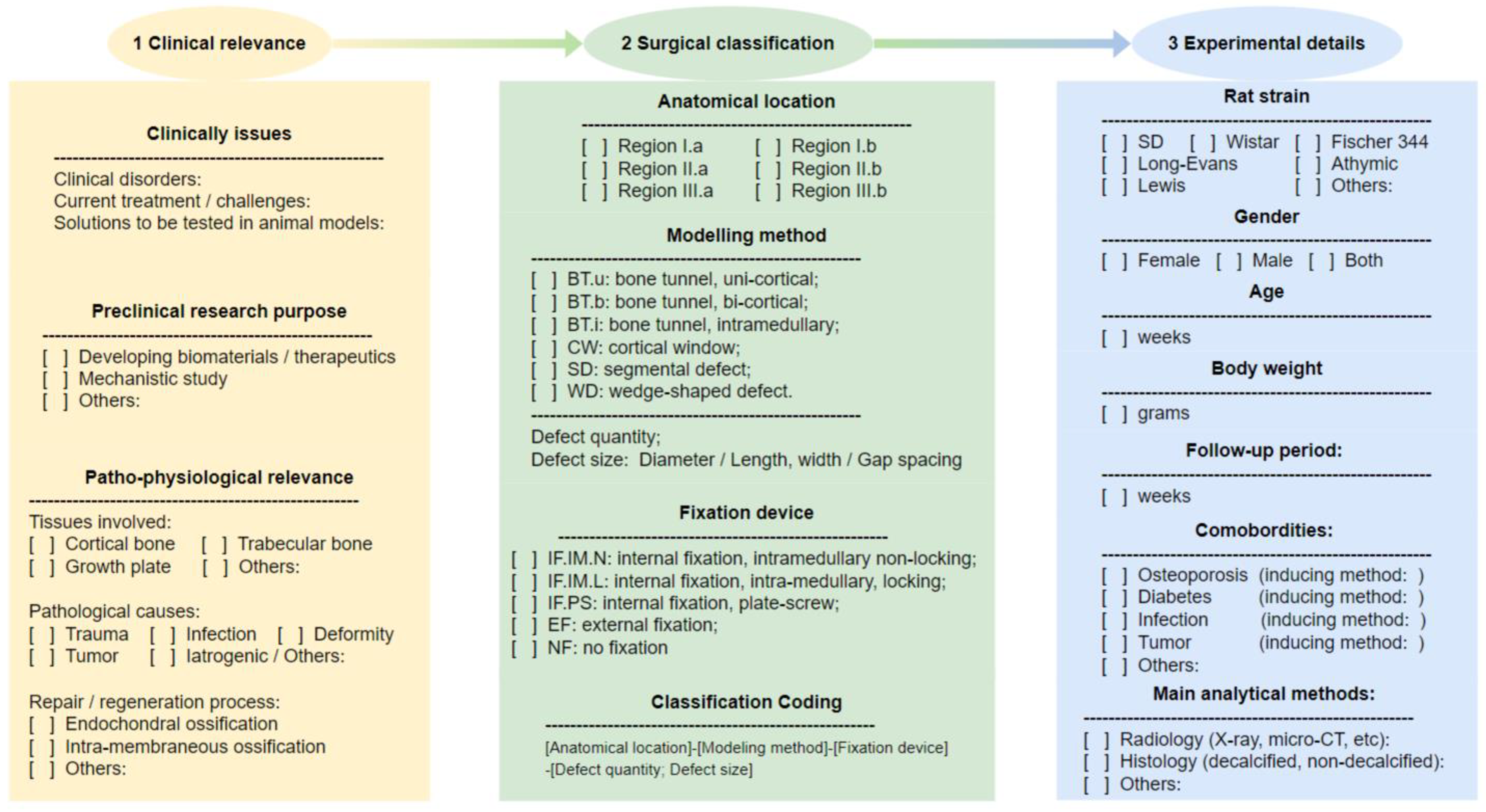
3.2.2. Definition of Anatomical Locations
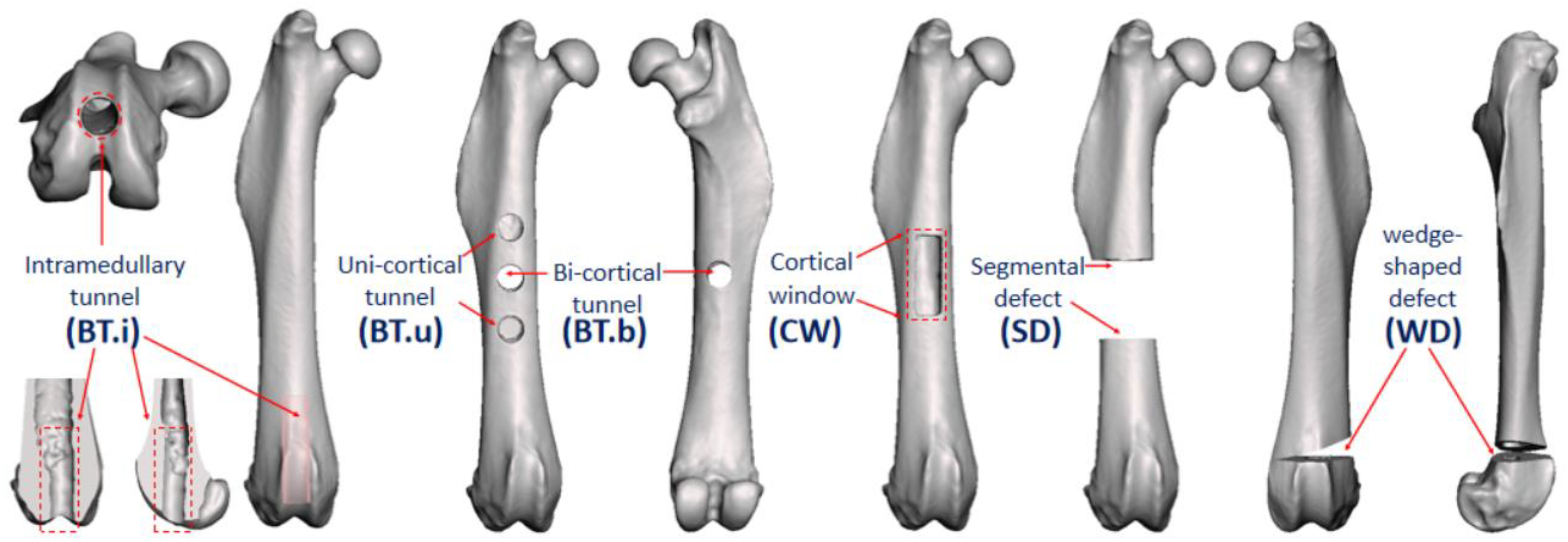
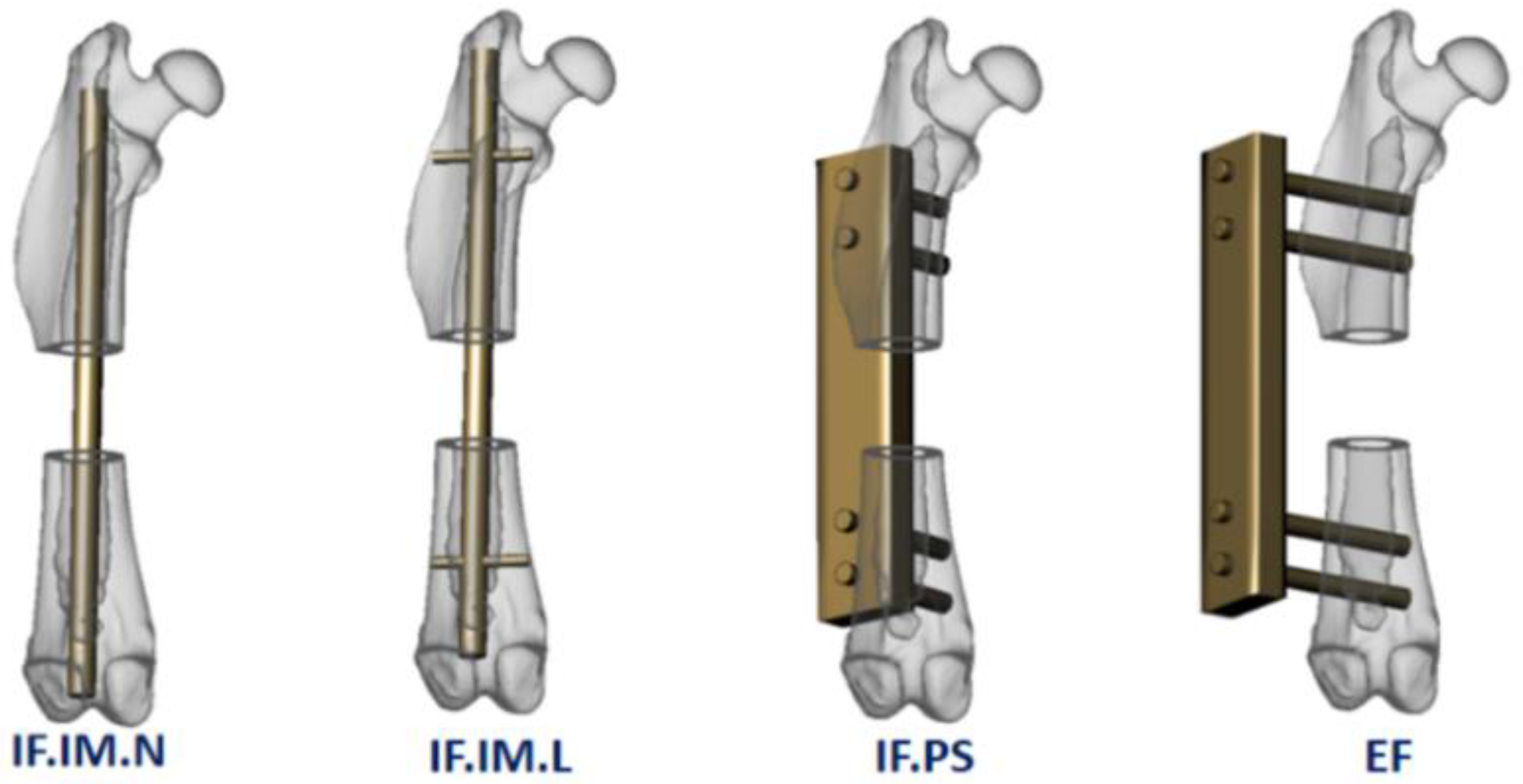
3.2.3. Classification of Modeling Methods and Fixation Devices
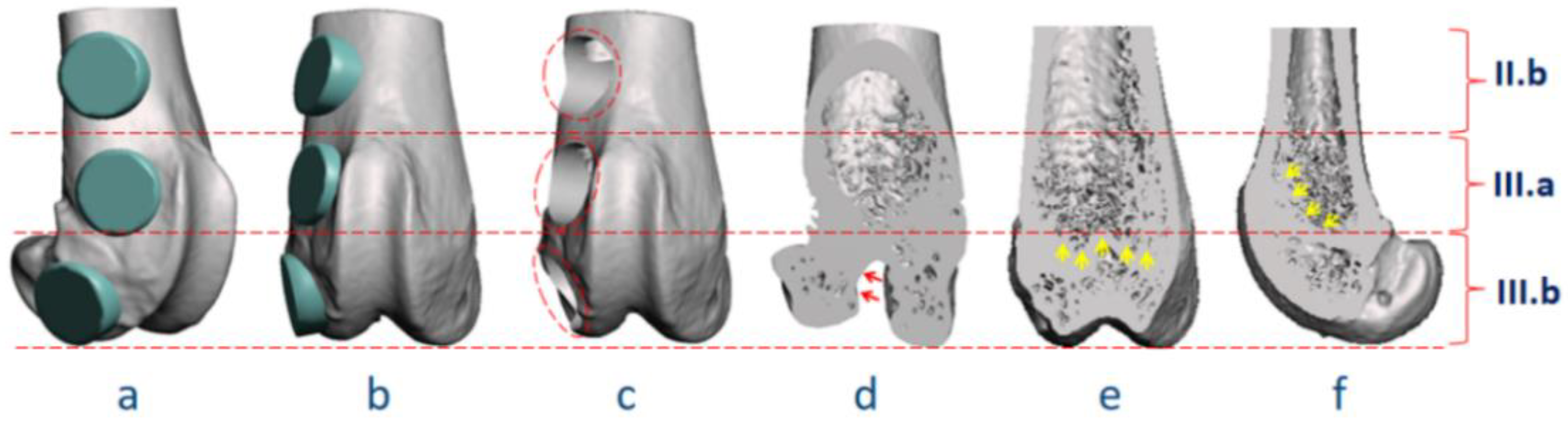
3.3. Analysis of Potential Surgical Pitfalls
3.4. Detection of Gender-Related Bone Size Difference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Migliorini, F.; La Padula, G.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 118. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Dziuba, D.; Meyer-Lindenberg, A.; Seitz, J.M.; Waizy, H.; Angrisani, N.; Reifenrath, J. Long-term in vivo degradation behaviour and biocompatibility of the magnesium alloy ZEK100 for use as a biodegradable bone implant. Acta Biomater. 2013, 9, 8548–8560. [Google Scholar] [CrossRef]
- Schaller, B.; Burkhard, J.P.M.; Chagnon, M.; Beck, S.; Imwinkelried, T.; Assad, M. Fracture Healing and Bone Remodeling With Human Standard-Sized Magnesium Versus Polylactide-Co-Glycolide Plate and Screw Systems Using a Mini-Swine Craniomaxillofacial Osteotomy Fixation Model. J. Oral Maxillofac. Surg. 2018, 76, 2138–2150. [Google Scholar] [CrossRef]
- Meyers, N.; Sukopp, M.; Jäger, R.; Steiner, M.; Matthys, R.; Lapatki, B.; Ignatius, A.; Claes, L. Characterization of interfragmentary motion associated with common osteosynthesis devices for rat fracture healing studies. PLoS ONE 2017, 12, e0176735. [Google Scholar] [CrossRef]
- Glatt, V.; Evans, C.H.; Matthys, R. Design, characterisation and in vivo testing of a new, adjustable stiffness, external fixator for the rat femur. Eur. Cell Mater. 2012, 23, 289–298. [Google Scholar] [CrossRef]
- Histing, T.; Garcia, P.; Holstein, J.H.; Klein, M.; Matthys, R.; Nuetzi, R.; Steck, R.; Laschke, M.W.; Wehner, T.; Bindl, R.; et al. Small animal bone healing models: Standards, tips, and pitfalls results of a consensus meeting. Bone 2011, 49, 591–599. [Google Scholar] [CrossRef]
- Sun, Y.; Helmholz, H.; Will, O.; Damm, T.; Wiese, B.; Luczak, M.; Peschke, E.; Luthringer-Feyerabend, B.; Ebel, T.; Hövener, J.B.; et al. Dynamic in vivo monitoring of fracture healing process in response to magnesium implant with multimodal imaging: Pilot longitudinal study in a rat external fixation model. Biomater. Sci. 2022, 10, 1532–1543. [Google Scholar] [CrossRef]
- Wang, H.; Fu, X.; Shi, J.; Li, L.; Sun, J.; Zhang, X.; Han, Q.; Deng, Y.; Gan, X. Nutrient Element Decorated Polyetheretherketone Implants Steer Mitochondrial Dynamics for Boosted Diabetic Osseointegration. Adv. Sci. 2021, 8, e2101778. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, L.; Ray, S.; Thormann, U.; Hillengass, J.; Delorme, S.; Schnettler, R.; Alt, V.; Bäuerle, T. Osteoporosis influences osteogenic but not angiogenic response during bone defect healing in a rat model. Injury 2013, 44, 923–929. [Google Scholar] [CrossRef]
- Chen, X.; Kidder, L.S.; Lew, W.D. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J. Orthop. Res. 2002, 20, 142–150. [Google Scholar] [CrossRef]
- ISO 10993-6; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. International Organization for Standardization: Geneva, Switzerland, 2016.
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The Impact of Bioceramic Scaffolds on Bone Regeneration in Preclinical In Vivo Studies: A Systematic Review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Marcazzan, S.; Weinstein, R.L.; Del Fabbro, M. Efficacy of platelets in bone healing: A systematic review on animal studies. Platelets 2018, 29, 326–337. [Google Scholar] [CrossRef]
- Peric, M.; Dumic-Cule, I.; Grcevic, D.; Matijasic, M.; Verbanac, D.; Paul, R.; Grgurevic, L.; Trkulja, V.; Bagi, C.M.; Vukicevic, S. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 2015, 70, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Chi, X.; Leu, M.C.; Ochoa, J. Image processing, geometric modeling and data management for development of a virtual bone surgery system. Comput. Aided Surg. 2008, 13, 30–40. [Google Scholar] [CrossRef]
- Sharkh, H.A.; Makhoul, N. In-House Surgeon-Led Virtual Surgical Planning for Maxillofacial Reconstruction. J. Oral Maxillofac. Surg. 2020, 78, 651–660. [Google Scholar] [CrossRef]
- Stamm, T.; Böttcher, D.; Kleinheinz, J. The University Münster model surgery system for orthognathic surgery—The digital update. Head Face Med. 2021, 17, 31. [Google Scholar] [CrossRef]
- Singh, G.D.; Singh, M. Virtual Surgical Planning: Modeling from the Present to the Future. J. Clin. Med. 2021, 10, 5655. [Google Scholar] [CrossRef] [PubMed]
- Lohre, R.; Warner, J.J.P.; Athwal, G.S.; Goel, D.P. The evolution of virtual reality in shoulder and elbow surgery. JSES Int. 2020, 4, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Debbaut, C.; De Wilde, D.; Casteleyn, C.; Cornillie, P.; Van Loo, D.; Van Hoorebeke, L.; Monbaliu, D.; Fan, Y.D.; Segers, P. Modeling the impact of partial hepatectomy on the hepatic hemodynamics using a rat model. IEEE Trans. Biomed. Eng. 2012, 59, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- van Houdt, C.I.A.; Ulrich, D.J.O.; Jansen, J.A.; van den Beucken, J.J.J.P. The performance of CPC/PLGA and Bio-Oss® for bone regeneration in healthy and osteoporotic rats. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 131–142. [Google Scholar] [CrossRef]
- Qing, W.; Guang-Xing, C.; Lin, G.; Liu, Y. The osteogenic study of tissue engineering bone with BMP2 and BMP7 gene-modified rat adipose-derived stem cell. J. Biomed. Biotechnol. 2012, 2012, 410879. [Google Scholar] [CrossRef]
- Oizumi, I.; Hamai, R.; Shiwaku, Y.; Mori, Y.; Anada, T.; Baba, K.; Miyatake, N.; Hamada, S.; Tsuchiya, K.; Nishimura, S.N.; et al. Impact of simultaneous hydrolysis of OCP and PLGA on bone induction of a PLGA-OCP composite scaffold in a rat femoral defect. Acta Biomater. 2021, 124, 358–373. [Google Scholar] [CrossRef]
- Liao, L.; Yang, S.; Miron, R.J.; Wei, J.; Zhang, Y.; Zhang, M. Osteogenic properties of PBLG-g-HA/PLLA nanocomposites. PLoS ONE 2014, 9, e105876. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xia, L.; Han, P.; Mao, L.; Wang, J.; Zhai, D.; Fang, B.; Chang, J.; Xiao, Y. Europium-Containing Mesoporous Bioactive Glass Scaffolds for Stimulating in Vitro and in Vivo Osteogenesis. ACS Appl. Mater. Interfaces 2016, 8, 11342–11354. [Google Scholar] [CrossRef] [PubMed]
- Hulsart-Billström, G.; Bergman, K.; Andersson, B.; Hilborn, J.; Larsson, S.; Jonsson, K.B. A uni-cortical femoral defect model in the rat: Evaluation using injectable hyaluronan hydrogel as a carrier for bone morphogenetic protein-2. J. Tissue Eng. Regen. Med. 2015, 9, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Han, L.; Lin, H.; Guo, Z.; Huang, Y.; Li, S.; Long, T.; Tang, W.; Tian, W.; Long, J. The role of antimiR-26a-5p/biphasic calcium phosphate in repairing rat femoral defects. Int. J. Mol. Med. 2019, 44, 857–870. [Google Scholar] [CrossRef]
- Pedriali, M.B.B.P.; Junior, W.T.; de Andrade, F.G.; Sangiorgio, J.P.M.; Pires, W.R.; De Paula Ramos, S. Bone regeneration in rat femoral defects after osteotomy with surgical ultrasound. Minerva Stomatol. 2016, 65, 1–10. [Google Scholar]
- Preininger, B.; Gerigk, H.; Bruckner, J.; Perka, C.; Schell, H.; Ellinghaus, A.; Schmidt-Bleek, K.; Duda, G. An experimental setup to evaluate innovative therapy options for the enhancement of bone healing using BMP as a benchmark—A pilot study. Eur. Cell Mater. 2012, 23, 262–271. [Google Scholar] [CrossRef]
- Liu, W.C.; Robu, I.S.; Patel, R.; Leu, M.C.; Velez, M.; Chu, T.M. The effects of 3D bioactive glass scaffolds and BMP-2 on bone formation in rat femoral critical size defects and adjacent bones. Biomed. Mater. 2014, 9, 045013. [Google Scholar] [CrossRef]
- Uchihara, Y.; Akahane, M.; Shimizu, T.; Ueha, T.; Morita, Y.; Nakasaki, S.; Kura, T.; Tohma, Y.; Kido, A.; Kawate, K.; et al. Osteogenic Matrix Cell Sheets Facilitate Osteogenesis in Irradiated Rat Bone. Biomed. Res. Int. 2015, 2015, 629168. [Google Scholar] [CrossRef]
- Gruber, H.E.; Gettys, F.K.; Montijo, H.E.; Starman, J.S.; Bayoumi, E.; Nelson, K.J.; Hoelscher, G.L.; Ramp, W.K.; Zinchenko, N.; Ingram, J.A.; et al. Genomewide molecular and biologic characterization of biomembrane formation adjacent to a methacrylate spacer in the rat femoral segmental defect model. J. Orthop. Trauma 2013, 27, 290–297. [Google Scholar] [CrossRef]
- Nau, C.; Seebach, C.; Trumm, A.; Schaible, A.; Kontradowitz, K.; Meier, S.; Buechner, H.; Marzi, I.; Henrich, D. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury 2016, 47, 325–334. [Google Scholar] [CrossRef]
- Leiblein, M.; Koch, E.; Winkenbach, A.; Schaible, A.; Nau, C.; Büchner, H.; Schröder, K.; Marzi, I.; Henrich, D. Size matters: Effect of granule size of the bone graft substitute (Herafill®) on bone healing using Masquelet’s induced membrane in a critical size defect model in the rat’s femur. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1469–1482. [Google Scholar] [CrossRef]
- Harrison, L.J.; Cunningham, J.L.; Strömberg, L.; Goodship, A.E. Controlled induction of a pseudarthrosis: A study using a rodent model. J. Orthop. Trauma 2003, 17, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Watanabe, Y.; Harada, N.; Abe, S.; Matsushita, T.; Yamanaka, K.; Kaneko, T.; Sakai, Y. Establishment of reproducible, critical-sized, femoral segmental bone defects in rats. Tissue Eng. Part C Methods. 2014, 20, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Alt, V.; Pan, L.; Thormann, U.; Schnettler, R.; Strauss, L.G.; Heinemann, S.; Schumacher, M.; Gelinsky, M.; Nies, B.; et al. Application of F-18-sodium fluoride (NaF) dynamic PET-CT (dPET-CT) for defect healing: A comparison of biomaterials in an experimental osteoporotic rat model. Med. Sci. Monit. 2014, 20, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Thormann, U.; Sommer, U.; Khassawna, T.E.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Lips, K.S.; Heiss, C.; Heinemann, S.; et al. Effects of macroporous, strontium loaded xerogel-scaffolds on new bone formation in critical-size metaphyseal fracture defects in ovariectomized rats. Injury 2016, 47 (Suppl. S1), S52–S61. [Google Scholar] [CrossRef]
- Alt, V.; Thormann, U.; Ray, S.; Zahner, D.; Dürselen, L.; Lips, K.; El Khassawna, T.; Heiss, C.; Riedrich, A.; Schlewitz, G.; et al. A new metaphyseal bone defect model in osteoporotic rats to study biomaterials for the enhancement of bone healing in osteoporotic fractures. Acta Biomater. 2013, 9, 7035–7042. [Google Scholar] [CrossRef]
- Montijo, H.E.; Kellam, J.F.; Gettys, F.K.; Starman, J.S.; Nelson, M.K.; Bayoumi, E.M.; Bosse, M.J.; Gruber, H.E. Utilization of the AO LockingRatNail in a novel rat femur critical defect model. J. Investig. Surg. 2012, 25, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.; Rotter, R.; Schattner, S.; Mittlmeier, T.; Claes, L.; Vollmar, B.; Gradl, G. Introduction of a new interlocked intramedullary nailing device for stabilization of critically sized femoral defects in the rat: A combined biomechanical and animal experimental study. J. Orthop. Res. 2008, 26, 184–189. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Salazar, B.P.; Bai, Y.; Gardner, M.J.; Yang, Y.P.; Stanford iTEAM Group; Pan, C.C.; Stahl, A.M.; Moeinzadeh, S.; Kim, S.; et al. A bioactive synthetic membrane improves bone healing in a preclinical nonunion model. Injury 2022, 53, 1368–1374. [Google Scholar] [CrossRef]
- Gallardo-Calero, I.; Barrera-Ochoa, S.; Manzanares, M.C.; Sallent, A.; Vicente, M.; López-Fernández, A.; De Albert, M.; Aguirre, M.; Soldado, F.; Vélez, R. Vascularized Periosteal Flaps Accelerate Osteointegration and Revascularization of Allografts in Rats. Clin. Orthop. Relat. Res. 2019, 477, 741–755. [Google Scholar] [CrossRef]
- Hara, K.; Hellem, E.; Yamada, S.; Sariibrahimoglu, K.; Mølster, A.; Gjerdet, N.R.; Hellem, S.; Mustafa, K.; Yassin, M.A. Efficacy of treating segmental bone defects through endochondral ossification: 3D printed designs and bone metabolic activities. Mater. Today Bio. 2022, 14, 100237. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Van der Jagt, O.P.; Amin Yavari, S.; De Haas, M.F.; Waarsing, J.H.; Jahr, H.; Van Lieshout, E.M.; Patka, P.; Verhaar, J.A.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2013, 31, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.R.; Smith, B.T.; Tatara, A.M.; Molina, E.R.; Lee, E.J.; Piepergerdes, T.C.; Uhrig, B.A.; Guldberg, R.E.; Bennett, G.N.; Wenke, J.C.; et al. Effects of Local Antibiotic Delivery from Porous Space Maintainers on Infection Clearance and Induction of an Osteogenic Membrane in an Infected Bone Defect. Tissue Eng. Part A 2017, 23, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Dupont, K.M.; Boerckel, J.D.; Stevens, H.Y.; Diab, T.; Kolambkar, Y.M.; Takahata, M.; Schwarz, E.M.; Guldberg, R.E. Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair. Cell Tissue Res. 2012, 347, 575–588. [Google Scholar] [CrossRef]
- Morishita, Y.; Naito, M.; Miyazaki, M.; He, W.; Wu, G.; Wei, F.; Sintuu, C.; Hymanson, H.; Brochmann, E.J.; Murray, S.S.; et al. Enhanced effects of BMP-binding peptide combined with recombinant human BMP-2 on the healing of a rodent segmental femoral defect. J. Orthop. Res. 2010, 28, 258–264. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Yu, L.; Zou, L.; Yang, L.; Lin, S.; Wang, J.; Yuan, Z.; Dai, J. Exosomal miR-335 derived from mature dendritic cells enhanced mesenchymal stem cell-mediated bone regeneration of bone defects in athymic rats. Mol. Med. 2021, 27, 20. [Google Scholar] [CrossRef]
- Burastero, G.; Scarfì, S.; Ferraris, C.; Fresia, C.; Sessarego, N.; Fruscione, F.; Monetti, F.; Scarfò, F.; Schupbach, P.; Podestà, M.; et al. The association of human mesenchymal stem cells with BMP-7 improves bone regeneration of critical-size segmental bone defects in athymic rats. Bone 2010, 47, 117–126. [Google Scholar] [CrossRef]
- Glatt, V.; Miller, M.; Ivkovic, A.; Liu, F.; Parry, N.; Griffin, D.; Vrahas, M.; Evans, C. Improved healing of large segmental defects in the rat femur by reverse dynamization in the presence of bone morphogenetic protein-2. J. Bone Jt. Surg. Am. 2012, 94, 2063–2073. [Google Scholar] [CrossRef]
- Širka, A.; Raina, D.B.; Isaksson, H.; Tanner, K.E.; Smailys, A.; Kumar, A.; Tarasevičius, Š.; Tägil, M.; Lidgren, L. Calcium Sulphate/Hydroxyapatite Carrier for Bone Formation in the Femoral Neck of Osteoporotic Rats. Tissue Eng. Part A 2018, 24, 1753–1764. [Google Scholar] [CrossRef]
- Raina, D.B.; Širka, A.; Qayoom, I.; Teotia, A.K.; Liu, Y.; Tarasevicius, S.; Tanner, K.E.; Isaksson, H.; Kumar, A.; Tägil, M.; et al. Long-Term Response to a Bioactive Biphasic Biomaterial in the Femoral Neck of Osteoporotic Rats. Tissue Eng. Part A 2020, 26, 1042–1051. [Google Scholar] [CrossRef]
- Qayoom, I.; Teotia, A.K.; Kumar, A. Nanohydroxyapatite Based Ceramic Carrier Promotes Bone Formation in a Femoral Neck Canal Defect in Osteoporotic Rats. Biomacromolecules 2020, 21, 328–337. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, T.; Li, C.; Shi, X.; Liu, X.; Yang, X.; Sun, H. Improvement of intertrochanteric bone quality in osteoporotic female rats after injection of polylactic acid-polyglycolic acid copolymer/collagen type I microspheres combined with bone mesenchymal stem cells. Int. Orthop. 2012, 36, 2163–2171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Zötsch, S.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Song, D.H.; Kim, S.H.; Jung, Y.; Kim, S.J. Development and characterization of various osteoarthritis models for tissue engineering. PLoS ONE 2018, 13, e0194288. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Shimizu, Y.; Imai, Y.; Mukai, T.; Yamamoto, A.; Sano, Y.; Ikeo, N.; Isozaki, S.; Takahashi, T.; Oikawa, M.; et al. In vivo corrosion behaviour of magnesium alloy in association with surrounding tissue response in rats. Biomed. Mater. 2016, 11, 025001. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.; Adam, R.; Biță, A.; Miculescu, M.; Trante, O.; Petrescu, I.M.; Pogărășteanu, M. Comparative Assessment of In Vitro and In Vivo Biodegradation of Mg-1Ca Magnesium Alloys for Orthopedic Applications. Materials 2020, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Slob, A.K.; Van der Werff Ten Bosch, J.J. Sex differences in body growth in the rat. Physiol. Behav. 1975, 14, 353–361. [Google Scholar] [CrossRef]
- Santiago, H.A.; De Pierro, L.R.; Reis, R.M.; Caluz, A.G.; Ribeiro, V.B.; Volpon, J.B. Allometric relationships among body mass, MUZZLE-tail length, and tibia length during the growth of Wistar rats. Acta Cir. Bras. 2015, 30, 743–748. [Google Scholar] [CrossRef]
- Sontag, W. Age-dependent morphometric alterations in the distal femora of male and female rats. Bone 1992, 13, 297–310. [Google Scholar] [CrossRef]
- Iida, H.; Fukuda, S. Age-related changes in bone mineral density, cross-sectional area and strength at different skeletal sites in male rats. J. Vet. Med. Sci. 2002, 64, 29–34. [Google Scholar] [CrossRef][Green Version]
- Fukuda, S.; Iida, H. Age-related changes in bone mineral density, cross-sectional area and the strength of long bones in the hind limbs and first lumbar vertebra in female Wistar rats. J. Vet. Med. Sci. 2004, 66, 755–760. [Google Scholar] [CrossRef] [PubMed]
- M’gregor, A.N. The Repair of Bone, with special reference to Transplantation and other Artificial Aids. J. Anat. Physiol. 1892, 26, 220–230. [Google Scholar] [PubMed]
- Huntington, T.W. VI. Case of Bone Transference: Use of a Segment of Fibula to Supply a Defect in the Tibia. Ann. Surg. 1905, 41, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar] [CrossRef]
- Strech, D.; Dirnagl, U. 3Rs missing: Animal research without scientific value is unethical. BMJ Open Sci. 2019, 3, e000035. [Google Scholar] [CrossRef]
- Spanagel, R. Ten Points to Improve Reproducibility and Translation of Animal Research. Front. Behav. Neurosci. 2022, 16, 869511. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Shang, Z.; Zhao, B.; Jiao, M.; Liu, W.; Cheng, M.; Zhai, B.; Guo, Y.; Liu, B.; et al. Biodegradable metals for bone defect repair: A systematic review and meta-analysis based on animal studies. Bioact. Mater. 2021, 6, 4027–4052. [Google Scholar] [CrossRef]
- de Vries, R.B.; Wever, K.E.; Avey, M.T.; Stephens, M.L.; Sena, E.S.; Leenaars, M. The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies. ILAR J. 2014, 55, 427–437. [Google Scholar] [CrossRef]
- van Luijk, J.; Bakker, B.; Rovers, M.M.; Ritskes-Hoitinga, M.; de Vries, R.B.; Leenaars, M. Systematic reviews of animal studies; missing link in translational research? PLoS ONE 2014, 9, e89981. [Google Scholar] [CrossRef]
- Roach, H.I.; Mehta, G.; Oreffo, R.O.; Clarke, N.M.; Cooper, C. Temporal analysis of rat growth plates: Cessation of growth with age despite presence of a physis. J. Histochem. Cytochem. 2003, 51, 373–383. [Google Scholar] [CrossRef]
- Wu, P.K.; Chen, C.F.; Chen, C.M.; Tsai, S.W.; Cheng, Y.C.; Chang, M.C.; Chen, W.M. Grafting for bone defects after curettage of benign bone tumor—Analysis of factors influencing the bone healing. J. Chin. Med. Assoc. 2018, 81, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Strube, P.; Mehta, M.; Baerenwaldt, A.; Trippens, J.; Wilson, C.J.; Ode, A.; Perka, C.; Duda, G.N.; Kasper, G. Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone 2009, 45, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.A.; Yu, Y.; Yee, G.; Low, A.K.; Diwan, A.D.; Walsh, W.R. Poor histological healing of a femoral fracture following 12 months of oestrogen deficiency in rats. Osteoporos. Int. 2013, 24, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- La Fontaine, J.; Chen, C.; Hunt, N.; Jude, E.; Lavery, L. Type 2 Diabetes and Metformin Influence on Fracture Healing in an Experimental Rat Model. J. Foot Ankle Surg. 2016, 55, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Hadeed, A.; Werntz, R.L.; Varacallo, M. External Fixation Principles and Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Histing, T.; Holstein, J.H.; Garcia, P.; Matthys, R.; Kristen, A.; Claes, L.; Menger, M.D.; Pohlemann, T. Ex vivo analysis of rotational stiffness of different osteosynthesis techniques in mouse femur fracture. J. Orthop. Res. 2009, 27, 1152–1156. [Google Scholar] [CrossRef]
- Claes, L.E.; Heigele, C.A.; Neidlinger-Wilke, C.; Kaspar, D.; Seidl, W.; Margevicius, K.J.; Augat, P. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 1998, 355, S132–S147. [Google Scholar] [CrossRef]
- Bekos, A.; Sioutis, S.; Kostroglou, A.; Saranteas, T.; Mavrogenis, A.F. The history of intramedullary nailing. Int. Orthop. 2021, 45, 1355–1361. [Google Scholar] [CrossRef]
- Grant, W.T.; Wang, G.J.; Balian, G. Type X collagen synthesis during endochondral ossification in fracture repair. J. Biol. Chem. 1987, 262, 9844–9849. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef]



| Figure | Classification Code | Detailed Explanation | |||
|---|---|---|---|---|---|
| Anatomical Region | Surgical Method | Fixation Device | Defect Quantity; Defect Size | ||
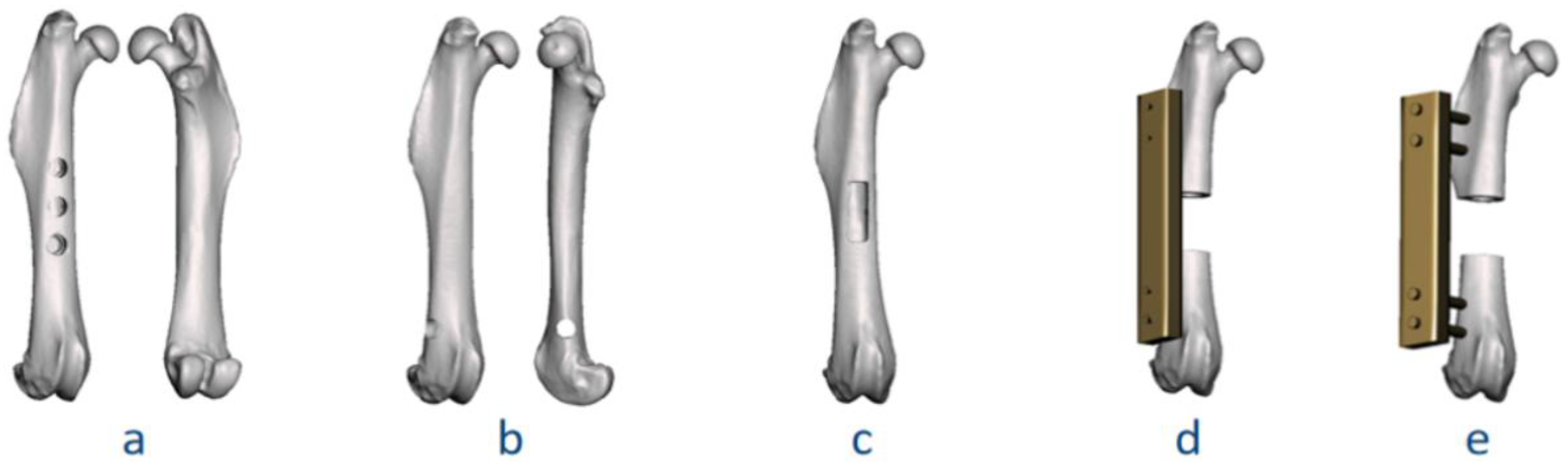
| 7a | II.a-BT.u-NF-(3; 2 mm) | II.a | BT.u: uni-cortical tunnel | NF: no fixation | Quantity: 3; Diameter: 2 mm |
| 7b | II.b-BT.b-NF-(1; 2 mm) | II.b | BT.b: bi-cortical tunnel | NF: no fixation | Quantity: 1; Diameter: 2 mm |
| 7c | II.a-CW-NF-(1; 6 mm, 2 mm) | II.a | CW: cortical window | NF: no fixation | Quantity: 1; Length: 6 mm, width: 2 mm |
| 7d | II.a-SD-IF.PS-(1; 6 mm) | II.a | SD: segmental defect | IF.PS: internal fixation, plate-screw | Quantity: 1; Gap size: 6 mm |
| 7e | II.a-SD-EF-(1; 6 mm) | II.a | SD: segmental defect | EF: external fixation | Quantity: 1; Gap size: 6 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Helmholz, H.; Willumeit-Römer, R. Surgical Classification for Preclinical Rat Femoral Bone Defect Model: Standardization Based on Systematic Review, Anatomical Analysis and Virtual Surgery. Bioengineering 2022, 9, 476. https://doi.org/10.3390/bioengineering9090476
Sun Y, Helmholz H, Willumeit-Römer R. Surgical Classification for Preclinical Rat Femoral Bone Defect Model: Standardization Based on Systematic Review, Anatomical Analysis and Virtual Surgery. Bioengineering. 2022; 9(9):476. https://doi.org/10.3390/bioengineering9090476
Chicago/Turabian StyleSun, Yu, Heike Helmholz, and Regine Willumeit-Römer. 2022. "Surgical Classification for Preclinical Rat Femoral Bone Defect Model: Standardization Based on Systematic Review, Anatomical Analysis and Virtual Surgery" Bioengineering 9, no. 9: 476. https://doi.org/10.3390/bioengineering9090476
APA StyleSun, Y., Helmholz, H., & Willumeit-Römer, R. (2022). Surgical Classification for Preclinical Rat Femoral Bone Defect Model: Standardization Based on Systematic Review, Anatomical Analysis and Virtual Surgery. Bioengineering, 9(9), 476. https://doi.org/10.3390/bioengineering9090476






