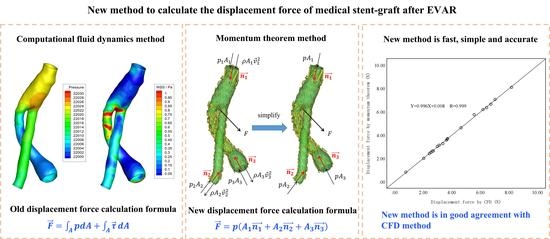
Fast and Accurate Computation of the Displacement Force of Stent Grafts after Endovascular Aneurysm Repair
Abstract
1. Introduction
2. Materials and Methods
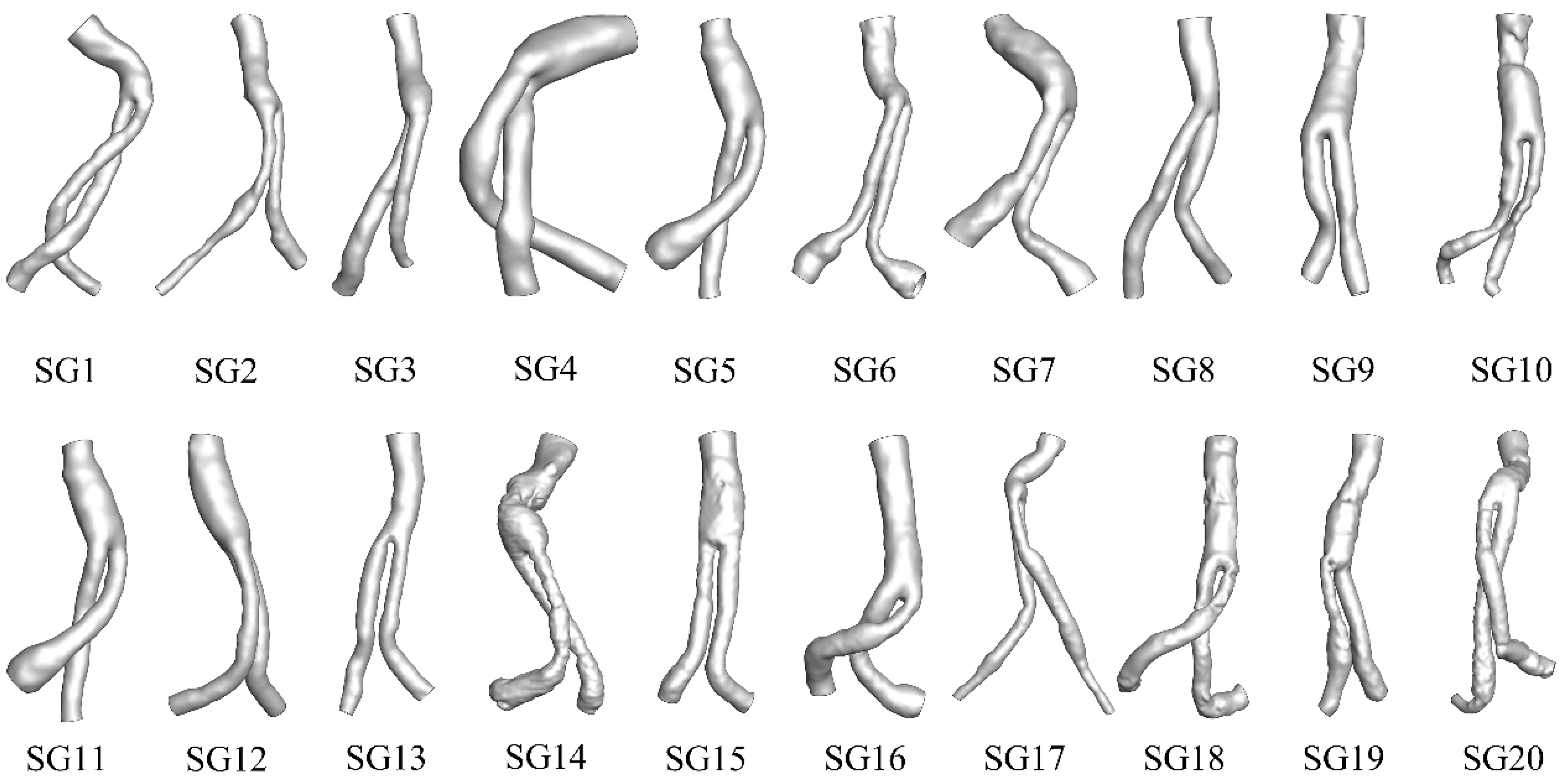
2.1. Geometry
2.2. CFD Computation
2.2.1. Governing Equations
2.2.2. Boundary Conditions
2.2.3. Meshing and Computing
2.2.4. Calculation of Displacement Force
2.3. The Proposed Approach to Computing DF
2.3.1. Governing Equations
2.3.2. Measurement of the Cross-Sectional Area and Angles of SG Ends
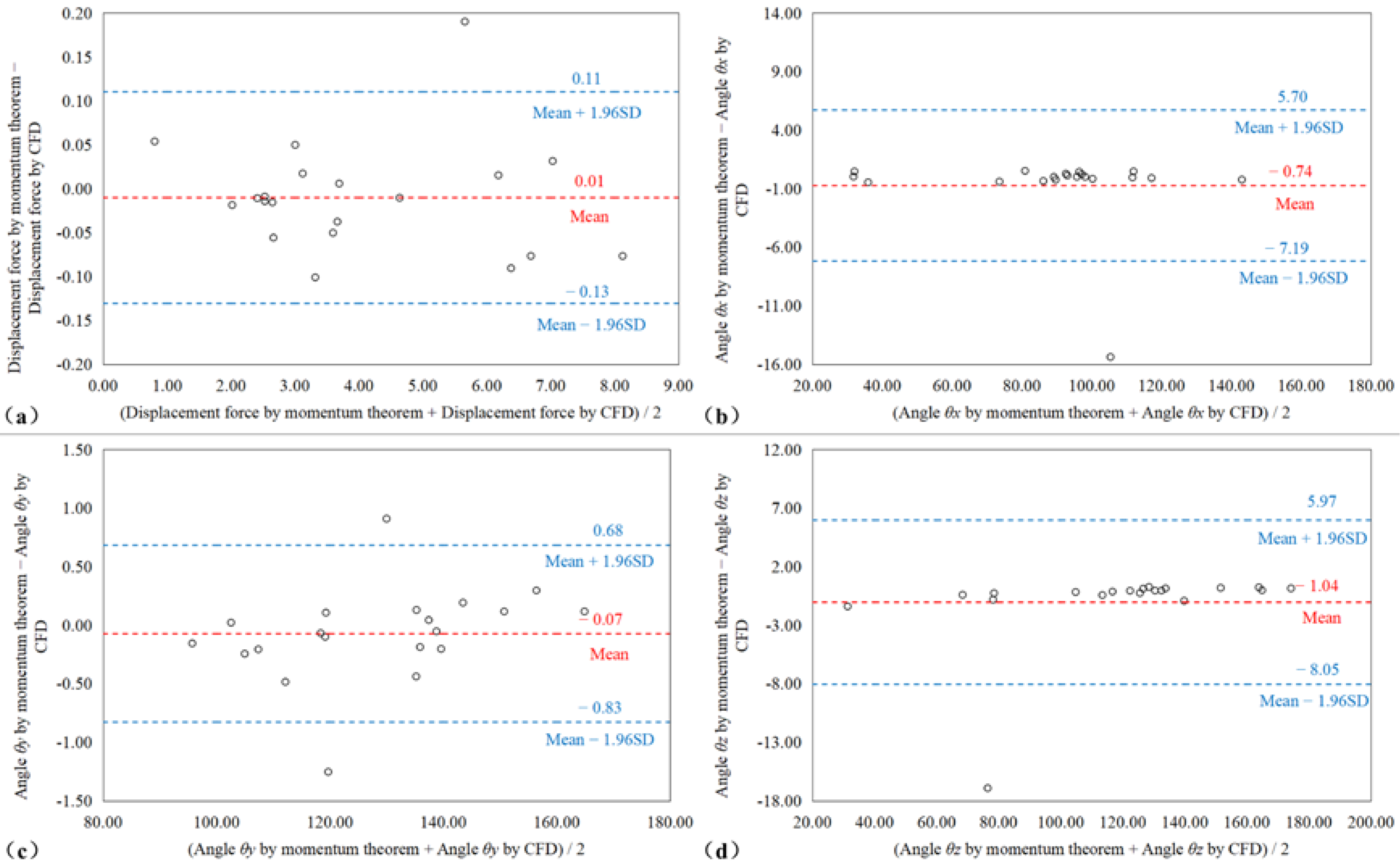
2.4. Statistical Analysis
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanhainen, A.; Verzini, F.; van herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2018, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Upchurch, G. The Society of Vascular Surgery Practice Guidelines on the Care of Patients with Abdominal Aortic Aneurysms. J. Vasc. Surg. 2019, 154, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, R.; Brown, L.; Kwong, G.P.S.; Powell, J.; Thompson, S. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet 2004, 364, 843–848. [Google Scholar] [CrossRef]
- Becquemin, J.P.; Pillet, J.C.; Lescalie, F.; Sapoval, M.; Goueffic, Y.; Lermusiaux, P.; Steinmetz, E.; Marzelle, J.; ACE Trialists. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J. Vasc. Surg. 2011, 53, 1167–1173.e1. [Google Scholar] [CrossRef]
- De Bruin, J.L.; Baas, A.F.; Buth, J.; Prinssen, M.; Verhoeven, E.L.; Cuypers, P.W.; van Sambeek, M.R.H.M.; Balm, R.; Grobbee, D.E.; Blankensteijn, J.D.; et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N. Engl. J. Med. 2010, 362, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Sweeting, M.J.; Ulug, P.; Blankensteijn, J.D.; Lederle, F.A.; Becquemin, J.P.; Greenhalgh, R.M.; Greenhalgh, R.M.; Beard, J.D.; Buxton, M.J.; et al. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br. J. Surg. 2017, 104, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.; Goldberg, J.; Macsweeney, S. A UK Multi-centre Experience with a Second-generation Endovascular Stent-graft: Results from the Zenith Users Group. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2004, 27, 51–55. [Google Scholar] [CrossRef]
- Rödel, S.; Geelkerken, R.; Prescott, R.; Florek, H.; Kasprzak, P.; Brunkwall, J. The Anaconda (TM) AAA Stent Graft System: 2-Year Clinical and Technical Results of a Multicentre Clinical Evaluation. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2009, 38, 732–740. [Google Scholar] [CrossRef]
- Sternbergh Iii, W.C.; Carter, G.; York, J.; Yoselevitz, M.; Money, S. Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. Off. Publ. Soc. Vasc. Surg. Int. Soc. Cardiovasc. Surg. N. Am. Chapter 2002, 35, 482–486. [Google Scholar] [CrossRef]
- Figueroa, C.; Taylor, C.; Yeh, V.; Chiou, A.; Gorrepati, M.; Zarins, C. Preliminary 3D Computational Analysis of the Relationship between Aortic Displacement Force and Direction of Endograft Movement. J. Vasc. Surg. 2010, 51, 1488–1497; discussion 1497. [Google Scholar] [CrossRef]
- Harris, P.; Vallabhaneni, S.R.; Desgranges, P.; Becquemin, J.-P.; van Marrewijk, C.; Laheij, R.J.F. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: The EUROSTAR experience. J. Vasc. Surg. 2000, 32, 739–749. [Google Scholar] [CrossRef]
- Sampram, E.; Karafa, M.; Mascha, E.; Clair, D.; Greenberg, R.; Lyden, S.; O’Hara, P.J.; Sarac, T.P.; Srivastava, S.D.; Butler, B.; et al. Nature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm * ** *. J. Vasc. Surg. Off. Publ. Soc. Vasc. Surg. Int. Soc. Cardiovasc. Surg. N. Am. Chapter 2003, 37, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Resch, T.; Ivancev, K.; Brunkwall, J.; Nyman, U.; Malina, M.; Lindblad, B. Distal Migration of Stent-Grafts after Endovascular Repair of Abdominal Aortic Aneurysms. J. Vasc. Interv. Radiol. 1999, 10, 257–264. [Google Scholar] [CrossRef]
- Krsmanovic, D.; Koncar, I.; Petrovic, D.; Milašinović, D.; Davidovic, L.; Filipovic, N. Computer modelling of maximal displacement forces in endoluminal thoracic aortic stent graft. Comput. Methods Biomech. Biomed. Eng. 2012, 17, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, L.; Wang, Y.; Zhao, J.; Zheng, T.; Yuan, D. Predictor of false lumen thrombosis after thoracic endovascular aortic repair for type B dissection. J. Thorac. Cardiovasc. Surg. 2019, 160, 360–367. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, J.; Zhao, J.; Wang, T.; Zheng, T.; Yuan, D. Association between blood flow pattern and rupture risk of abdominal aortic aneurysm based on computational fluid dynamics. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2022. [Google Scholar] [CrossRef]
- Qing, M.; Qiu, Y.; Wang, J.; Zheng, T.; Yuan, D. A Comparative Study on the Hemodynamic Performance within Cross and Non-cross Stent-Grafts for Abdominal Aortic Aneurysms with an Angulated Neck. Front. Physiol. 2021, 12, 795085. [Google Scholar] [CrossRef]
- Xiong, Z.; Yang, P.; Li, D.; Qiu, Y.; Zheng, T.; Hu, J. A computational fluid dynamics analysis of a patient with acute non-A-non-B aortic dissection after type I hybrid arch repair. Med. Eng. Phys. 2020, 77, 43–52. [Google Scholar] [CrossRef]
- Figueroa, C.; Taylor, C.; Yeh, V.; Chiou, A.; Zarins, C. Effect of Curvature on Displacement Forces Acting on Aortic Endografts: A 3-Dimensional Computational Analysis. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2009, 16, 284–294. [Google Scholar]
- Georgakarakos, E.; Xenakis, A.; Manopoulos, C.; Georgiadis, G.; Tsangaris, S.; Lazarides, M. Modeling and Computational Analysis of the Hemodynamic Effects of Crossing the Limbs in an Aortic Endograft (“Ballerina” Position). J. Endovasc. Ther. 2012, 19, 549–557. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Ma, Y.; Huang, B.; Yang, Y.; Yuan, D.; Weng, C.; Wang, T. Mid Term Outcomes of Crossed Limb vs Standard Limb Configuration in Endovascular Abdominal Aortic Aneurysm Repair: A Propensity Score Analysis. J. Vasc. Surg. 2021, 73, 1832. [Google Scholar] [CrossRef]
- Mohan, I.; Harris, P.; van Marrewijk, C.; Laheij, R.J.F.; How, T. Factors and Forces Influencing Stent-Graft Migration after Endovascular Aortic Aneurysm Repair. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2002, 9, 748–755. [Google Scholar]
- Liffman, K.; Lawrence-Brown, M.; Semmens, J.; Bui, A.; Rudman, M.; Hartley, D. Analytical Modeling and Numerical Simulation of Forces in an Endoluminal Graft. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2001, 8, 358–371. [Google Scholar]
- Iasiello, M.; Vafai, K.; Andreozzi, A.; Bianco, N. Analysis of non-Newtonian effects within an aorta-iliac bifurcation region. J. Biomech. 2017, 64, 153–163. [Google Scholar] [CrossRef]
- Johnston, B.; Johnston, P.; Corney, S.; Kilpatrick, D. Non-Newtonian blood flow in human right coronary arteries: Steady state simulations. J. Biomech. 2004, 37, 709–720. [Google Scholar] [CrossRef]
- Qiu, Y.; Yuan, D.; Wang, Y.; Wen, J.; Zheng, T. Hemodynamic investigation of a patient-specific abdominal aortic aneurysm with iliac artery tortuosity. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 1–10. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.; Fan, Y.; Peng, L.; Liu, R.; Zhao, J.; Yuan, D.; Zheng, T. Role of intraluminal thrombus in abdominal aortic aneurysm ruptures: A hemodynamic point of view. Med. Phys. 2019, 46, 4263–4275. [Google Scholar] [CrossRef]
- Formaggia, L.; Quarteroni, A.; Veneziani, A. Cardiovascular Mathematics: Modeling and Simulation of the Circulatory System; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Roos, H.; Tokarev, M.; Chernoray, V.; Ghaffari, M.; Falkenberg, M.; Jeppsson, A.; Nilsson, H. Displacement Forces in Stent Grafts: Influence of Diameter Variation and Curvature Asymmetry. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 150–156. [Google Scholar] [CrossRef]
- Liu, M.; Sun, A.; Deng, X. Hemodynamic performance within crossed stent grafts: Computational and experimental study on the effect of cross position and angle. BioMed. Eng. OnLine 2018, 17, 85. [Google Scholar] [CrossRef]
- Mills, C.; Gabe, I.; Gault, J.; Mason, D.; Ross, J., Jr.; Braunwald, E.; Shillingford, J.P. Pressure flow relationship and vascular impedance in man. Cardiovasc. Res. 1970, 4, 405–417. [Google Scholar] [CrossRef]
- Roache, P.J. Perspective: A Method for Uniform Reporting of Grid Refinement Studies. J. Fluids Eng.-Trans. Asme 1994, 116, 405–413. [Google Scholar] [CrossRef]
- Kelsey, L.; Powell, J.; Norman, P.; Miller, K.; Doyle, B. A comparison of hemodynamic metrics and intraluminal thrombus burden in a common iliac artery aneurysm: Comparing Hemodynamic Metrics with Thrombus in Iliac Artery Aneurysms. Int. J. Numer. Methods Biomed. Eng. 2016, 33, e02821. [Google Scholar] [CrossRef]
- Celik, I.; Ghia, U.; Roache, P.J.; Freitas, C.; Coloman, H.; Raad, P. Procedure of Estimation and Reporting of Uncertainty Due to Discretization in CFD Applications. J. Fluids Eng. 2008, 130, 078001. [Google Scholar]
- Vieira, S.; Corrente, J. Statistical methods for assessing agreement between double readings of clinical measurements. J. Appl. Oral Sci. Rev. FOB 2011, 19, 488–492. [Google Scholar] [CrossRef]
- Jiang, J.; Li, C.; Hu, Y.; Li, C.; He, J.; Leng, X.; Xiang, J.; Geb, J.; Wang, J. A Novel CFD-based Computed Index of Microcirculatory Resistance (IMR) Derived from Coronary Angiography to Assess Coronary Microcirculation. Comput. Methods Programs Biomed. 2022, 221, 106897. [Google Scholar] [CrossRef]
- Volodos, N.; Karpovich, I.P.; Shekhanin, V.E.; Troian, V.; Iakovenko, L.F. A case of distant transfemoral endoprosthesis of the thoracic artery using a self-fixing synthetic prosthesis in traumatic aneurysm. Grud. Khirurgiia 1988, 6, 84–86. [Google Scholar]
- Kalteis, M.; Benedikt, P.; Huber, F.; Haller, F.; Kastner, M.; Lugmayr, H. Looking for a Learning Curve in EVAR Based on the Zenith Stent Graft. Int. J. Angiol. Off. Publ. Int. Coll. Angiol. Inc. 2012, 21, 223–228. [Google Scholar]
- Zettervall, S.; Schermerhorn, M.; Soden, P.; McCallum, J.; Shean, K.; Deery, S.; O’Malley, A.J.; Landon, B. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J. Vasc. Surg. 2016, 65, 626–634. [Google Scholar] [CrossRef]
- Wang, S.; Hicks, C.; Malas, M. Neck diameter and inner curve seal zone predict endograft-related complications in highly angulated necks after endovascular aneurysm repair using the Aorfix endograft. J. Vasc. Surg. 2018, 67, 760–769. [Google Scholar] [CrossRef]
- Howard, D.; Marron, C.; Sideso, E.; Puckridge, P.; Verhoeven, E.; Spark, J. Influence of Proximal Aortic Neck Diameter on Durability of Aneurysm Sealing and Overall Survival in Patients Undergoing Endovascular Aneurysm Repair. Real World Data from the Gore Global Registry for Endovascular Aortic Treatment (GREAT). Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2018, 56, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Domanin, M.; Bissacco, D.; Romarowsky, R.; Conti, M.; Auricchio, F.; Ferraresi, M.; Trimarchi, S. Drag Forces after Thoracic Endovascular Aortic Repair. Gen. Rev. Literature. Ann. Vasc. Surg. 2021, 75, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, F.; McGloughlin, T.; Morris, L. A computational assessment of the hemodynamic effects of crossed and non-crossed bifurcated stent-graft devices for the treatment of abdominal aortic aneurysms. Med. Eng. Phys. 2016, 38, 1458–1573. [Google Scholar] [PubMed]
- Li, Z.; Kleinstreuer, C. Analysis of biomechanical factors affecting stent-graft migration in an abdominal aortic aneurysm. J. Biomech. 2006, 39, 2264–2273. [Google Scholar] [CrossRef]
- Roos, H.; Ghaffari, M.; Falkenberg, M.; Chernoray, V.; Jeppsson, A.; Nilsson, H. Displacement Forces in Iliac Landing Zones and Stent Graft Interconnections in Endovascular Aortic Repair: An Experimental Study. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2014, 47, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Asenbaum, U.; Schoder, M.; Schwartz, E.; Langs, G.; Baltzer, P.; Wolf, F.; Prusa, A.M.; Loewe, C.; Nolz, R. Stent-graft surface movement after endovascular aneurysm repair: Baseline parameters for prediction, and association with migration and stent-graft-related endoleaks. Eur. Radiol. 2019, 29, 6385–6395. [Google Scholar] [CrossRef]
- Sternbergh Iii, W.C.; Money, S.; Greenberg, R.; Chuter, T. Influence of endograft oversizing on device migration, endoleak, aneurysm shrinkage, and aortic neck dilation: Results from the Zenith Multicenter Trial. J. Vasc. Surg. Off. Publ. Soc. Vasc. Surg. Int. Soc. Cardiovasc. Surg. N. Am. Chapter 2004, 39, 20–26. [Google Scholar] [CrossRef]
- Prehn, J.; Schlösser, F.; Muhs, B.; Verhagen, H.; Moll, F.; Herwaarden, J.A. Oversizing of Aortic Stent Grafts for Abdominal Aneurysm Repair: A Systematic Review of the Benefits and Risks. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2009, 38, 42–53. [Google Scholar] [CrossRef]
- Rouby, A.F.; Kuntz, S.; Delay, C.; Thaveau, F.; Georg, Y.; Lejay, A.; Chakfe, N. Volume Change after Endovascular Treatment of Common Iliac Arteries ≥ 17 mm Diameter: Assessment of Type 1b Endoleak Risk Factors. Eur. J. Vasc. Endovasc. Surg. 2019, 59, 51–58. [Google Scholar] [CrossRef]
- Rahmani, S.; Grewal, I.; Nabovati, A.; Doyle, M.; Roche-Nagle, G.; Tse, L. Increasing angulation decreases measured aortic stent graft pullout forces. J. Vasc. Surg. 2014, 63, 493–499. [Google Scholar] [CrossRef][Green Version]
- Belvroy, V.; Romarowski, R.; van Bakel, T.; Herwaarden, J.; Bismuth, J.; Auricchio, F.; Moll, F.L.; Trimarchi, S. Impact of Aortic Tortuosity on Displacement Forces in Descending Thoracic Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surgery 2020, 59, 557–564. [Google Scholar] [CrossRef]



| Heading | CFD Method (N) | Simplified Momentum Quantitative (N) | Relative Error (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fx | Fy | Fz | F | Fx | Fy | Fz | F | ||
| SG1 | 4.49 | −2.12 | −2.51 | 5.56 | 4.67 | −2.15 | −2.58 | 5.75 | 3.42 |
| SG2 | –0.37 | −2.39 | −1.74 | 2.98 | −0.38 | −2.44 | −1.76 | 3.03 | 1.67 |
| SG3 | 0.87 | −2.72 | –1.25 | 3.11 | 0.9 | −2.73 | −1.24 | 3.13 | 0.55 |
| SG4 | −5.62 | −3.34 | 2.56 | 7.02 | −5.62 | −3.35 | 2.62 | 7.05 | 0.45 |
| SG5 | 3.14 | −1.80 | 0.73 | 3.69 | 3.12 | −1.81 | 0.78 | 3.7 | 0.16 |
| SG6 | −0.31 | −0.71 | 0.07 | 0.78 | −0.11 | −0.76 | 0.31 | 0.83 | 6.91 |
| SG7 | 0.59 | −1.83 | 3.07 | 3.62 | 0.55 | −1.74 | 3.07 | 3.57 | 1.39 |
| SG8 | −0.13 | −1.61 | −1.95 | 2.53 | −0.14 | −1.64 | −1.92 | 2.53 | 0.36 |
| SG9 | 0.01 | −0.24 | −2.40 | 2.42 | 0.02 | −0.24 | −2.39 | 2.41 | 0.45 |
| SG10 | −0.48 | −0.73 | −3.25 | 3.37 | −0.46 | −0.71 | −3.16 | 3.27 | 3 |
| SG11 | 3.13 | −1.80 | 0.72 | 3.68 | 3.1 | −1.78 | 0.73 | 3.65 | 1.02 |
| SG12 | 0.16 | −1.83 | −1.75 | 2.54 | 0.18 | −1.81 | −1.75 | 2.52 | 0.56 |
| SG13 | −0.20 | −0.53 | −1.95 | 2.03 | −0.20 | −0.52 | −1.93 | 2.01 | 0.92 |
| SG14 | −0.27 | −5.96 | −1.59 | 6.18 | −0.30 | −5.98 | −1.58 | 6.19 | 0.25 |
| SG15 | −0.73 | −5.07 | −4.37 | 6.73 | −0.77 | −5.00 | −4.31 | 6.65 | 1.14 |
| SG16 | −2.12 | −3.30 | −2.49 | 4.64 | −2.11 | −3.30 | −2.48 | 4.63 | 0.23 |
| SG17 | −1.00 | −0.81 | −2.37 | 2.69 | −0.98 | −0.78 | −2.32 | 2.64 | 2.08 |
| SG18 | −3.03 | −5.82 | −4.86 | 8.17 | −3.06 | −5.72 | −4.83 | 8.09 | 0.94 |
| SG19 | −0.48 | −2.03 | −1.65 | 2.66 | −0.47 | −2.01 | −1.65 | 2.64 | 0.59 |
| SG20 | 0.09 | −4.73 | −4.35 | 6.43 | 0.09 | −4.67 | −4.28 | 6.34 | 1.41 |
| Heading | CFD Method (°) | Simplified Momentum Quantitative (°) | Relative Error (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| θx | θy | θz | θx | θy | θz | ||||
| SG1 | 35.71 | 111.92 | 116.66 | 36.18 | 112.41 | 116.79 | 1.29 | 0.43 | 0.11 |
| SG2 | 97.26 | 143.59 | 125.45 | 97.04 | 143.4 | 125.7 | 0.22 | 0.13 | 0.2 |
| SG3 | 73.37 | 150.82 | 113.25 | 73.77 | 150.7 | 113.68 | 0.54 | 0.08 | 0.38 |
| SG4 | 142.94 | 118.35 | 68.21 | 143.17 | 118.42 | 68.62 | 0.16 | 0.06 | 0.6 |
| SG5 | 32.28 | 119.37 | 77.82 | 31.81 | 119.27 | 78.64 | 1.46 | 0.09 | 1.04 |
| SG6 | 97.72 | 156.59 | 68.04 | 113.1 | 156.29 | 84.96 | 13.6 | 0.19 | 19.91 |
| SG7 | 81.18 | 119.09 | 30.65 | 80.67 | 120.35 | 32.05 | 0.64 | 1.04 | 4.36 |
| SG8 | 93.08 | 130.47 | 139.36 | 92.96 | 129.56 | 140.28 | 0.13 | 0.7 | 0.66 |
| SG9 | 89.57 | 95.65 | 174.34 | 89.81 | 95.8 | 174.2 | 0.26 | 0.16 | 0.08 |
| SG10 | 98.15 | 102.58 | 164.94 | 98.15 | 102.56 | 164.96 | 0 | 0.02 | 0.01 |
| SG11 | 31.78 | 119.14 | 78.42 | 31.76 | 119.24 | 78.68 | 0.07 | 0.08 | 0.34 |
| SG12 | 85.98 | 135.83 | 133.89 | 86.33 | 136.01 | 133.75 | 0.4 | 0.14 | 0.1 |
| SG13 | 95.77 | 104.86 | 164.01 | 95.77 | 105.1 | 163.78 | 0.01 | 0.23 | 0.14 |
| SG14 | 92.74 | 164.98 | 104.76 | 92.46 | 164.86 | 104.93 | 0.3 | 0.07 | 0.16 |
| SG15 | 96.69 | 138.78 | 130.43 | 96.25 | 138.83 | 130.48 | 0.46 | 0.04 | 0.04 |
| SG16 | 117.11 | 135.35 | 122.34 | 117.21 | 135.22 | 122.4 | 0.08 | 0.1 | 0.05 |
| SG17 | 111.7 | 107.28 | 151.68 | 111.75 | 107.49 | 151.5 | 0.04 | 0.19 | 0.12 |
| SG18 | 112.26 | 135.01 | 126.65 | 111.78 | 135.45 | 126.54 | 0.42 | 0.32 | 0.09 |
| SG19 | 100.19 | 139.54 | 128.63 | 100.35 | 139.74 | 128.37 | 0.16 | 0.15 | 0.2 |
| SG20 | 89.18 | 137.48 | 132.51 | 89.19 | 137.44 | 132.55 | 0 | 0.03 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qing, M.; Liu, Z.; Zheng, T. Fast and Accurate Computation of the Displacement Force of Stent Grafts after Endovascular Aneurysm Repair. Bioengineering 2022, 9, 447. https://doi.org/10.3390/bioengineering9090447
Qing M, Liu Z, Zheng T. Fast and Accurate Computation of the Displacement Force of Stent Grafts after Endovascular Aneurysm Repair. Bioengineering. 2022; 9(9):447. https://doi.org/10.3390/bioengineering9090447
Chicago/Turabian StyleQing, Ming, Zhan Liu, and Tinghui Zheng. 2022. "Fast and Accurate Computation of the Displacement Force of Stent Grafts after Endovascular Aneurysm Repair" Bioengineering 9, no. 9: 447. https://doi.org/10.3390/bioengineering9090447
APA StyleQing, M., Liu, Z., & Zheng, T. (2022). Fast and Accurate Computation of the Displacement Force of Stent Grafts after Endovascular Aneurysm Repair. Bioengineering, 9(9), 447. https://doi.org/10.3390/bioengineering9090447