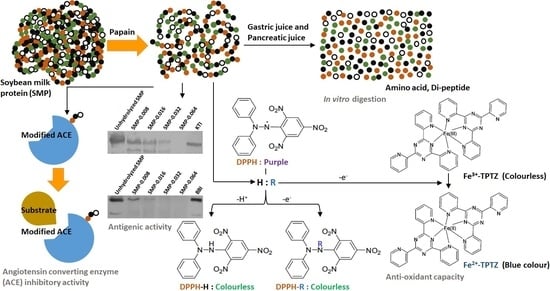
Hydrolysis of Soybean Milk Protein by Papain: Antioxidant, Anti-Angiotensin, Antigenic and Digestibility Perspectives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soybean Milk
2.2. Chemicals and Reagents
2.3. Hydrolysis of SMP by Papain
2.4. Analytical Methods
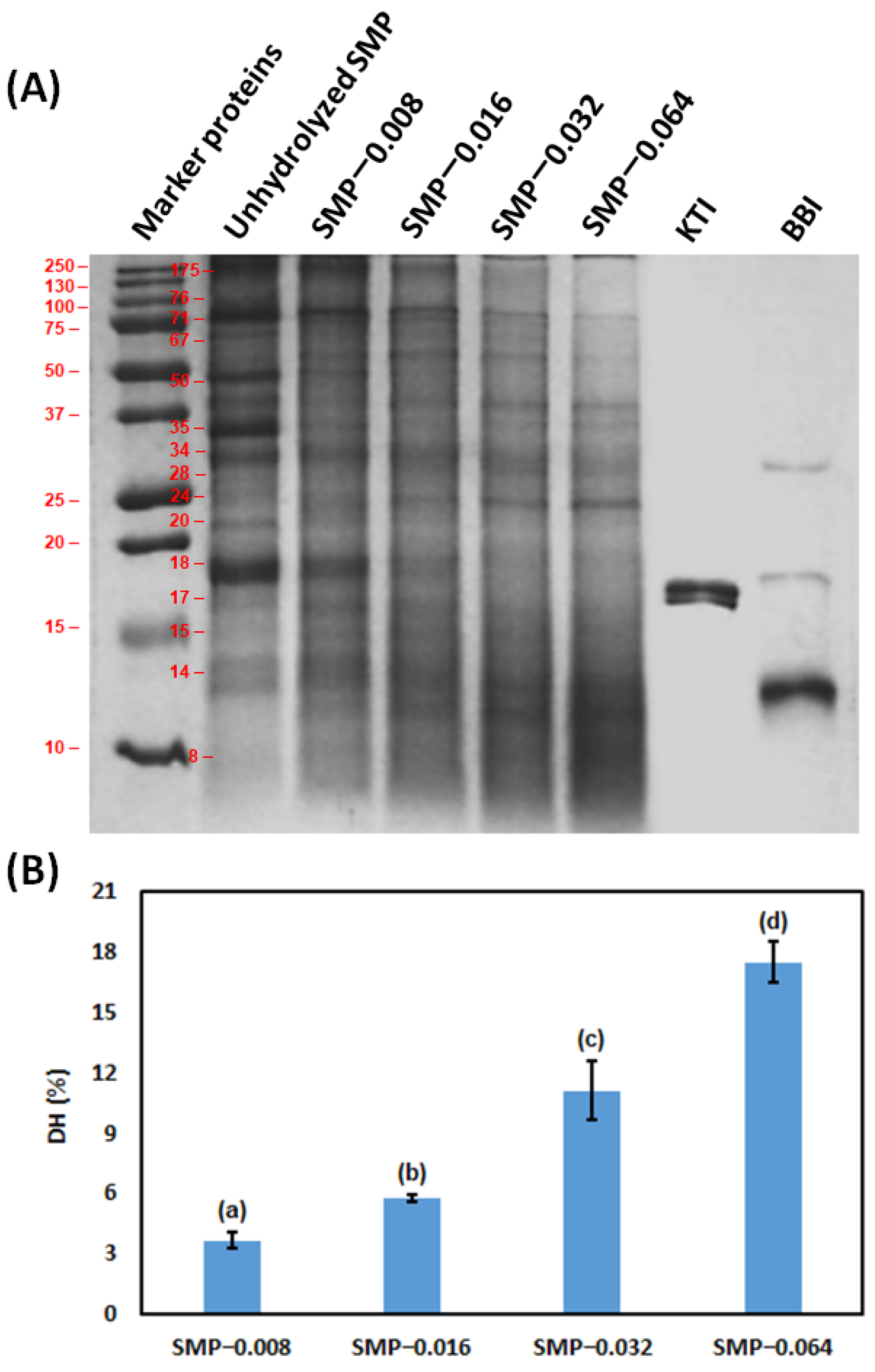
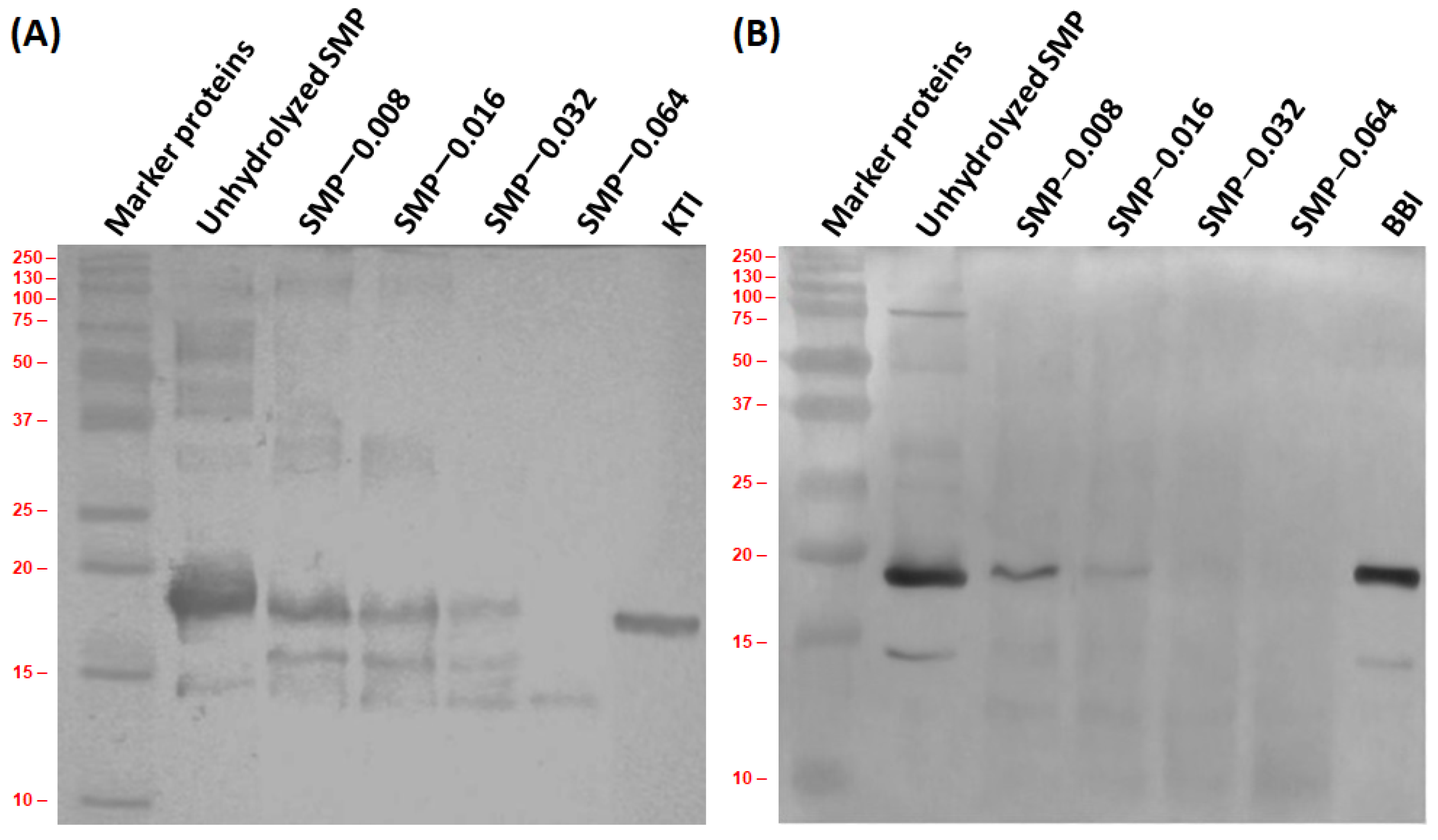
2.4.1. Molecular Weight of SMP and Peptides by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4.2. Immunoblotting
2.4.3. Degree of Hydrolysis (DH) of SMP
2.4.4. Determination of Protein Concentration
2.4.5. Determination of Total Carbohydrate Concentration
2.4.6. Determination of Total Fat Concentration
2.4.7. Determination of the Activity of Papain
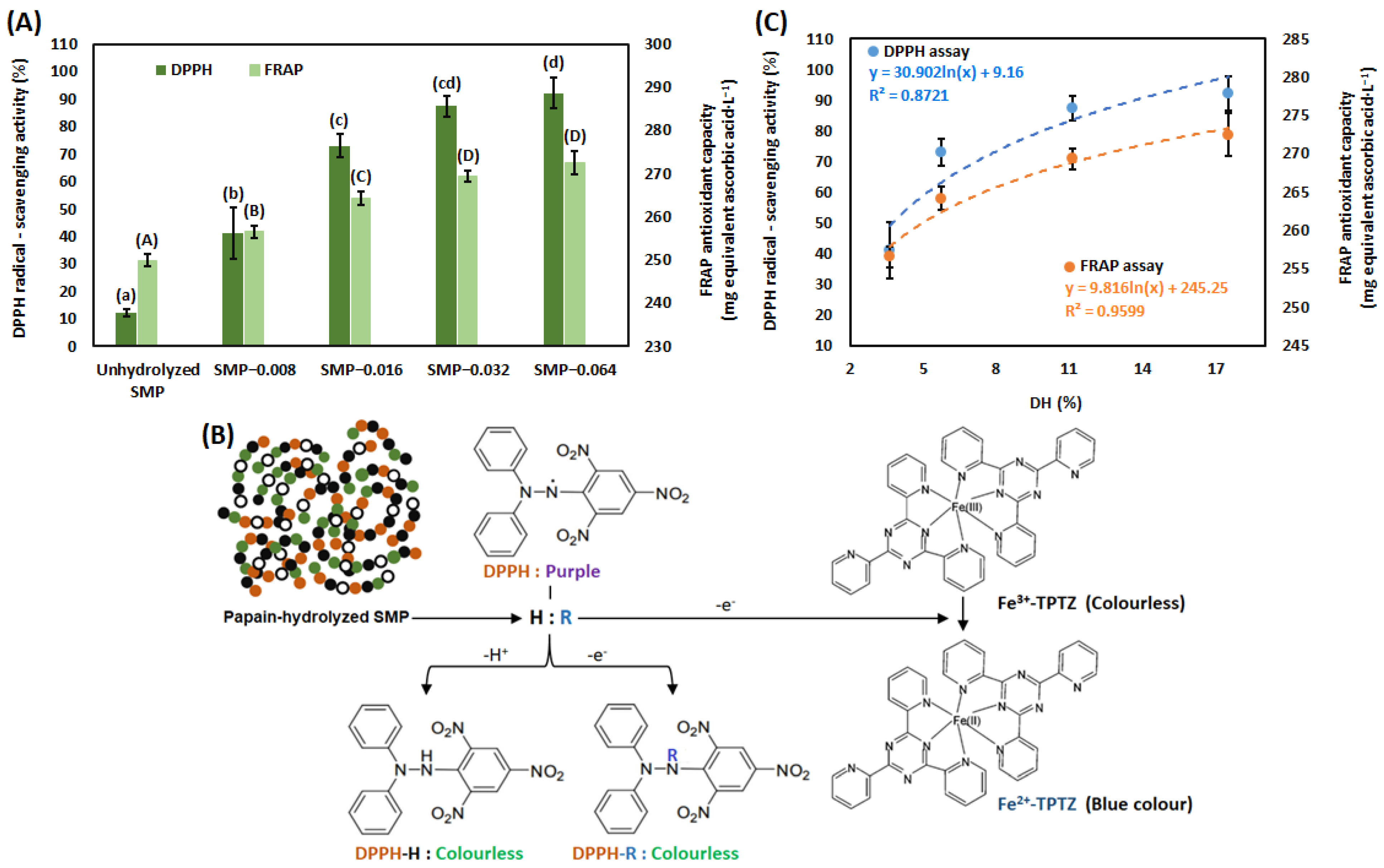
2.4.8. Determination of the Antioxidant Capacity
Ferric-Reducing Ability of Plasma (FRAP) Assay
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical-Scavenging Assay
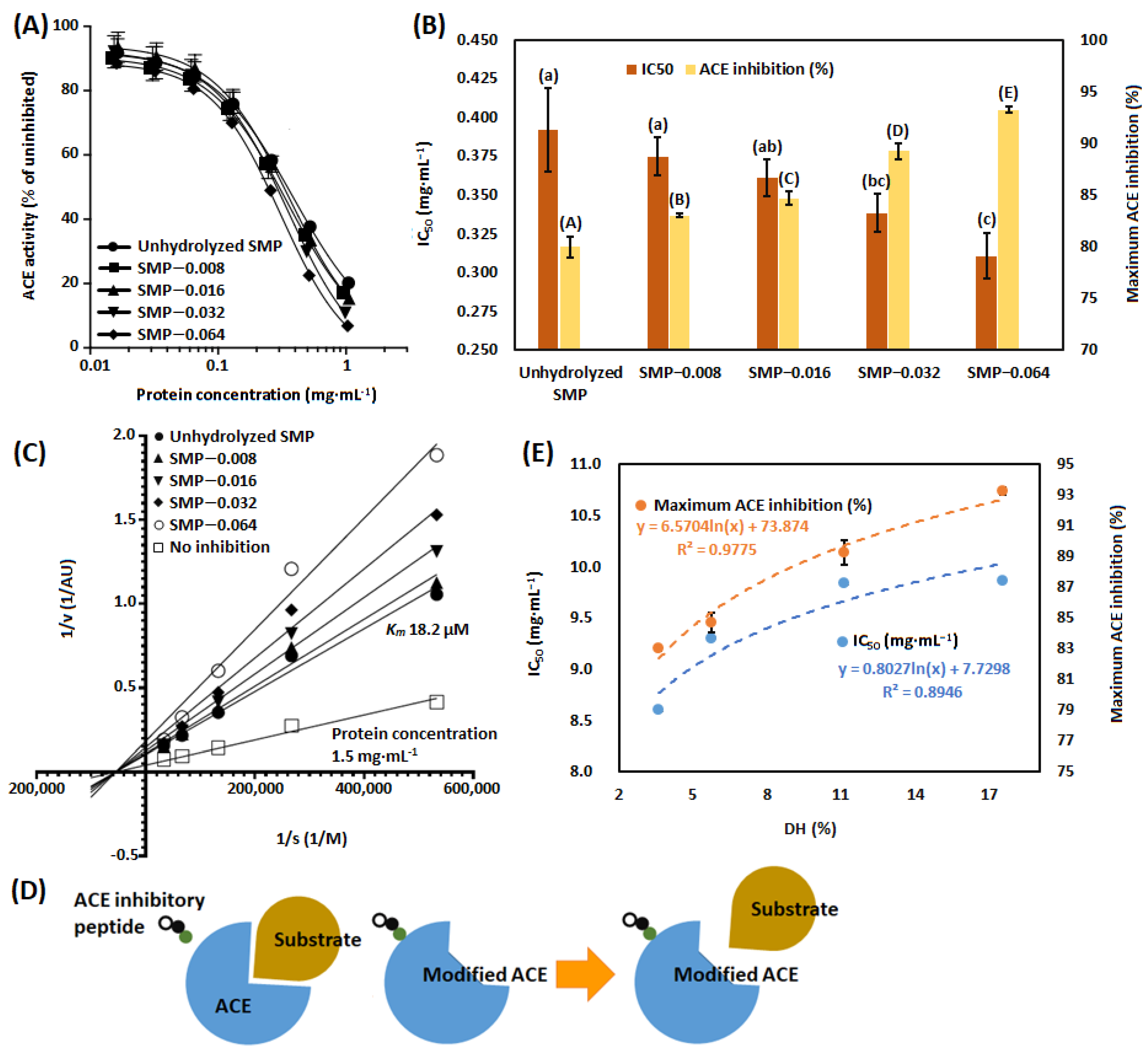
2.4.9. Determination of ACE Inhibitory Activity
2.4.10. Inhibitory Activities of KTI and BBI in Native (Non-Denaturing Condition) Polyacrylamide Gels
2.4.11. In Vitro Digestion
2.5. Statistical Analysis
3. Results and Discussion
3.1. Hydrolysis of SMP by Papain
3.2. Antioxidant Capacity
3.3. ACE-Inhibitory Activity
3.4. Antigenic Activity
3.5. Digestibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salter, A.M. Impact of Consumption of Animal Products on Cardiovascular Disease, Diabetes, and Cancer in Developed Countries. Anim. Front. 2013, 3, 20–27. [Google Scholar] [CrossRef]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow Milk versus Plant-Based Milk Substitutes: A Comparison of Product Image and Motivational Structure of Consumption. Sustainability 2019, 11, 5046. [Google Scholar] [CrossRef]
- McKevith, B. Nutritional Aspects of Oilseeds. Nutr. Bull. 2005, 30, 13–26. [Google Scholar] [CrossRef]
- L’Hocine, L.; Boye, J.I. Allergenicity of Soybean: New Developments in Identification of Allergenic Proteins, Cross-Reactivities and Hypoallergenization Technologies. Crit. Rev. Food Sci. Nutr. 2007, 47, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Huby, R.D.J.; Dearman, R.J.; Kimber, I. Why Are Some Proteins Allergens? Toxicol. Sci. 2000, 55, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Astwood, J.D.; Leach, J.N.; Fuchs, R.L. Stability of Food Allergens to Digestion in Vitro. Nat. Biotechnol. 1996, 14, 1269–1273. [Google Scholar] [CrossRef]
- Song, H.K.; Suh, S.W. Kunitz-Type Soybean Trypsin Inhibitor Revisited: Refined Structure of Its Complex with Porcine Trypsin Reveals an Insight into the Interaction between a Homologous Inhibitor from Erythrina Caffra and Tissue-Type Plasminogen Activator. J. Mol. Biol. 1998, 275, 347–363. [Google Scholar] [CrossRef]
- Liener, I.E. Implications of Antinutritional Components in Soybean Foods. Crit. Rev. Food Sci. Nutr. 1994, 34, 31–67. [Google Scholar] [CrossRef]
- Matsuo, A.; Matsushita, K.; Fukuzumi, A.; Tokumasu, N.; Yano, E.; Zaima, N.; Moriyama, T. Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans. Foods 2020, 9, 522. [Google Scholar] [CrossRef]
- Cordle, C.T. Soy Protein Allergy: Incidence and Relative Severity. J. Nutr. 2004, 134, 1213S–1219S. [Google Scholar] [CrossRef] [Green Version]
- Mulalapele, L.T.; Xi, J. Detection and Inactivation of Allergens in Soybeans: A Brief Review of Recent Research Advances. Grain Oil Sci. Technol. 2021, 4, 191–200. [Google Scholar] [CrossRef]
- L’Hocine, L.; Boye, J.I.; Jouve, S. Ionic Strength and PH-Induced Changes in the Immunoreactivity of Purified Soybean Glycinin and Its Relation to Protein Molecular Structure. J. Agric. Food Chem. 2007, 55, 5819–5826. [Google Scholar] [CrossRef] [PubMed]
- Franck, P.; Moneret Vautrin, D.A.; Dousset, B.; Kanny, G.; Nabet, P.; Guénard-Bilbaut, L.; Parisot, L. The Allergenicity of Soybean-Based Products Is Modified by Food Technologies. Int. Arch. Allergy Immunol. 2002, 128, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.-G.; Ko, Y.-J.; Kim, E.-J.; Lee, G.-L.; Kim, D.-G.; Lee, J.-O.; Ahn, K.-M.; Ryu, C.-H. Allergenicity Change of Soybean Proteins by Thermal Treatment Methods. J. Life Sci. 2012, 22, 524–531. [Google Scholar] [CrossRef]
- Žilić, S.; Božović, I.; Savić, S.; Šobajić, S. Heat Processing of Soybean Kernel and Its Effect on Lysine Availability and Protein Solubility. Open Life Sci. 2006, 1, 572–583. [Google Scholar] [CrossRef]
- Yin, H.; Jia, F.; Huang, J.; Zhang, Y.; Zheng, X.; Zhang, X. Effect of Extrusion on the Structure and Antigenicity of Soybean β-Conglycinin. Grain Oil Sci. Technol. 2019, 2, 67–72. [Google Scholar] [CrossRef]
- Cabanillas, B.; Cuadrado, C.; Rodriguez, J.; Dieguez, M.C.; Crespo, J.F.; Novak, N. Boiling and Pressure Cooking Impact on IgE Reactivity of Soybean Allergens. Int. Arch. Allergy Immunol. 2018, 175, 36–43. [Google Scholar] [CrossRef]
- Pi, X.; Sun, Y.; Guo, X.; Chen, Q.; Cheng, J.; Guo, M. Effects of Thermal Sterilization on the Allergenicity of Soybeans. LWT 2022, 154, 112678. [Google Scholar] [CrossRef]
- Xi, J.; He, M. High Hydrostatic Pressure (HHP) Effects on Antigenicity and Structural Properties of Soybean β-Conglycinin. J. Food Sci. Technol. 2018, 55, 630–637. [Google Scholar] [CrossRef]
- Peñas, E.; Gomez, R.; Frias, J.; Baeza, M.L.; Vidal-Valverde, C. High Hydrostatic Pressure Effects on Immunoreactivity and Nutritional Quality of Soybean Products. Food Chem. 2011, 125, 423–429. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W.; Guo, X. Comparative Study of Four Physical Approaches about Allergenicity of Soybean Protein Isolate for Infant Formula. Food Agric. Immunol. 2016, 27, 604–623. [Google Scholar] [CrossRef]
- Haddad, J.; Allaf, K. A Study of the Impact of Instantaneous Controlled Pressure Drop on the Trypsin Inhibitors of Soybean. J. Food Eng. 2007, 79, 353–357. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Singh, A.; Gariepy, Y.; Raghavan, V. Comparison of Conventional and Microwave Treatment on Soymilk for Inactivation of Trypsin Inhibitors and In Vitro Protein Digestibility. Foods 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schlüter, O.; Schweiggert-Weisz, U.; Eisner, P. The Effects of Pulsed Ultraviolet Light, Cold Atmospheric Pressure Plasma, and Gamma-Irradiation on the Immunoreactivity of Soy Protein Isolate. Innov. Food Sci. Emerg. Technol. 2016, 38, 374–383. [Google Scholar] [CrossRef]
- Yang, W.W.; Chung, S.-Y.; Ajayi, O.; Krishnamurthy, K.; Konan, K.; Goodrich-Schneider, R. Use of Pulsed Ultraviolet Light to Reduce the Allergenic Potency of Soybean Extracts. Int. J. Food Eng. 2010, 6, 1–12. [Google Scholar] [CrossRef]
- Moriyama, T.; Yano, E.; Kitta, K.; Kawamoto, S.; Kawamura, Y.; Todoriki, S. Effect of Gamma Irradiation on Soybean Allergen Levels. Biosci. Biotechnol. Biochem. 2013, 77, 2371–2377. [Google Scholar] [CrossRef]
- Zheng, T.; Li, X.; Taha, A.; Wei, Y.; Hu, T.; Fatamorgana, P.B.; Zhang, Z.; Liu, F.; Xu, X.; Pan, S.; et al. Effect of High Intensity Ultrasound on the Structure and Physicochemical Properties of Soy Protein Isolates Produced by Different Denaturation Methods. Food Hydrocoll. 2019, 97, 105216. [Google Scholar] [CrossRef]
- Magishi, N.; Yuikawa, N.; Kobayashi, M.; Taniuchi, S. Degradation and Removal of Soybean Allergen in Japanese Soy Sauce. Mol. Med. Rep. 2017, 16, 2264–2268. [Google Scholar] [CrossRef]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of Major Allergens and Allergenicity Reduction of Soybean Meal through Solid-State Fermentation with Microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Huang, J. Study on Enzymatic Hydrolysis of Soybean β-Conglycinin Using Alkaline Protease from Bacillus Subtilis ACCC 01746 and Antigenicity of Its Hydrolysates. Grain Oil Sci. Technol. 2021, 4, 18–25. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Yuan, D.; Zhao, X.; Cui, S.; Hu, J.; Wang, J. Reduction of the Allergenic Protein in Soybean Meal by Enzymatic Hydrolysis. Food Agric. Immunol. 2014, 25, 301–310. [Google Scholar] [CrossRef]
- Lee, M.R.; Chiu, T.C.; Dou, J. Determination of 1,3-Dichloro-2-Propanol and 3-Chloro-1,2-Propandiol in Soy Sauce by Headspace Derivatization Solid-Phase Microextraction Combined with Gas Chromatography–Mass Spectrometry. Anal. Chim. Acta 2007, 591, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.D.; Cromie, D.D.O.; Davies, A.P. Mechanism of Formation of Chloropropanols Present in Protein Hydrolysates. J. Am. Oil Chem. Soc. 1991, 68, 785–790. [Google Scholar] [CrossRef]
- Kerezsi, A.D.; Jacquet, N.; Blecker, C. Advances on Physical Treatments for Soy Allergens Reduction—A Review. Trends Food Sci. Technol. 2022, 122, 24–39. [Google Scholar] [CrossRef]
- Shibasaki, M.; Suzuki, S.; Tajima, S.; Nemoto, H.; Kuroume, T. Allergenicity of Major Component Proteins of Soybean. Int. Arch. Allergy Appl. Immunol. 1980, 61, 441–448. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and Health Benefits of Soy Proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- van Boxtel, E.L.; van den Broek, L.A.M.; Koppelman, S.J.; Gruppen, H. Legumin Allergens from Peanuts and Soybeans: Effects of Denaturation and Aggregation on Allergenicity. Mol. Nutr. Food Res. 2008, 52, 674–682. [Google Scholar] [CrossRef]
- Evans, C.E.; Garlich, J.D.; Stark, C.R.; Grimes, J.L. The Effect of Feed Processing of Novel Unheated, Low Trypsin Inhibitor Soybeans on the Performance of Young Female Turkeys Reared from Hatch to 21 Days of Age. Poult. Sci. 2021, 100, 101399. [Google Scholar] [CrossRef]
- Barac, M.B.; Jovanovic, S.T.; Stanojevic, S.P.; Pesic, M.B. Effect of Limited Hydrolysis on Traditional Soy Protein Concentrate. Sensors 2006, 6, 1087–1101. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, X.; Wei, M.; Zhao, W.; He, B.; Lu, Q.; Huo, Q.; Ma, C. Optimization of Glutamine Peptide Production from Soybean Meal and Analysis of Molecular Weight Distribution of Hydrolysates. Int. J. Mol. Sci. 2012, 13, 7483–7495. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Corrêa, A.P.F.; Coletto, D.; Daroit, D.J.; Cladera-Olivera, F.; Brandelli, A. Soy Protein Hydrolysis with Microbial Protease to Improve Antioxidant and Functional Properties. J. Food Sci. Technol. 2015, 52, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Hrčková, M.; Rusňáková, M.; Zemanovič, J. Enzymatic Hydrolysis of Defatted Soy Flour by Three Different Proteases and Their Effect on the Functional Properties of Resulting Protein Hydrolysates. Czech J. Food Sci. 2011, 20, 7–14. [Google Scholar] [CrossRef]
- Banan-Mwine Daliri, E.; Oh, D.H.; Lee, B.H. Bioactive Peptides—Review. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive Peptides: A Review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A Clinical Perspective of Obesity, Metabolic Syndrome and Cardiovascular Disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef] [PubMed]
- Ferder, L.; Inserra, F.; Martínez-Maldonado, M. Inflammation and the Metabolic Syndrome: Role of Angiotensin II and Oxidative Stress. Curr. Hypertens. Rep. 2006, 8, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Tham, D.M.; Martin-McNulty, B.; Wang, Y.; Wilson, D.W.; Vergona, R.; Sullivan, M.E.; Dole, W.; Rutledge, J.C. Angiotensin II Is Associated with Activation of NF-KappaB-Mediated Genes and Downregulation of PPARs. Physiol. Genom. 2002, 11, 21–30. [Google Scholar] [CrossRef]
- Kato, S.; Luyckx, V.A.; Ots, M.; Lee, K.W.; Ziai, F.; Troy, J.L.; Brenner, B.M.; MacKenzie, H.S. Renin-Angiotensin Blockade Lowers MCP-1 Expression in Diabetic Rats. Kidney Int. 1999, 56, 1037–1048. [Google Scholar] [CrossRef]
- Jahandideh, F.; Wu, J. Perspectives on the Potential Benefits of Antihypertensive Peptides towards Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2192. [Google Scholar] [CrossRef] [Green Version]
- Nath, A.; Szécsi, G.; Csehi, B.; Mednyánszky, Z.; Kiskó, G.; Bányai, É.; Dernovics, M.; Koris, A. Production of Hypoallergenic Antibacterial Peptides from Defatted Soybean Meal in Membrane Bioreactor: A Bioprocess Engineering Study with Comprehensive Product Characterization. Food Technol. Biotechnol. 2017, 55, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hou, Y. Limited Hydrolysis of Soybean Protein Concentrate and Isolate with Two Proteases and the Impact on Emulsifying Activity Index of Hydrolysates. Afr. J. Biotechnol. 2009, 8, 3314–3319. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-Oxidative Capacity of Enzymatically Released Peptides from Soybean Protein Isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Reitmeier, C.; Murphy, P.A.; Johnson, L.A. Enzymatic Hydrolysis of Extruded-Expelled Soy Flour and Resulting Functional Properties. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 731–737. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Amorim, M.M.; Voss, G.B.; Nerli, B.B.; Picó, G.A.; Pintado, M.E. Bioactive Properties of Peptides Obtained from Argentinian Defatted Soy Flour Protein by Corolase PP Hydrolysis. Food Chem. 2016, 198, 36–44. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and Characterization of Bioactive Peptides from Soy Hydrolysate and Soy-Fermented Food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.S.; Baek, H.H.; Lee, H.G. Purification and Characterization of Antioxidant Peptides from Soy Protein Hydrolysate. J. Food Biochem. 2010, 34, 120–132. [Google Scholar] [CrossRef]
- Maehashi, K.; Arai, S. Taste evaluation for peptides in protein hydrolysates from soybean and other plants. In Analysis of Taste and Aroma; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Ishibashi, N.; Ono, I.; Kato, K.; Shigenaga, T.; Shinoda, I.; Okai, H.; Fukui, S. Role of the Hydrophobic Amino Acid Residue in the Bitterness of Peptides. Agric. Biol. Chem. 1988, 52, 91–94. [Google Scholar] [CrossRef]
- Ha, M.; Bekhit, A.E.D.A.; Carne, A.; Hopkins, D.L. Characterisation of Commercial Papain, Bromelain, Actinidin and Zingibain Protease Preparations and Their Activities toward Meat Proteins. Food Chem. 2012, 134, 95–105. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Hopkins, D.L.; Geesink, G.; Bekhit, A.A.; Franks, P. Exogenous Proteases for Meat Tenderization. Crit. Rev. Food Sci. Nutr. 2014, 54, 1012–1031. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Kailo, G.G.; Mednyánszky, Z.; Kiskó, G.; Csehi, B.; Pásztorné-Huszár, K.; Gerencsér-Berta, R.; Galambos, I.; Pozsgai, E.; Bánvölgyi, S.; et al. Antioxidant and Antibacterial Peptides from Soybean Milk through Enzymatic- and Membrane-Based Technologies. Bioengineering 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Z.; Guo, M.M.; Hua, Y.F.; Cao, D.; Zhang, C.M. Enzymatic Preparation of Immunomodulating Hydrolysates from Soy Proteins. Bioresour. Technol. 2008, 99, 8873–8879. [Google Scholar] [CrossRef]
- Zeng, M.; Adhikari, B.; He, Z.; Qin, F.; Huang, X.; Chen, J. Improving the Foaming Properties of Soy Protein Isolate Through Partial Enzymatic Hydrolysis. Dry. Technol. 2013, 31, 1545–1552. [Google Scholar] [CrossRef]
- Marinova, M.; Cuc, N.T.K.; Tchorbanov, B. Enzymatic Hydrolysis of Soy Protein Isolate by Food Grade Proteinases and Aminopeptidases of Plant Origin. Biotechnol. Biotechnol. Equip. 2008, 22, 835–838. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xiong, Y.L. Antioxidant Activity of Soy Protein Hydrolysates in a Liposomal System. J. Food Sci. 2002, 67, 2952–2956. [Google Scholar] [CrossRef]
- Lee, J.S.; Mi, A.Y.; Seung, H.K.; Baek, H.H.; Hyeon, G.L. Antioxidant and ACE Inhibitory Activities of Soybean Hydrolysates: Effect of Enzyme and Degree of Hydrolysis. Food Sci. Biotechnol. 2008, 17, 873–877. [Google Scholar]
- Meinlschmidt, P.; Sussmann, D.; Schweiggert-Weisz, U.; Eisner, P. Enzymatic Treatment of Soy Protein Isolates: Effects on the Potential Allergenicity, Technofunctionality, and Sensory Properties. Food Sci. Nutr. 2016, 4, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Margatan, W.; Ruud, K.; Wang, Q.; Markowski, T.; Ismail, B. Angiotensin Converting Enzyme Inhibitory Activity of Soy Protein Subjected to Selective Hydrolysis and Thermal Processing. J. Agric. Food Chem. 2013, 61, 3460–3467. [Google Scholar] [CrossRef]
- Csehi, B.; Szerdahelyi, E.; Pásztor-Huszár, K.; Salamon, B.; Tóth, A.; Zeke, I.; Jónás, G.; Friedrich, L. Changes of Protein Profiles in Pork and Beef Meat Caused by High Hydrostatic Pressure Treatment. Acta Aliment. 2016, 45, 565–571. [Google Scholar] [CrossRef]
- Matuz, J.; Póka, R.; Boldizsár, I.; Szerdahelyi, E.; Hajós, G. Structure and Potential Allergenic Character of Cereal Proteins II. Potential Allergens in Cereal Samples. Cereal Res. Commun. 2000, 28, 433–442. [Google Scholar] [CrossRef]
- Mahdavi-Yekta, M.; Nouri, L.; Azizi, M.H. The Effects of Hydrolysis Condition on Antioxidant Activity of Protein Hydrolyzate from Quinoa. Food Sci. Nutr. 2019, 7, 930–936. [Google Scholar] [CrossRef] [PubMed]
- ISO-20483; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- Rutherfurd, S.M. Methodology for Determining Degree of Hydrolysis of Proteins in Hydrolysates: A Review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kleyn, D.H.; Lynch, J.M.; Barbano, D.M.; Bloom, M.J.; Mitchell, M.W. Determination of Fat in Raw and Processed Milks by the Gerber Method: Collaborative Study. J. AOAC Int. 2001, 84, 1499–1508. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, V.; Majumdar, D.K. Influence of Tableting on Enzymatic Activity of Papain along with Determination of Its Percolation Threshold with Microcrystalline Cellulose. Int. Sch. Res. Not. 2014, 2014, 140891. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Arfaoui, L. Total Polyphenol Content and Radical Scavenging Activity of Functional Yogurt Enriched with Dates. Czech J. Food Sci. 2020, 38, 287–292. [Google Scholar] [CrossRef]
- Fagyas, M.; Úri, K.; Siket, I.M.; Daragó, A.; Boczán, J.; Bányai, E.; Édes, I.; Papp, Z.; Tóth, A. New Perspectives in the Renin-Angiotensin-Aldosterone System (RAAS) I: Endogenous Angiotensin Converting Enzyme (ACE) Inhibition. PLoS ONE 2014, 9, e87843. [Google Scholar] [CrossRef]
- LAEMMLI, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, K.P.; Hymowitz, T. Characterization of Trypsin and Chymotrypsin Inhibitors in the Wild Perennial Glycine Species. J. Agric. Food Chem. 1992, 40, 2356–2363. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J.; Butts, C.A.; Van Wijk, H.; Rowan, A.M.; Reynolds, G.W. An Acute Ileal Amino Acid Digestibility Assay Is a Valid Procedure for Use in Human Ileostomates. J. Nutr. 2005, 135, 404–409. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Jaudzems, G.; Guthrie, J.; Lahrichi, S.; Fuerer, C. Total Amino Acids by UHPLC-UV in Infant Formulas and Adult Nutritionals, First Action 2018.06. J. AOAC Int. 2019, 102, 1574–1588. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Zhang, C.; Kong, X.; Hua, Y. Heat-Induced Inactivation Mechanisms of Kunitz Trypsin Inhibitor and Bowman-Birk Inhibitor in Soymilk Processing. Food Chem. 2014, 154, 108–116. [Google Scholar] [CrossRef]
- Ogawa, T.; Bando, N.; Tsuji, H.; Okajima, H.; Nishikawa, K.; Sasaoka, K. Investigation of the IgE-Binding Proteins in Soybeans by Immunoblotting with the Sera of the Soybean-Sensitive Patients with Atopic Dermatitis. J. Nutr. Sci. Vitaminol. 1991, 37, 555–565. [Google Scholar] [CrossRef]
- Panthee, D.R.; Kwanyuen, P.; Sams, C.E.; West, D.R.; Saxton, A.M.; Pantalone, V.R. Quantitative Trait Loci for β-Conglycinin (7S) and Glycinin (11S) Fractions of Soybean Storage Protein. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 1005–1012. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Stãnciuc, N.; Grigore-Gurgu, L.; Aprodu, I. Spectroscopic and Molecular Modeling Investigations on Heat Induced Behaviour of Soy Proteins. Emirates J. Food Agric. 2019, 31, 569–577. [Google Scholar] [CrossRef]
- Adachi, M.; Kanamori, J.; Masuda, T.; Yagasaki, K.; Kitamura, K.; Mikami, B.; Utsumi, S. Crystal Structure of Soybean 11S Globulin: Glycinin A3B4 Homohexamer. Proc. Natl. Acad. Sci. USA 2003, 100, 7395–7400. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, W.M.; Dick, W.E.J.; Cavins, J.F.; Dombrink-Kurtzman, M.A.; Slodki, M.E. Isolation and Characterization of a Soybean Lectin Having 4-O-Methylglucuronic Acid Specificity. Biochemistry 1986, 25, 952–958. [Google Scholar] [CrossRef]
- Riascos, J.J.; Weissinger, S.M.; Weissinger, A.K.; Kulis, M.; Burks, A.W.; Pons, L. The Seed Biotinylated Protein of Soybean (Glycine max): A Boiling-Resistant New Allergen (Gly m 7) with the Capacity To Induce IgE-Mediated Allergic Responses. J. Agric. Food Chem. 2016, 64, 3890–3900. [Google Scholar] [CrossRef]
- Wilson, S.; Blaschek, K.; de Mejia, E. Allergenic Proteins in Soybean: Processing and Reduction of P34 Allergenicity. Nutr. Rev. 2005, 63, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Bando, N.; Hiemori, M.; Yamanishi, R.; Kimoto, M.; Nishikawa, K.; Ogawa, T. Purification of Characterization of Soybean Allergen Gly m Bd 28K. Biosci. Biotechnol. Biochem. 1997, 61, 942–947. [Google Scholar] [CrossRef]
- Sugawara, M.; Ito, D.; Akita, M.; Oguri, S.; Momonoki, Y. Kunitz Soybean Trypsin Inhibitor Is Modified at Its C-Terminus by Novel Soybean Thiol Protease (Protease T1). Plant Prod. Sci. 2007, 10, 314–321. [Google Scholar] [CrossRef]
- Chaudhry, R.Q.; Bigelsen, R.; Wolff, A. Reaction To Soymilk But Not Other Soy Products In Gly m 4 Sensitized Birch Pollen-Allergic Patients. J. Allergy Clin. Immunol. 2012, 129, AB34. [Google Scholar] [CrossRef]
- Amnuaycheewa, P.; de Mejia, E.G. Purification, Characterisation, and Quantification of the Soy Allergen Profilin (Gly m 3) in Soy Products. Food Chem. 2010, 119, 1671–1680. [Google Scholar] [CrossRef]
- DiPietro, C.M.; Liener, I.E. Heat Inactivation of the Kunitz and Bowman-Birk Soybean Protease Inhibitors. J. Agric. Food Chem. 1989, 37, 39–44. [Google Scholar] [CrossRef]
- Birk, Y. The Bowman-Birk Inhibitor. Trypsin- and Chymotrypsin-inhibitor from Soybeans. Int. J. Pept. Protein Res. 1985, 25, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Gitlin-Domagalska, A.; Maciejewska, A.; Dębowski, D. Bowman-Birk Inhibitors: Insights into Family of Multifunctional Proteins and Peptides with Potential Therapeutical Applications. Pharmaceuticals 2020, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Hua, Y. Improvement of Soybean Product Flavor and Quality as Affected by Extraction of Soybean Oil Bodies Based on a Soymilk Model System. Int. J. Food Prop. 2021, 24, 895–905. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Zhang, C.; Kong, X.; Hua, Y. The Heat-Induced Protein Aggregate Correlated with Trypsin Inhibitor Inactivation in Soymilk Processing. J. Agric. Food Chem. 2012, 60, 8012–8019. [Google Scholar] [CrossRef]
- Fujii, T. Coagulation and Rheological Behaviors of Soy Milk Colloidal Dispersions. Biosci. Biotechnol. Biochem. 2017, 81, 680–686. [Google Scholar] [CrossRef]
- Murata, K.; Kusakabe, I.; Kobayashi, H.; Akaike, M.; Murakami, K. Coagulation of Proteins in Leguminous Milk by Commercial Proteinases. Agric. Biol. Chem. 1988, 52, 1317–1318. [Google Scholar] [CrossRef]
- Garavand, F.; Daly, D.F.M.; Gómez-Mascaraque, L.G. Biofunctional, Structural, and Tribological Attributes of GABA-Enriched Probiotic Yoghurts Containing Lacticaseibacillus Paracasei Alone or in Combination with Prebiotics. Int. Dairy J. 2022, 129, 105348. [Google Scholar] [CrossRef]
- Silvestre, M.P.C. Review of Methods for the Analysis of Protein Hydrolysates. Food Chem. 1997, 60, 263–271. [Google Scholar] [CrossRef]
- Mamboya, F.; Amri, E. Papain, a Plant Enzyme of Biological Importance: A Review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar] [CrossRef]
- Samaei, S.P.; Martini, S.; Tagliazucchi, D.; Gianotti, A.; Babini, E. Antioxidant and Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides Obtained from Alcalase Protein Hydrolysate Fractions of Hemp (Cannabis sativa L.) Bran. J. Agric. Food Chem. 2021, 69, 9220–9228. [Google Scholar] [CrossRef]
- Bamdad, F.; Shin, S.H.; Suh, J.-W.; Nimalaratne, C.; Sunwoo, H. Anti-Inflammatory and Antioxidant Properties of Casein Hydrolysate Produced Using High Hydrostatic Pressure Combined with Proteolytic Enzymes. Molecules 2017, 22, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzamirad, V.; Aluko, R.E. Angiotensin-Converting Enzyme Inhibition and Free-Radical Scavenging Properties of Cationic Peptides Derived from Soybean Protein Hydrolysates. Int. J. Food Sci. Nutr. 2008, 59, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, V.; Rainha, N.; Paiva, L.; Lima, E.; Baptista, J. Bovine Milk Formula Based on Partial Hydrolysis of Caseins by Bromelain Enzyme: Better Digestibility and Angiotensin-Converting Enzyme-Inhibitory Properties. Int. J. Food Prop. 2014, 17. [Google Scholar] [CrossRef]
- Asoodeh, A.; Homayouni-Tabrizi, M.; Shabestarian, H.; Emtenani, S.; Emtenani, S. Biochemical Characterization of a Novel Antioxidant and Angiotensin I-Converting Enzyme Inhibitory Peptide from Struthio Camelus Egg White Protein Hydrolysis. J. Food Drug Anal. 2016, 24, 332–342. [Google Scholar] [CrossRef]
- Pripp, A.H.; Sørensen, R.; Stepaniak, L.; Sørhaug, T. Relationship between Proteolysis and Angiotensin-I-Converting Enzyme Inhibition in Different Cheeses. LWT Food Sci. Technol. 2006, 39, 677–683. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of Peptide Substrates and Inhibitors of Angiotensin-Converting Enzyme. Importance of the COOH-Terminal Dipeptide Sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [CrossRef]
- Dellafiora, L.; Pugliese, R.; Bollati, C.; Gelain, F.; Galaverna, G.; Arnoldi, A.; Lammi, C. “Bottom-Up” Strategy for the Identification of Novel Soybean Peptides with Angiotensin-Converting Enzyme Inhibitory Activity. J. Agric. Food Chem. 2020, 68, 2082–2090. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Otte, J.; van Camp, J. Physiological, Chemical and Technological Aspects of Milk-Protein-Derived Peptides with Antihypertensive and ACE-Inhibitory Activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Ogawa, A.; Samoto, M.; Takahashi, K. Soybean Allergens and Hypoallergenic Soybean Products. J. Nutr. Sci. Vitaminol. 2000, 46, 271–279. [Google Scholar] [CrossRef]
- Xu, L.; Song, Y.; Liu, L.; Song, S.; Zhu, J.; Kuang, H.; Xu, C. Sandwich ELISA and Immunochromatographic Strip of Kunitz Trypsin Inhibitor Using Sensitive Monoclonal Antibodies. Food Agric. Immunol. 2016, 27, 772–782. [Google Scholar] [CrossRef]
- De La Barca, A.M.C.; Wall, A.; López-Díaz, J.A. Allergenicity, Trypsin Inhibitor Activity and Nutritive Quality of Enzymatically Modified Soy Proteins. Int. J. Food Sci. Nutr. 2005, 56, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kunitz, M. Crystalline soybean trypsin inhibitor: II. general properties. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, A.; Ahmad, A.S.; Amankwaa, A.; Csehi, B.; Mednyánszky, Z.; Szerdahelyi, E.; Tóth, A.; Tormási, J.; Truong, D.H.; Abrankó, L.; et al. Hydrolysis of Soybean Milk Protein by Papain: Antioxidant, Anti-Angiotensin, Antigenic and Digestibility Perspectives. Bioengineering 2022, 9, 418. https://doi.org/10.3390/bioengineering9090418
Nath A, Ahmad AS, Amankwaa A, Csehi B, Mednyánszky Z, Szerdahelyi E, Tóth A, Tormási J, Truong DH, Abrankó L, et al. Hydrolysis of Soybean Milk Protein by Papain: Antioxidant, Anti-Angiotensin, Antigenic and Digestibility Perspectives. Bioengineering. 2022; 9(9):418. https://doi.org/10.3390/bioengineering9090418
Chicago/Turabian StyleNath, Arijit, Abubakar Saleh Ahmad, Abraham Amankwaa, Barbara Csehi, Zsuzsanna Mednyánszky, Emőke Szerdahelyi, Attila Tóth, Judit Tormási, Duy Hoàng Truong, László Abrankó, and et al. 2022. "Hydrolysis of Soybean Milk Protein by Papain: Antioxidant, Anti-Angiotensin, Antigenic and Digestibility Perspectives" Bioengineering 9, no. 9: 418. https://doi.org/10.3390/bioengineering9090418
APA StyleNath, A., Ahmad, A. S., Amankwaa, A., Csehi, B., Mednyánszky, Z., Szerdahelyi, E., Tóth, A., Tormási, J., Truong, D. H., Abrankó, L., & Koris, A. (2022). Hydrolysis of Soybean Milk Protein by Papain: Antioxidant, Anti-Angiotensin, Antigenic and Digestibility Perspectives. Bioengineering, 9(9), 418. https://doi.org/10.3390/bioengineering9090418