Synergetic Thermal Therapy for Cancer: State-of-the-Art and the Future
Abstract
:1. Introduction
2. Current Thermal Therapy Technique for Cancer
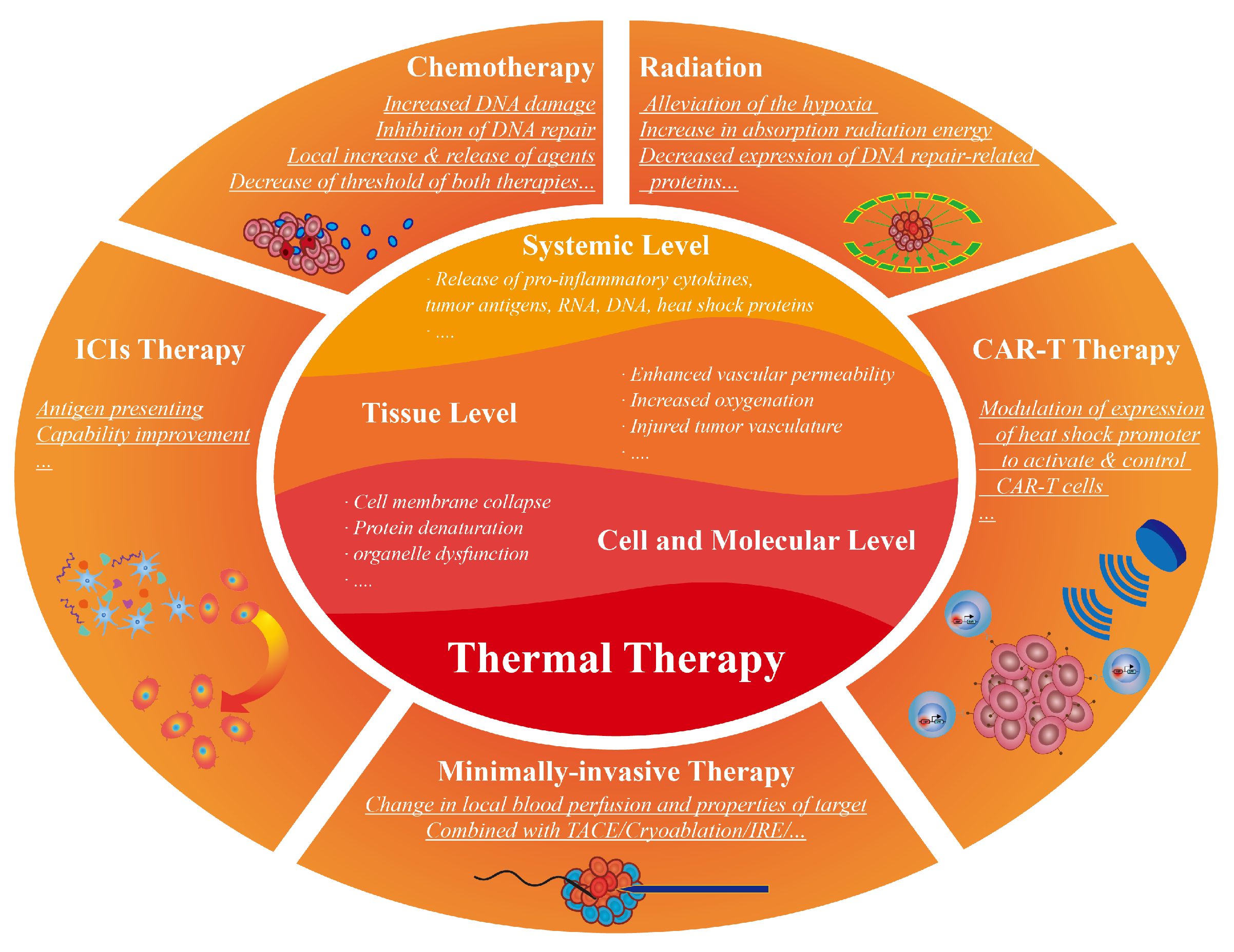
3. Cancer Treatments Combined with Thermal Therapy
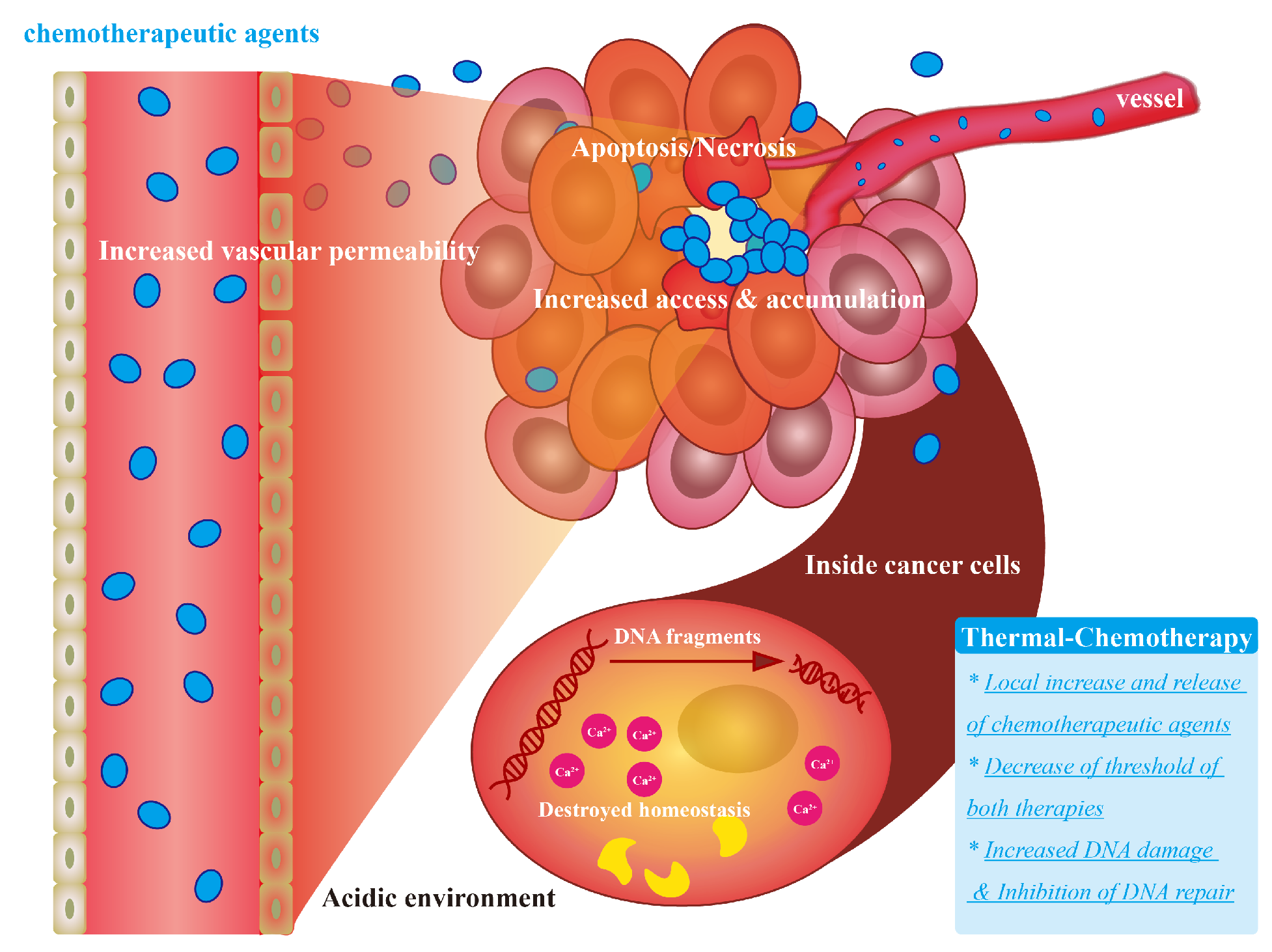
3.1. Chemotherapy Combined with Thermal Therapy
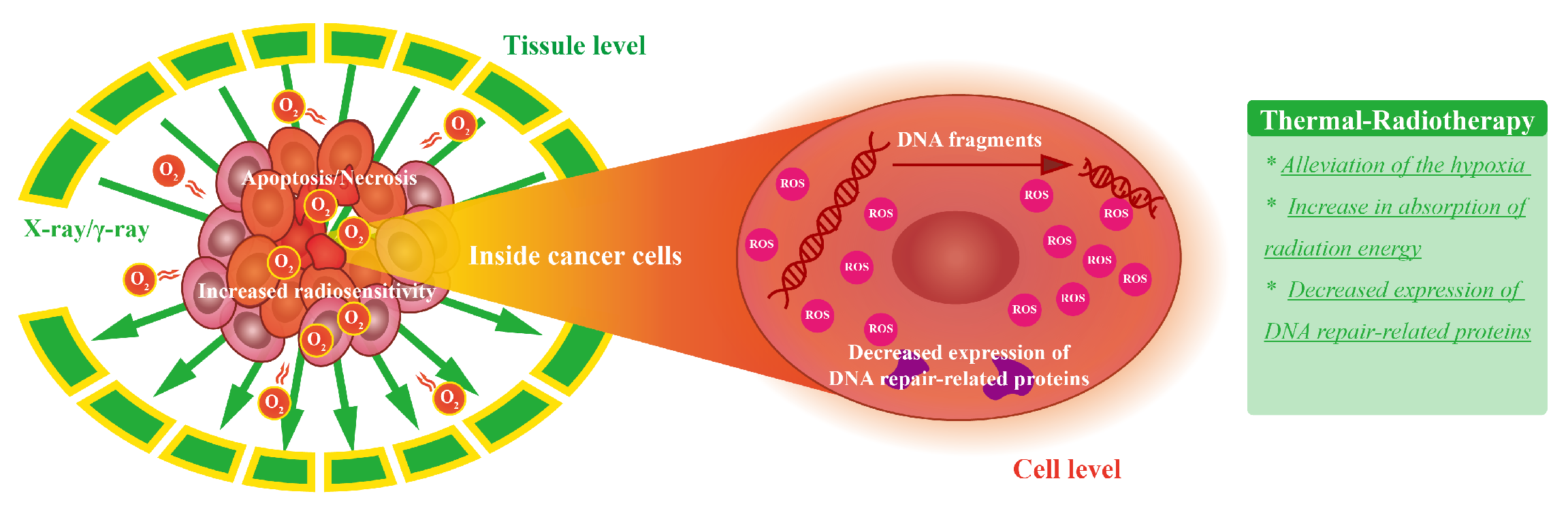
3.2. Radiation Therapy Combined with Thermal Therapy
3.3. Immunotherapy Combined with Thermal Therapy
3.3.1. Immune Checkpoint Blockade Therapy Combined with Thermal Therapy
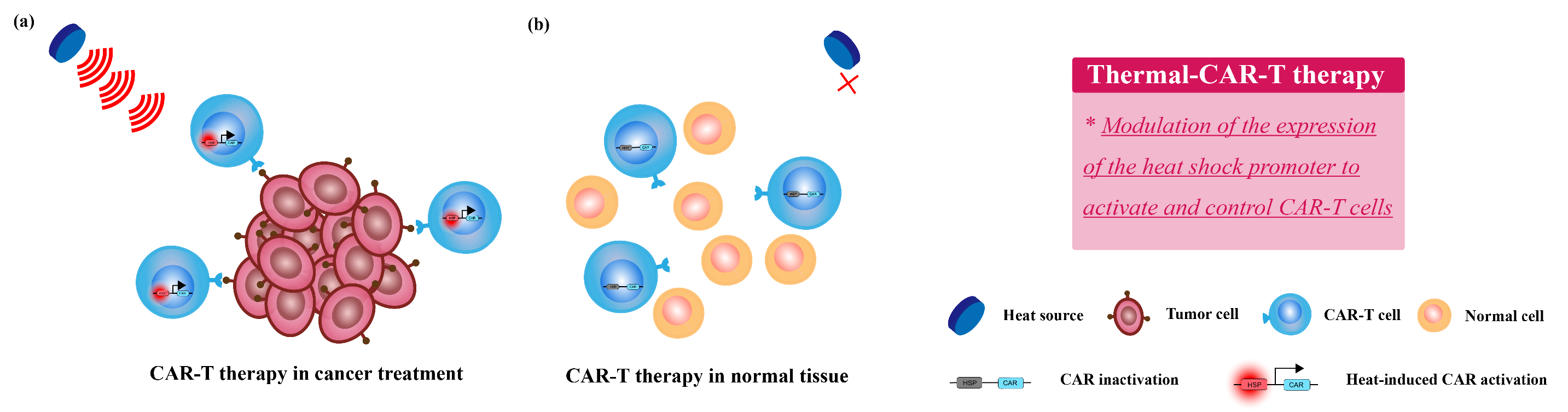
3.3.2. CAR-T Therapy Combined with Thermal Therapy
3.3.3. Other Immunotherapies Combined with Thermal Therapy
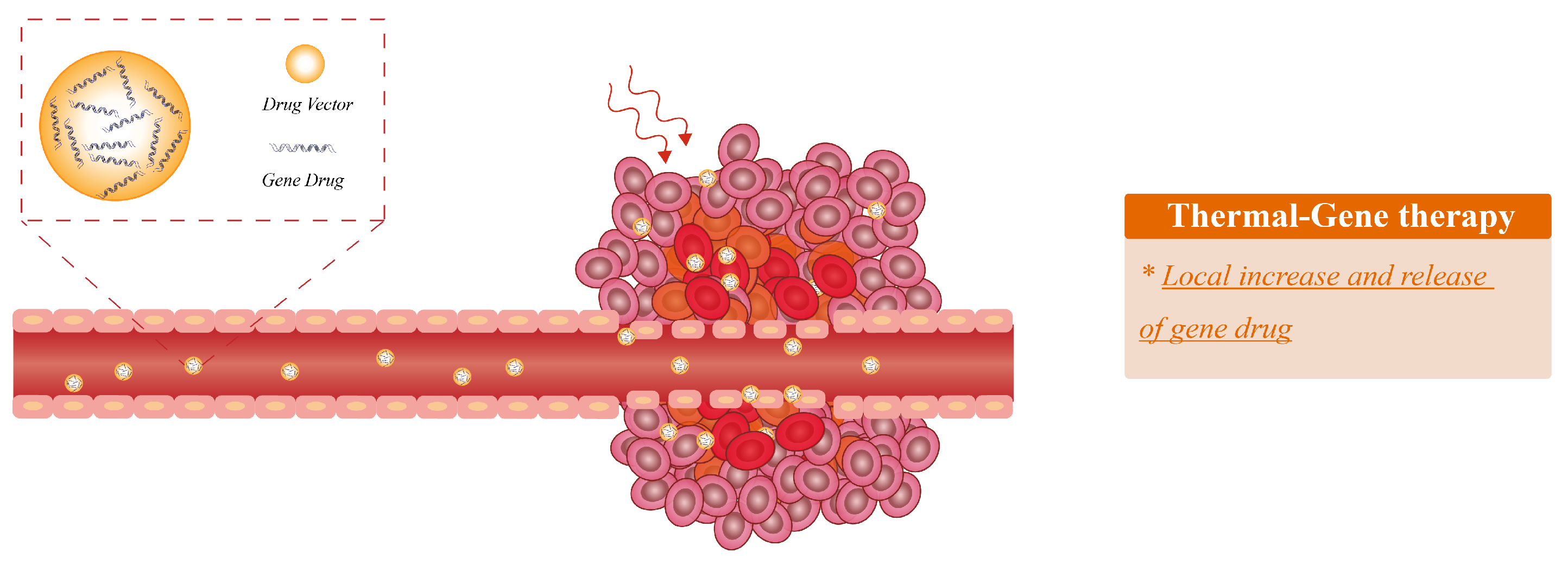
3.4. Gene Therapy Combined with Thermal Therapy
3.5. Other Minimally Invasive Therapy Combined with Thermal Therapy
4. Combined Multi-Therapies
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAR-T | Chimeric Antigen Receptor T-Cell |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| DOX | Doxorubicin |
| HIFU | High intensity focused ultrasound |
| HMGB1 | High mobility group protein B1 |
| HSPs | Heat shock proteins |
| ICIs | Immune checkpoint inhibitors |
| LITT | Laser interstitial thermal therapy |
| MPH | magnetic particle hyperthermia |
| MWA | Microwave ablation |
| OS | Overall survival |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PTT | Photothermal therapy |
| RFA | Radiofrequency ablation |
| TACE | Transcatheter arterial chemoembolization |
| TAMs | Tumor-associated macrophages |
| TARE | Transarterial radioembolization |
References
- Picchi, S.G.; Lassandro, G.; Bianco, A.; Coppola, A.; Ierardi, A.M.; Rossi, U.G.; Lassandro, F. RFA of primary and metastatic lung tumors: Long-term results. Med. Oncol. 2020, 37, 35. [Google Scholar] [CrossRef]
- Steinke, K.; King, J.; Glenn, D.; Morris, D.L. Radiofrequency ablation (RFA) of lung metastases from colovectal cancer (CRC)—One-year follow-up. Radiologe 2004, 44, 687–692. [Google Scholar] [PubMed]
- Gollapudi, L.A.; Tyberg, A. EUS-RFA of the pancreas: Where are we and future directions. Transl. Gastroenterol. Hepatol. 2022, 7, 18. [Google Scholar] [CrossRef]
- Smulian, A.G.; Moore, D.M.; Robertson, J.C.; Kralovic, S.M. Phase I Study Demonstrates Safety and Tolerability of Radiofrequency Ablation (RFA) of the Anal Mucosa. HIV Clin. Trials 2014, 15, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.P.; Chandan, S.; Khan, S.R.; Kassab, L.L.; Ponnada, S.; Artifon, E.L.A.; Otoch, J.P.; McDonough, S.; Adler, D.G. Photodynamic Therapy (PDT), Radiofrequency Ablation (RFA) With Biliary Stents in Palliative Treatment of Unresectable Extrahepatic Cholangiocarcinoma: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2022, 56, e153. [Google Scholar] [CrossRef]
- Kinoshita, T. RFA experiences, indications and clinical outcomes. Int. J. Clin. Oncol. 2019, 24, 603–607. [Google Scholar] [CrossRef]
- Muhammad, H.; Santhanam, P.; Russell, J.O.; Kuo, J.H. RFA and benign thyroid nodules: Review of the current literature. Laryngoscope Investig. Otolaryngol. 2021, 6, 155–165. [Google Scholar] [CrossRef]
- Hoffmann, R.-T.; Jakobs, T.F.; Trumm, C.; Helmberger, T.K.; Reiser, M.F. RFA of renal cell carcinoma in a solitary kidney. Abdom. Imaging 2008, 33, 230–236. [Google Scholar] [CrossRef]
- Cabibbo, G.; Maida, M.; Genco, C.; Alessi, N.; Peralta, M.; Butera, G.; Galia, M.; Brancatelli, G.; Genova, C.; Raineri, M.; et al. Survival of Patients with Hepatocellular Carcinoma (HCC) Treated by Percutaneous Radio-Frequency Ablation (RFA) Is Affected by Complete Radiological Response. PLoS ONE 2013, 8, e70016. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P. Hyperthermia in Combined Treatment of Cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Sen, K.; Sheppe, A.E.F.; Singh, I.; Hui, W.W.; Edelmann, M.J.; Rinaldi, C. Exosomes released by breast cancer cells under mild hyperthermic stress possess immunogenic potential and modulate polarization in vitro in macrophages. Int. J. Hyperth. 2020, 37, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yu, M.; Mao, X.; Pan, H.; Tang, X.; Wang, J.; Che, N.; Xie, H.; Ling, L.; Zhao, Y.; et al. Landscape of the Peripheral Immune Response Induced by Local Microwave Ablation in Patients with Breast Cancer. Adv. Sci. 2022, 9, 2200033. [Google Scholar] [CrossRef] [PubMed]
- Rangamuwa, K.; Leong, T.; Bozinovski, S.; Christie, M.; John, T.; Antippa, P.; Irving, L.; Steinfort, D. Increase in tumour PD-L1 expression in non-small cell lung cancer following bronchoscopic thermal vapour ablation. Transl. Lung Cancer Res. 2021, 10, 2858–2864. [Google Scholar] [CrossRef]
- Pang, H.; Hu, K.; Li, F.; Duan, H.; Chen, Y.; Hu, Y.; Wang, D.; Jiang, M. Untargeted metabolomics profiling in a mouse model of lung cancer treated with thermal ablation. Bioengineered 2022, 13, 11258–11268. [Google Scholar] [CrossRef]
- Fite, B.Z.; Wang, J.; Kare, A.J.; Ilovitsh, A.; Chavez, M.; Ilovitsh, T.; Zhang, N.; Chen, W.; Robinson, E.; Zhang, H.; et al. Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci. Rep. 2021, 11, 927. [Google Scholar] [CrossRef]
- Carter, T.J.; Agliardi, G.; Lin, F.; Ellis, M.; Jones, C.; Robson, M.; Richard-Londt, A.; Southern, P.; Lythgoe, M.; Zaw Thin, M.; et al. Potential of Magnetic Hyperthermia to Stimulate Localized Immune Activation. Small 2021, 17, 2005241. [Google Scholar] [CrossRef]
- Ito, F.; Vardam, T.D.; Appenheimer, M.M.; Eng, K.H.; Gollnick, S.O.; Muhitch, J.B.; Evans, S.S. In situ thermal ablation augments antitumor efficacy of adoptive T cell therapy. Int. J. Hyperth. 2019, 36, 22–36. [Google Scholar] [CrossRef]
- Skitzki, J.J.; Repasky, E.A.; Evans, S.S. Hyperthermia as an Immunotherapy Strategy for Cancer. Curr. Opin. Investig. Drugs 2010, 10, 550–558. [Google Scholar]
- Tan, S.K.; Luther, E.; Eichberg, D.; Shah, A.; Khan, K.; Jamshidi, A.; Ivan, M.; Gultekin, S.H.; Komotar, R. Complete Regression of a Solitary Cholangiocarcinoma Brain Metastasis Following Laser Interstitial Thermal Therapy. World Neurosurg. 2020, 144, 94–98. [Google Scholar] [CrossRef]
- Salem, U.; Kumar, V.A.; Madewell, J.E.; Schomer, D.F.; de Almeida Bastos, D.C.; Zinn, P.O.; Weinberg, J.S.; Rao, G.; Prabhu, S.S.; Colen, R.R. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.T.; Ma, K.W.; She, W.H. A review on radiofrequency, microwave and high-intensity focused ultrasound ablations for hepatocellular carcinoma with cirrhosis. Hepatobiliary Surg. Nutr. 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Bartek, J.; Chen, C.C. Cost-effectiveness of stereotactic laser ablation (SLA) for brain tumors. Int. J. Hyperth. 2020, 37, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Teraphongphom, N.; Kong, C.S.; Warram, J.M.; Rosenthal, E.L. Specimen Mapping in Head and Neck Cancer Using Fluorescence Imaging. Laryngoscope Investig. Otolaryngol. 2017, 2, 447–452. [Google Scholar] [CrossRef]
- Sadeghi-Goughari, M.; Jeon, S.; Kwon, H.J. Enhancing Thermal Effect of Focused Ultrasound Therapy Using Gold Nanoparticles. IEEE Trans. Nanobiosci. 2019, 18, 661–668. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Jiang, P.S.; Tsai, H.Y.; Drake, P.; Wang, F.N.; Chiang, C.S. Gadolinium-doped iron oxide nanoparticles induced magnetic field hyperthermia combined with radiotherapy increases tumour response by vascular disruption and improved oxygenation. Int. J. Hyperth. 2017, 33, 770–778. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Dong, X.; Zhang, C.; Mei, L.; Zang, Y.; Yan, L.; Zhang, H.; Gu, Z. Enhanced radiosensitization of ternary Cu3BiSe3 nanoparticles by photo-induced hyperthermia in the second near-infrared biological window. Nanoscale 2019, 11, 7157–7165. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, C.; Sun, C.; Yu, C. Polyphosphoester-Based Nanocarrier for Combined Radio-Photothermal Therapy of Breast Cancer. Acs Biomater. Sci. Eng. 2019, 5, 1868–1877. [Google Scholar] [CrossRef]
- Hiremath, N.; Kumar, R.; Hwang, K.C.; Banerjee, I.; Thangudu, S.; Vankayala, R. Near-Infrared Light Activatable Two-Dimensional Nanomaterials for Theranostic Applications: A Comprehensive Review. ACS Appl. Nano Mater. 2022, 5, 1719–1733. [Google Scholar] [CrossRef]
- Thangudu, S.; Kaur, N.; Korupalli, C.; Sharma, V.; Kalluru, P.; Vankayala, R. Recent advances in near infrared light responsive multi-functional nanostructures for phototheranostic applications. Biomater. Sci. 2021, 9, 5432–5443. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Yadav, H.P.; Gupta, N.; Deo, S.V.S.; Bhatnagar, S. Multidisciplinary Perioperative Management of Hyperthermic-Isolated Limb Perfusion for Malignant Melanoma: A Case Report. Indian J. Surg. Oncol. 2021, 12, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Cao, X.; Liu, X.; Qin, J.; Zhang, S.; Li, Q.; Liu, J. The Effect of Pancreatoduodenectomy Plus Intraperitoneal Hyperthermic Perfusion on Resectable Pancreatic Head Cancer: Cohort Study. Ann. Surg. Oncol. 2021, 28, 2337–2345. [Google Scholar] [CrossRef]
- Ye, M.; Shen, J.; Kong, M.; Lv, D.; Yang, H. Short-Term Effificacy of Intrapleural Hyperthermic Perfusion for Malignant Pleural Effusion in Lung Carcinoma. J. Thorac. Oncol. 2021, 16, S244. [Google Scholar] [CrossRef]
- Lim, D.; Namgung, B.; Woo, D.G.; Choi, J.S.; Kim, H.S.; Tack, G.R. Effect of Input Waveform Pattern and Large Blood Vessel Existence on Destruction of Liver Tumor Using Radiofrequency Ablation: Finite Element Analysis. J. Biomech. Eng. Trans. ASME 2010, 132, 8. [Google Scholar] [CrossRef]
- Ashour, A.S.; Asran, M.; Mohamed, W.S.; Fotiadis, D.I. Optimal Localization of a Novel Shifted 1T-Ring Based Microwave Ablation Probe in Hepatocellular Carcinoma. IEEE Trans. Biomed. Eng. 2021, 68, 505–514. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef]
- Bozinov, O.; Yang, Y.; Oertel, M.F.; Neidert, M.C.; Nakaji, P. Laser interstitial thermal therapy in gliomas. Cancer Lett. 2020, 474, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Li, C.; Xu, A.; Qiu, B.; Li, F.; Ding, W. Flav7+DOX co-loaded separable microneedle for light-triggered chemo-thermal therapy of superficial tumors. Chem. Eng. J. 2022, 428, 131913. [Google Scholar] [CrossRef]
- Ter Haar, G.; Coussios, C. High intensity focused ultrasound: Physical principles and devices. Int. J. Hyperth. 2007, 23, 89–104. [Google Scholar] [CrossRef]
- Roebuck, J.R.; Osterberg, H. The Joule-Thomson Effect in Argon. Phys. Rev. 1934, 46, 785–790. [Google Scholar] [CrossRef]
- Qi, S.L.; Zhang, P.; Wang, R.Z.; Xu, L.X. Flow boiling of liquid nitrogen in micro-tubes: Part I—The onset of nucleate boiling, two-phase flow instability and two-phase. Int. J. Heat Mass Transf. 2007, 50, 4999–5016. [Google Scholar] [CrossRef]
- Qi, S.L.; Zhang, P.; Wang, R.Z.; Xu, L.X. Flow boiling of liquid nitrogen in micro-tubes: Part II—Heat transfer characteristics and critical heat flux. Int. J. Heat Mass Transf. 2007, 50, 5017–5030. [Google Scholar] [CrossRef]
- Takahashi, Y.; Izumi, Y.; Matsutani, N.; Dejima, H.; Nakayama, T.; Okamura, R.; Uehara, H.; Kawamura, M. Optimized magnitude of cryosurgery facilitating anti-tumor immunoreaction in a mouse model of Lewis lung cancer. Cancer Immunol. Immunother. 2016, 65, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Morizane, C.; Ueno, M.; Okusaka, T.; Ishii, H.; Furuse, J. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Jpn. J. Clin. Oncol. 2018, 48, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Liu, J.; Jin, Z.; Qiu, G.; Xie, Q.; Mi, S.; Huang, J. Use of chemotherapy to treat hepatocellular carcinoma. Aims Math. 2022, 7, 31–45. [Google Scholar] [CrossRef]
- Yan, X.; Zhuang, L.P.; Ning, Z.Y.; Wang, P.; Meng, Z.Q. Addition of thermal ablation to systemic chemotherapy for the treatment of unresectable intrahepatic cholangiocarcinoma: A propensity score matching analysis. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 81–88. [Google Scholar] [CrossRef]
- Xu, F.; Song, J.; Lu, Y.; Wang, J.; Wang, J.; Xiao, H.; Li, Z. Clinical efficacy of systemic chemotherapy combined with radiofrequency ablation and microwave ablation for lung cancer: A comparative study. Int. J. Hyperth. 2021, 38, 900–906. [Google Scholar] [CrossRef]
- Wu, C.X.; Chen, M.L.; Zhang, H.; Han, J.J. Percutaneous Radiofrequency Ablation Combined with Chemotherapy Versus Chemotherapy Only for Ovarian Cancer Liver Metastasis. Front. Oncol. 2022, 11, 793024. [Google Scholar] [CrossRef]
- Torres-Lugo, M.; Rinaldi, C. Thermal potentiation of chemotherapy by magnetic nanoparticles. Nanomedicine 2013, 8, 1689–1707. [Google Scholar] [CrossRef]
- Shah, M.A.; Schwartz, G.K. Cell cycle-mediated drug resistance: An emerging concept in cancer therapy. Clin. Cancer Res. 2001, 7, 2168–2181. [Google Scholar] [PubMed]
- Issels, R.D. Hyperthermia Adds to Chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Wu, Q.; Fu, C.; Zhang, D.; Yu, J.; Meng, X.; Liang, P. Amplified intracellular Ca2+ for synergistic anti-tumor therapy of microwave ablation and chemotherapy. J. Nanobiotechnol. 2019, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.S.; Murad, H.Y.; Ashe, S.; Halliburton, G.; Yu, H.; He, J.; John, V.T.; Khismatullin, D.B. Ablative focused ultrasound synergistically enhances thermally triggered chemotherapy for prostate cancer in vitro. Mol. Pharm. 2016, 13, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Santucci, K.L.; Baust, J.; Snyder, K.; Buskirk, R.V.V.; Katz, A.; Corcoran, A.; Baust, J. Investigation of Bladder Cancer Cell Response to Cryoablation and Adjunctive Cisplatin Based Cryo/Chemotherapy. Clin. Res. 2020, 6. [Google Scholar] [CrossRef]
- Baust, J.M.; Santucci, K.L.; Van Buskirk, R.G.; Raijman, I.; Fisher, W.E.; Baust, J.G.; Snyder, K.K. An In Vitro Investigation into Cryoablation and Adjunctive Cryoablation/Chemotherapy Combination Therapy for the Treatment of Pancreatic Cancer Using the PANC-1 Cell Line. Biomedicines 2022, 10, 450. [Google Scholar] [CrossRef]
- Clarke, D.M.; Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: Implications of mitochondrial involvement. Cryobiology 2004, 49, 45–61. [Google Scholar] [CrossRef]
- Shi, D.; Zhuang, J.; Fan, Z.; Zhao, H.; Zhang, X.; Su, G.; Xie, L.; Ge, D.; Hou, Z. Self-targeting nanotherapy based on functionalized graphene oxide for synergistic thermochemotherapy. J. Colloid Interface Sci. 2021, 603, 70–84. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, N.; Yuan, W. NIR/Thermoresponsive Injectable Self-Healing Hydrogels Containing Polydopamine Nanoparticles for Efficient Synergistic Cancer Thermochemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 9118–9131. [Google Scholar] [CrossRef]
- Moros, M.; Idiago-López, J.; Asín, L.; Moreno-Antolín, E.; Beola, L.; Grazú, V.; Fratila, R.M.; Gutiérrez, L.; de la Fuente, J.M. Triggering Antitumoural Drug Release and Gene Expression by Magnetic Hyperthermia. Adv. Drug Deliv. Rev. 2019, 138, 326–343. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, H.; Wan, J.; Zhou, Y.; Xu, Q.; Zhao, Y.; Yang, X.; Gan, L. PH- and Photothermal-Driven Multistage Delivery Nanoplatform for Overcoming Cancer Drug Resistance. Theranostics 2019, 9, 3825–3839. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Methotrexate-Conjugated Chitosan-Grafted PH- and Thermo-Responsive Magnetic Nanoparticles for Targeted Therapy of Ovarian Cancer. Int. J. Biol. Macromol. 2020, 154, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.W.; Varma, V.B.; Ramanujan, R.V.; Miserez, A. Magnetically responsive peptide coacervates for dual hyperthermia and chemotherapy treatments of liver cancer. Acta Biomater. 2020, 110, 221–230. [Google Scholar] [CrossRef]
- Wang, X.; Gao, S.; Qin, Z.; Tian, R.; Wang, G.; Zhang, X.; Zhu, L.; Chen, X. Evans Blue Derivative-Functionalized Gold Nanorods for Photothermal Therapy-Enhanced Tumor Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15140–15149. [Google Scholar] [CrossRef]
- Yang, J.C.; Chen, Y.; Li, Y.H.; Yin, X.B. Magnetic Resonance Imaging-Guided Multi-Drug Chemotherapy and Photothermal Synergistic Therapy with pH and NIR-Stimulation Release. ACS Appl. Mater. Interfaces 2017, 9, 22278–22288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, M.; Ouyang, N.; Tang, Y.; Miao, P. Synergistic Chemo-thermal Therapy of Cancer by DNA-Templated Silver Nanoclusters and Polydopamine Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 21653–21660. [Google Scholar] [CrossRef]
- Yang, S.; Palanikumar, L.; Jeong, S.; Kim, K.; Lee, J.; Jeoung, E.; Kim, C.; Ryu, J.H.; Park, M.H. Synergistic Effect of Photothermal Therapy and Chemotherapy Using Camptothecin-Conjugated Gold Nanorods. Part. Part. Syst. Charact. 2018, 35, 1700307. [Google Scholar] [CrossRef]
- Su, L.; Wu, Q.; Tan, L.; Huang, Z.; Fu, C.; Ren, X.; Xia, N.; Chen, Z.; Ma, X.; Lan, X.; et al. High Biocompatible ZIF-8 Coated by ZrO2 for Chemo-microwave Thermal Tumor Synergistic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 10520–10531. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, P.; Zhang, A.; Xu, L.X. Study on tumor microvasculature damage induced by alternate cooling and heating. Ann. Biomed. Eng. 2008, 36, 1409–1419. [Google Scholar] [CrossRef]
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv. Drug Deliv. Rev. 2020, 163, 98–124. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Chen, M.; Wang, W.; Li, X.; Yang, H.; Yang, S.; Zhou, Z. Self-Amplified Apoptosis Targeting Nanoplatform for Synergistic Magnetic–Thermal/Chemo Therapy In Vivo. Adv. Healthc. Mater. 2020, 9, 2000202. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, Y.; Mao, J.; Lei, X.; Yang, Q.; Cui, C. Dendrimer-Modified Gold Nanorods as a Platform for Combinational Gene Therapy and Photothermal Therapy of Tumors. J. Exp. Clin. Cancer Res. 2021, 40, 303. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Yao, Q.; Zhang, H.; Chu, M.; Bhutia, Y.D.; Chen, R.; Ganapathy, V. Transporter-Targeted Nano-Sized Vehicles for Enhanced and Site-Specific Drug Delivery. Cancers 2020, 12, 2837. [Google Scholar] [CrossRef]
- Chen, X.; Fu, C.; Wang, Y.; Wu, Q.; Meng, X.; Xu, K. Mitochondria-targeting nanoparticles for enhanced microwave ablation of cancer. Nanoscale 2018, 10, 15677–15685. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as Photothermal Therapeutic Agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Wei, C.; Wang, P.; Huang, Z.; He, D.; Zhu, W.; Liu, H.; Chen, Z.; Wang, W.; Li, Y.; Shen, J.; et al. Construction of Surface-Modified Polydopamine Nanoparticles for Sequential Drug Release and Combined Chemo-Photothermal Cancer Therapy. Mol. Pharm. 2021, 18, 1327–1343. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Gillette, E.L.; Dewey, W.C. Effect of heat and radiation on synchronous Chinese hamster cells: Killing and repair. Radiat. Res. 1975, 64, 611–623. [Google Scholar] [CrossRef]
- Cheng, X.; Yong, Y.; Dai, Y.; Song, X.; Yang, G.; Pan, Y.; Ge, C. Enhanced Radiotherapy using Bismuth Sulfide Nanoagents Combined with Photo-thermal Treatment. Theranostics 2017, 7, 4087–4098. [Google Scholar] [CrossRef]
- Prezzano, K.M.; Prasad, D.; Hermann, G.M.; Belal, A.N.; Alberico, R.A. Radiofrequency Ablation and Radiation Therapy Improve Local Control in Spinal Metastases Compared to Radiofrequency Ablation Alone. Am. J. Hosp. Palliat. Med. 2019, 36, 417–422. [Google Scholar] [CrossRef]
- Verduijn, G.M.; deWee, E.M.; Rijnen, Z.; Togni, P.; Hardillo, J.A.U.; ten Hove, I.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Deep hyperthermia with the HYPERcollar system combined with irradiation for advanced head and neck carcinoma—A feasibility study. Int. J. Hyperth. 2011, 37, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhu, M.; Lin, G.; Liu, J.; Zhou, Z.; Tian, X.; Pan, Y. PEGylated Au@Pt Nanodendrites as Novel Theranostic Agents for Computed Tomography Imaging and Photothermal/Radiation Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 279–285. [Google Scholar] [CrossRef]
- Wang, J.; Tan, X.; Pang, X.; Liu, L.; Tan, F.; Li, N. MoS2 Quantum Dot@Polyaniline Inorganic-Organic Nanohybrids for in Vivo Dual-Modal Imaging Guided Synergistic Photothermal/Radiation Therapy. ACS Appl. Mater. Interfaces 2016, 8, 24331–24338. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Hou, X.; Yu, X.; Wei, X.; Li, Y.; Yang, D.; Jiang, X. Folic Acid-Conjugated Gold Nanostars for Computed Tomography Imaging and Photothermal/Radiation Combined Therapy. ACS Appl. Bio Mater. 2021, 4, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Xie, L.; Sang, W.; Wang, G.; Zhang, Z.; Li, B.; Tian, H.; Yan, J.; Tian, Y.; et al. A metal–polyphenolic nanosystem with NIR-II fluorescence-guided combined photothermal therapy and radiotherapy. Chem. Commun. 2021, 57, 11473–11476. [Google Scholar] [CrossRef]
- Liu, C.-X.; Gao, X.-S.; Xiong, L.-L.; Ge, H.-Y.; He, X.-Y.; Li, T.; Zhang, H.-J.; Bai, H.-Z.; Lin, Q.; Zhang, M.; et al. A Preclinical in Vivo Investigation of High-Intensity Focused Ultrasound Combined with Radiotherapy. Ultrasound Med. Biol. 2011, 37, 69–77. [Google Scholar] [CrossRef]
- Qian, L.; Shen, Y.; Xie, J.; Meng, Z. Immunomodulatory effects of ablation therapy on tumors: Potentials for combination with immunotherapy. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188385. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from immune checkpoint inhibitors. IJC Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, E447–E458. [Google Scholar] [CrossRef]
- Porcu, M.; Solinas, C.; Migali, C.; Battaglia, A.; Schena, M.; Mannelli, L.; Addeo, A.; Willard-Gallo, K.; Saba, L. Immune Checkpoint Inhibitor-Induced Pancreatic Injury: Imaging Findings and Literature Review. Target. Oncol. 2020, 15, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Feng, H.; Liu, H.; Guo, L.; Chen, C.; Yao, X.; Sun, S. Immune Checkpoint Inhibitors-Related Thyroid Dysfunction: Epidemiology, Clinical Presentation, Possible Pathogenesis, and Management. Front. Endocrinol. 2021, 12, 649863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Fu, Y.; Zhu, B.; Zhang, B.; Wang, J. Pneumonitis Induced by Immune Checkpoint Inhibitors: From Clinical Data to Translational Investigation. Front. Oncol. 2020, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.S.; Lionakis, M.S. Infectious Complications of Immune Checkpoint Inhibitors. Infect. Dis. Clin. North Am. 2020, 34, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.W.; Nussbaum, B.; Jäger, G.; Jurmeister, P.S.; Budczies, J.; Pereira, P.L.; Clasen, S.; Kowalewski, D.J.; Mühlenbruch, L.; Königsrainer, I.; et al. A Non-interventional Clinical Trial Assessing Immune Responses After Radiofrequency Ablation of Liver Metastases from Colorectal Cancer. Front. Immunol. 2019, 10, 2526. [Google Scholar] [CrossRef]
- Han, J.W.; Yoon, S.K. Immune Responses Following Locoregional Treatment for Hepatocellular Carcinoma: Possible Roles of Adjuvant Immunotherapy. Pharmaceutics 2021, 13, 1387. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.; Jiang, H.; Zhang, Y.; Lin, W.; Mo, J.; Jin, C. Combination of Ablation and Immunotherapy for Hepatocellular Carcinoma: Where We Are and Where to Go. Front. Immunol. 2021, 12, 792781. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Chen, S.; Bi, H.; Xia, F.; Feng, K.; Ma, K.; Ni, B. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: A propensity score matching analysis. Int. J. Hyperth. 2021, 38, 1519–1528. [Google Scholar] [CrossRef]
- Chang, X. Treatment of Kidney Tumors with Radiofrequency Ablation (rfa) Combined with Systemic Pd-1 Inhibition Results in Both Primary Tumor Control and Prevention of Lung Metastasis in a Preclinical Animal Model. J. Urol. 2017, 197, E187. [Google Scholar] [CrossRef]
- Lemdani, K.; Mignet, N.; Boudy, V.; Seguin, J.; Oujagir, E.; Bawa, O.; Peschaud, F.; Emile, J.-F.; Capron, C.; Malafosse, R. Local immunomodulation combined to radiofrequency ablation results in a complete cure of local and distant colorectal carcinoma. Oncoimmunology 2019, 8, e1550342. [Google Scholar] [CrossRef]
- den Brok, M.; Sutmuller, R.P.M.; van der Voort, R.; Bennink, E.J.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004, 64, 4024–4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagnoni, F.F.; Zerbini, A.; Pelosi, G.; Missale, G. Combination of radiofrequency ablation and immunotherapy. Front. Biosci. Landmark 2008, 13, 369–381. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Jiang, J.; Zhang, M.; Shen, J. CTLA-4 Blockade Suppresses Progression of Residual Tumors and Improves Survival After Insufficient Radiofrequency Ablation in a Subcutaneous Murine Hepatoma Model. Cardiovasc. Interv. Radiol. 2020, 43, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, J.; Ding, N.; Zhang, Y.; Zhu, Y.; Dong, S.; Wang, X.; Peng, C.; Zhou, C.; Zhou, L.; et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 2019, 10, 5421. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef]
- Eranki, A.; Srinivasan, P.; Ries, M.; Kim, A.; Lazarski, C.A.; Rossi, C.T.; Khokhlova, T.D.; Wilson, E.; Knoblach, S.M.; Sharma, K.; et al. High-Intensity Focused Ultrasound (HIFU) Triggers Immune Sensitization of Refractory Murine Neuroblastoma to Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2020, 26, 1152–1161. [Google Scholar] [CrossRef]
- Shi, G.; Zhong, M.; Ye, F.; Zhang, X. Low-frequency HIFU induced cancer immunotherapy: Tempting challenges and potential opportunities. Cancer Biol. Med. 2019, 16, 714–728. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Huang, Z.; Wang, X.; Jin, Z.; Li, J.; Limsakul, P.; Zhu, L.; Allen, M.; Pan, Y.; et al. Control of the Activity of CAR-T Cells within Tumours via Focused Ultrasound. Nat. Biomed. Eng. 2021, 5, 1336–1347. [Google Scholar] [CrossRef]
- Miller, I.C.; Zamat, A.; Sun, L.-K.; Phuengkham, H.; Harris, A.M.; Gamboa, L.; Yang, J.; Murad, J.P.; Priceman, S.J.; Kwong, G.A. Enhanced Intratumoural Activity of CAR T Cells Engineered to Produce Immunomodulators under Photothermal Control. Nat. Biomed. Eng. 2021, 5, 1348–1359. [Google Scholar] [CrossRef]
- Jansen, M.C.; van Hillegersberg, R.; Schoots, I.G.; Levi, M.; Beek, J.F.; Crezee, H.; van Gulik, T.M. Cryoablation Induces Greater Inflammatory and Coagulative Responses than Radiofrequency Ablation or Laser Induced Thermotherapy in a Rat Liver Model. Surgery 2010, 147, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; O’Flanagan, S.; Lam, T.; Roy, P.; Pelaez, F.; Burbach, B.J.; Azarin, S.M.; Shimizu, Y.; Bischof, J.C. Engineering T Cell Response to Cancer Antigens by Choice of Focal Therapeutic Conditions. Int. J. Hyperth. 2019, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Matin, S.F.; Tam, A.L.; Sheth, R.A.; Ahrar, K.; Tidwell, R.S.; Rao, P.; Karam, J.A.; Wood, C.G.; Tannir, N.M.; et al. Pilot Study of Tremelimumab with and without Cryoablation in Patients with Metastatic Renal Cell Carcinoma. Nat. Commun. 2021, 12, 6375. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Y.; Guo, Z.; Si, T.; Xing, W.; Yu, W.; Wang, Y. Cryoablation Inhibition of Distant Untreated Tumors (Abscopal Effect) Is Immune Mediated. Oncotarget 2019, 10, 4180–4191. [Google Scholar] [CrossRef]
- Benzon, B.; Glavaris, S.A.; Simons, B.W.; Hughes, R.M.; Ghabili, K.; Mullane, P.; Miller, R.; Nugent, K.; Shinder, B.; Tosoian, J.; et al. Combining Immune Check-Point Blockade and Cryoablation in an Immunocompetent Hormone Sensitive Murine Model of Prostate Cancer. Prostate Cancer Prostatic Dis. 2018, 21, 126–136. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Y.; Li, J.; Diao, L.; Shao, L.; Han-Zhang, H.; Zhang, L.; Kang, Q.; Yang, W. Exceptional Response of Cryoablation Followed by Pembrolizumab in a Patient with Metastatic Cervical Carcinosarcoma with High Tumor Mutational Burden: A Case Report. Oncol. 2020, 25, 15–18. [Google Scholar] [CrossRef]
- Den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Nierkens, S.; Bennink, E.J.; Toonen, L.W.J.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. Synergy between In Situ Cryoablation and TLR9 Stimulation Results in a Highly Effective In Vivo Dendritic Cell Vaccine. Cancer Res. 2006, 66, 7285–7292. [Google Scholar] [CrossRef]
- Niu, L.-Z. Combination Treatment with Comprehensive Cryoablation and Immunotherapy in Metastatic Hepatocellular Cancer. World J. Gastroenterol. 2013, 19, 3473. [Google Scholar] [CrossRef]
- Doshi, A.; Zhou, M.; Bui, N.; Wang, D.S.; Ganjoo, K.; Hwang, G.L. Safety and Feasibility of Cryoablation during Immunotherapy in Patients with Metastatic Soft Tissue Sarcoma. J. Vasc. Interv. Radiol. 2021, 32, 1688–1694. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, T.; Lu, Y.; Feng, H. The Application of Cytidyl Guanosyl Oligodeoxynucleotide Can Affect the Antitumor Immune Response Induced by a Combined Protocol of Cryoablation and Dendritic Cells in Lewis Lung Cancer Model. Med. Sci. Monit. 2016, 22, 1309–1317. [Google Scholar] [CrossRef]
- Friedmann, T.; Roblin, R. Gene therapy for human genetic disease? Science 1972, 175, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Murty, T.; Mackall, C.L. Gene editing to enhance the efficacy of cancer cell therapies. Mol. Ther. 2021, 29, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M. Cancer Gene Therapy: An Awkward Adolescence. Cancer Gene Ther. 2003, 10, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnot, P. Chapter 4—-Viral Vectors for Delivery of Antiviral Sequences. In Gene Therapy for Viral Infections; Arbuthnot, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 95–126. [Google Scholar]
- Bushman, F.D. Retroviral insertional mutagenesis in humans: Evidence for four genetic mechanisms promoting expansion of cell clones. Mol. Ther. 2020, 28, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Davé, U.P.; Jenkins, N.A.; Copeland, N.G. Gene therapy insertional mutagenesis insights. Science 2004, 303, 333. [Google Scholar] [CrossRef]
- Torres-Vanegas, J.D.; Cruz, J.C.; Reyes, L.H. Delivery systems for nucleic acids and proteins: Barriers, cell capture pathways and nanocarriers. Pharmaceutics 2021, 13, 428. [Google Scholar] [CrossRef]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef]
- Takai, T.; Ohmori, H. Enhancement of DNA transfection efficiency by heat treatment of cultured mammalian cells. Biochim. Biophys. Acta 1992, 1129, 161–165. [Google Scholar] [CrossRef]
- Madio, D.P.; van Gelderen, P.; DesPres, D.; Olson, A.W.; de Zwart, J.A.; Fawcett, T.W.; Holbrook, N.J.; Mandel, M.; Moonen, C.T. On the feasibility of MRI-guided focused ultrasound for local induction of gene expression. J. Magn. Reson. Imaging 1998, 8, 101–104. [Google Scholar] [CrossRef]
- Du, X.; Qiu, B.; Zhan, X.; Kolmakova, A.; Gao, F.; Hofmann, L.V.; Cheng, L.; Chatterjee, S.; Yang, X. Radiofrequency-Enhanced Vascular Gene Transduction and Expression for Intravascular MR Imaging–Guided Therapy: Feasibility Study in Pigs. Radiology 2005, 236, 939–944. [Google Scholar] [CrossRef]
- Hashiya, N.; Aoki, M.; Tachibana, K.; Taniyama, Y.; Yamasaki, K.; Hiraoka, K.; Makino, H.; Yasufumi, K.; Ogihara, T.; Morishita, R. Local Delivery of E2F Decoy Oligodeoxynucleotides Using Ultrasound with Microbubble Agent (Optison) Inhibits Intimal Hyperplasia after Balloon Injury in Rat Carotid Artery Model. Biochem. Biophys. Res. Commun. 2004, 317, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Shen, B.; Chao, L.; Chao, J. Kallikrein-Modified Mesenchymal Stem Cell Implantation Provides Enhanced Protection Against Acute Ischemic Kidney Injury by Inhibiting Apoptosis and Inflammation. Hum. Gene Ther. 2008, 19, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene Therapy Comes of Age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef] [PubMed]
- Zintchenko, A.; Ogris, M.; Wagner, E. Temperature Dependent Gene Expression Induced by PNIPAM-Based Copolymers: Potential of Hyperthermia in Gene Transfer. Bioconjugate Chem. 2006, 17, 766–772. [Google Scholar] [CrossRef]
- Yuan, G.; Zeng, C.-L.; Zhu, D.-D.; Shi, X.-J. Influences of RFA combined with TACE on the HIF-1 alpha and EGR level of patients with primary hepatic carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1738–1745. [Google Scholar] [PubMed]
- Di Costanzo, G.G.; Tortora, R. Intermediate hepatocellular carcinoma: How to choose the best treatment modality? World J. Hepatol. 2015, 7, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.T.; Jakobs, T.F.; Kubisch, C.H.; Stemmler, H.J.; Trumm, C.; Tatsch, K.; Helmberger, T.K.; Reiser, M.F. Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease-Is it feasible? Eur. J. Radiol. 2010, 74, 199–205. [Google Scholar] [CrossRef]
- Fang, Z.; Mao, H.; Moser, M.A.J.; Zhang, W.; Qian, Z.; Zhang, B. Irreversible Electroporation Enhanced by Radiofrequency Ablation: An In Vitro and Computational Study in a 3D Liver Tumor Model. Ann. Biomed. Eng. 2021, 49, 2126–2138. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.J.; Ding, L.J.; Moser, M.A.J.; Zhang, E.M.; Zhang, W.J. Tumor Ablation Enhancement by Combining Radiofrequency Ablation and Irreversible Electroporation: An In Vitro 3D Tumor Study. Ann. Biomed. Eng. 2019, 47, 694–705. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Pan, C.-C.; Wu, P.-H.; Zhao, M.; Li, W.; Huang, Z.-L.; Yi, R.-Y. Efficacy of minimally invasive therapies on unresectable pancreatic cancer. Chin. J. Cancer 2013, 32, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-W.; Zhang, Y.-J.; Chen, M.-S.; Xu, L.; Liang, H.-H.; Lin, X.-J.; Guo, R.-P.; Zhang, Y.-Q.; Lau, W.Y. Radiofrequency Ablation With or Without Transcatheter Arterial Chemoembolization in the Treatment of Hepatocellular Carcinoma: A Prospective Randomized Trial. J. Clin. Oncol. 2013, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Cho, S.K.; Shin, S.W.; Hyun, D.; Lee, M.W.; Rhim, H. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: Comparison with TACE or RFA monotherapy. Abdom. Radiol. 2019, 44, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhang, Z.; Kuai, J. Analysis of efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J. Buon 2019, 24, 150–157. [Google Scholar]
- Baydoun, H.; Meirovich, H.; Maroun, G.; Coburn, N.; David, E. Locoregional options in the management of cholangiocarcinoma: Single center experience. Ann. Palliat. Med. 2021, 10, 1784–1791. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, A.; Xu, L.X. Evaluation of alternate cooling and heating for tumor treatment. Int. J. Heat Mass Transf. 2008, 51, 5478–5485. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, A. Study of alternate cooling and heating treatment induced tumor microvasculature injury. Chin. Sci. Bull. 2010, 55, 172–178. [Google Scholar] [CrossRef]
- Cai, Z.; Song, M.; Zhang, A.; Sun, J.; Xu, L.X. Numerical Simulation of a New Probe for the Alternate Cooling and Heating of a Subcutaneous Mouse Tumor Model. Numer. Heat Transf. Part A Appl. 2013, 63, 534–548. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Wang, G.; He, X.; Xu, L.; Wang, S.; Peng, W. Multimode tumor ablation therapy induced different diffusion and microvasculature related parameters change on functional magnetic resonance imaging compared to radiofrequency ablation in liver tumor. Medicine 2020, 99, e20795. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Y.; Liu, P.; Xu, L.X. Tumor-related HSP70 released after cryo-thermal therapy targeted innate immune initiation in the antitumor immune response. Int. J. Hyperth. 2020, 37, 843–853. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, J.; Peng, P.; Wang, S.; Liu, P.; Xu, L.X. Downregulated TNF-α Levels after Cryo-Thermal Therapy Drive Tregs Fragility to Promote Long-Term Antitumor Immunity. Int. J. Mol. Sci. 2021, 22, 9951. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, M.; Peng, P.; Lou, Y.; Zhang, A.; Liu, P. Iron Released after Cryo-Thermal Therapy Induced M1 Macrophage Polarization, Promoting the Differentiation of CD4+ T Cells into CTLs. Int. J. Mol. Sci. 2021, 22, 7010. [Google Scholar] [CrossRef]
- Wang, H.; Agarwal, P.; Liang, Y.; Xu, J.; Zhao, G.; Tkaczuk, K.H.R.; Lu, X.; He, X. Enhanced cancer therapy with cold-controlled drug release and photothermal warming enabled by one nanoplatform. Biomaterials 2018, 180, 265–278. [Google Scholar] [CrossRef]
- Ektate, K.; Munteanu, M.C.; Ashar, H.; Malayer, J.; Ranjan, A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci. Rep. 2018, 8, 13062. [Google Scholar] [CrossRef] [PubMed]
- Koleoso, M.; Feng, X.; Xue, Y.; Li, Q.; Munshi, T.; Chen, X. Micro/nanoscale magnetic robots for biomedical applications. Mater. Today Bio 2020, 8, 100085. [Google Scholar] [CrossRef]
- Tolba, M.F.; Elghazaly, H.; Bousoik, E.; Elmazar, M.M.A.; Tolaney, S.M. Novel combinatorial strategies for boosting the efficacy of immune checkpoint inhibitors in advanced breast cancers. Clin. Transl. Oncol. 2021, 23, 1979–1994. [Google Scholar] [CrossRef]
- Ji, Q.; Fu, Y.; Zhu, X.; Wang, L.; Ling, C. Effect of RFA and TACE combined with postoperative cytokine-induced killer cell immunotherapy in primary hepatocellular carcinoma. J. Buon 2021, 26, 235–242. [Google Scholar]
- Lou, Y.; Jia, S.; Liu, P.; Xu, L.X. CCL5 Deficiency Enhanced Cryo–Thermal-Triggered Long-Term Anti-Tumor Immunity in 4T1 Murine Breast Cancer. Biomedicines 2022, 10, 559. [Google Scholar] [CrossRef] [PubMed]
- Skandalakis, G.P.; Rivera, D.R.; Rizea, C.D.; Bouras, A.; Jesu Raj, J.G.; Bozec, D.; Hadjipanayis, C.G. Hyperthermia treatment advances for brain tumors. Int. J. Hyperth. 2020, 37, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Hornowski, T.; Antal, I.; Rajnak, M.; Timko, M.; Józefczak, A. Sono-Magnetic Heating in Tumor Phantom. J. Magn. Magn. Mater. 2020, 500, 166396. [Google Scholar] [CrossRef]
- Lyng, H.; Rofstad, E.K. Treatment Failure following Sequential Thermoradiotherapy of Locally Advanced Breast Carcinoma Occurs Primarily in Poorly Vascularized Tumors. Oncology 1995, 52, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Li, Y.; Zhao, Y.; Shan, T.; Gong, X.; Li, F.; Tang, M.-X.; Wang, Z. Acoustic Beam Mapping for Guiding HIFU Therapy In Vivo Using Sub-Therapeutic Sound Pulse and Passive Beamforming. IEEE Trans. Biomed. Eng. 2021, 69, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
| Thermal Therapy | Thermal Source | Wavelength/Frequency |
|---|---|---|
| RFA | Electromagnetic Field | 200 kHz to 1200 kHz [35] |
| MWA | Electromagnetic Field | 300 MHz to 300 GHz [36] |
| MPH | Magnetic Field | 0.1 kHz to 4000 kHz [26] |
| LITT/PTT | Light | 532 nm to 2100 nm [37,38,39] |
| HIFU | Ultrasound | 0.5 MHz to 10 MHz [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Cao, B.; Zhao, S.; Zhang, A. Synergetic Thermal Therapy for Cancer: State-of-the-Art and the Future. Bioengineering 2022, 9, 474. https://doi.org/10.3390/bioengineering9090474
Dai Q, Cao B, Zhao S, Zhang A. Synergetic Thermal Therapy for Cancer: State-of-the-Art and the Future. Bioengineering. 2022; 9(9):474. https://doi.org/10.3390/bioengineering9090474
Chicago/Turabian StyleDai, Qizheng, Bo Cao, Shiqing Zhao, and Aili Zhang. 2022. "Synergetic Thermal Therapy for Cancer: State-of-the-Art and the Future" Bioengineering 9, no. 9: 474. https://doi.org/10.3390/bioengineering9090474







