Riehl’s Melanosis: A Multimodality, In Vivo, Real-Time Skin Imaging Study with Cellular Resolution Optical Coherence Tomography and Advanced Skin Diagnosis System in a Tertiary Medical Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Conventional Clinical and Dermoscopic Imaging
2.3. Histopathological Analysis
2.4. Multimodality Advanced Skin Imaging System
2.5. Statistical Analysis
3. Results
3.1. Clinical Features
3.2. Conventional Clinical and Dermoscopic Imaging
3.3. Histopathological Characteristics
3.4. Multimodality Advanced Skin Imaging System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riehl, G. Über eine eigenartige Melanose. Wien. Klin Wochenschr 1917, 30, 780–781. [Google Scholar]
- Park, J.Y.; Kim, Y.C.; Lee, E.S.; Park, K.C.; Kang, H.Y. Acquired bilateral melanosis of the neck in perimenopausal women. Br. J. Dermatol. 2011, 166, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Vinay, K.; Bishnoi, A.; Kamat, D.; Chatterjee, D.; Kumaran, M.S.; Parsad, D. Acquired Dermal Macular Hyperpigmentation: An Update. Indian Dermatol. Online J. 2021, 12, 663–673. [Google Scholar] [PubMed]
- Yim, J.H.; Kang, I.H.; Shin, M.K.; Lee, M.H. Differences among Dermoscopic Findings in Riehl’s Melanosis of the Cheek and Neck. Ann. Dermatol. 2019, 31, 460–463. [Google Scholar] [CrossRef]
- Woo, Y.R.; Park, H.E.; Jeong, S.W.; Park, H.J. Melanogenic Properties and Expression Profiles of Melanogenic Paracrine Molecules in Riehl’s Melanosis. Int. J. Mol. Sci. 2020, 21, 1695. [Google Scholar] [CrossRef]
- Smucker, J.E.; Kirby, J.S. Riehl Melanosis Treated Successfully with Q-Switch Nd:YAG Laser. J. Drugs Dermatol. 2014, 13, 356–358. [Google Scholar]
- Fadilla, Y.; Hindritiani, R.; Ruchiatan, K.; Gunawan, H.; Fakhrosa, I. The variability of clinical manifestation in Riehl’s melanosis: A two-case report. J. Egypt. Women’s Dermatol. Soc. 2020, 17, 57. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Kohli, I.; Chaowattanapanit, S.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J. Am. Acad. Dermatol. 2017, 77, 591–605. [Google Scholar] [CrossRef]
- Wang, K.X.; Meekings, A.; Fluhr, J.W.; McKenzie, G.; Lee, D.A.; Fisher, J.; Markowitz, O.; Siegel, D.M. Optical coherence tomography-based optimization of mohs micrographic surgery of Basal cell carcinoma: A pilot study. Dermatol. Surg. 2013, 39, 627–633. [Google Scholar] [CrossRef]
- Abignano, G.; Aydin, S.Z.; Castillo-Gallego, C.; Liakouli, V.; Woods, D.; Meekings, A.; Wakefield, R.J.; McGonagle, D.G.; Emery, P.; Del Galdo, F. Virtual skin biopsy by optical coherence tomography: The first quantitative imaging biomarker for scleroderma. Ann. Rheum. Dis. 2013, 72, 1845–1851. [Google Scholar] [CrossRef]
- Pomerantz, R.; Zell, D.; McKenzie, G.; Siegel, D.M. Optical Coherence Tomography Used as a Modality to Delineate Basal Cell Carcinoma prior to Mohs Micrographic Surgery. Case Rep. Dermatol. 2011, 3, 212–218. [Google Scholar] [CrossRef]
- Morsy, H.; Kamp, S.; Thrane, L.; Behrendt, N.; Saunder, B.; Zayan, H.; Elmagid, E.A.; Jemec, G.B. Optical coherence tomography imaging of psoriasis vulgaris: Correlation with histology and disease severity. Arch. Dermatol. Res. 2009, 302, 105–111. [Google Scholar] [CrossRef]
- Abuzahra, F.; Spoler, F.; Forst, M.; Brans, R.; Erdmann, S.; Merk, H.F.; Obrigkeit, D.H. Pilot study: Optical coherence tomography as a non-invasive diagnostic perspective for real time visualisation of onychomycosis. Mycoses 2009, 53, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-Y.; Jheng, D.-Y.; Liao, Y.-H.; Ho, T.-S. Diode-laser-pumped glass-clad Ti:sapphire crystal fiber based broadband light source. IEEE Photon Technol. Lett. 2012, 24, 854–856. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, C.-K.; Hsu, K.-Y.; Ho, T.-S.; Lin, M.-Y.; Tjiu, J.-W.; Huang, S.-L. Full-depth epidermis tomography using a Mirau-based full-field optical coherence tomography. Biomed. Opt. Express 2014, 5, 3001–3010. [Google Scholar] [CrossRef]
- Cunha, D.; Richardson, T.; Sheth, N.; Orchard, G.; Coleman, A.; Mallipeddi, R. Comparison of ex vivo optical coherence tomography with conventional frozen-section histology for visualizing basal cell carcinoma during Mohs micrographic surgery. Br. J. Dermatol. 2011, 165, 576–580. [Google Scholar] [CrossRef]
- Tsai, M.R.; Ho, T.S.; Wu, Y.H.; Lu, C.W. In vivo dual-mode full-field optical coherence tomography for differentiation of types of melanocytic nevi. J. Biomed. Opt. 2021, 26, 020501. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, J.Y.; Wu, Y.H. Application of Cellular Resolution Full-Field Optical Coherence Tomography in vivo for the Diagnosis of Skin Tumours and Inflammatory Skin Diseases: A Pilot Study. Dermatology 2021, 238, 121–131. [Google Scholar] [CrossRef]
- Woo, Y.R.; Jung, Y.; Kim, M.; Park, H.J. Impact of Riehl’s melanosis on quality of life in Korean patients: A cross-sectional comparative study. J. Dermatol. 2020, 47, 893–897. [Google Scholar] [CrossRef]
- Iwayama, T.; Oka, M.; Fukumoto, T. Treatment of henna-induced Riehl’s melanosis with a 755-nm picosecond alexandrite laser. Lasers Med. Sci. 2020, 35, 1659–1661. [Google Scholar] [CrossRef]
- Nakayama, H.; Harada, R.; Toda, M. Pigmented cosmetic dermatitis. Int. J. Dermatol. 1976, 15, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, J.H.; Lee, D.Y.; Lee, J.H. Acquired Bilateral Dyspigmentation on Face and Neck: Clinically Appropriate Approaches. J. Korean Med. Sci. 2016, 31, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, S.P.W.; Pandya, A.; Chandran, V.; Rodrigues, M.; Dlova, N.C.; Kang, H.Y.; Ramam, M.; Dayrit, J.F.; Goh, B.K.; Parsad, D. A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl’s melanosis. Int. J. Dermatol. 2018, 58, 263–272. [Google Scholar] [CrossRef]
- Pérez-Bernal, A.; Muñoz-Pérez, M.A.; Camacho, F. Management of facial hyperpigmentation. Am. J. Clin. Dermatol. 2000, 1, 261–268. [Google Scholar] [CrossRef]
- Khanna, N.; Rasool, S. Facial melanoses: Indian perspective. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 552–563. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Fitzpatrick, T.B.; Anderson, R.R.; Parrish, J.A. Localization of malanin pigmentation in the skin with Wood’s lamp. Br. J. Dermatol. 1977, 96, 245–248. [Google Scholar] [CrossRef]
- Grimes, P.E.; Yamada, N.; Bhawan, J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am. J. Dermatopathol. 2005, 27, 96–101. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef]
- Byers, H.R.; Maheshwary, S.; Amodeo, D.M.; Dykstra, S.G. Role of cytoplasmic dynein in perinuclear aggregation of phagocytosed melanosomes and supranuclear melanin cap formation in human keratinocytes. J. Investig. Dermatol. 2003, 121, 813–820. [Google Scholar] [CrossRef]
- Chung, B.Y.; Kim, J.E.; Ko, J.Y.; Chang, S.E. A pilot study of a novel dual-pulsed 1064 nm Q-switched Nd: YAG laser to treat Riehl’s melanosis. J. Cosmet. Laser Ther. 2014, 16, 290–292. [Google Scholar] [CrossRef]
- Wang, L.; Wen, X.; Hao, D.; Li, Y.; Du, D.; Jiang, X. Combination therapy with salicylic acid chemical peels, glycyrrhizin compound, and vitamin C for Riehl’s melanosis. J. Cosmet. Dermatol. 2019, 19, 1377–1380. [Google Scholar] [CrossRef]
- Cho, M.Y.; Roh, M.R. Successful Treatment of Riehl’s Melanosis With Mid-Fluence Q-Switched Nd:YAG 1064-nm Laser. Lasers Surg. Med. 2020, 52, 753–760. [Google Scholar] [CrossRef]
- Choi, C.W.; Jo, G.; Lee, D.H.; Jo, S.J.; Lee, C.; Mun, J.H. Analysis of Clinical Features and Treatment Outcomes Using 1,064-nm Nd-YAG Laser with Topical Hydroquinone in Patients with Riehl’s Melanosis: A Retrospective Study in 10 Patients. Ann. Dermatol. 2019, 31, 127–132. [Google Scholar] [CrossRef]
- Kim, S.M.; Hwang, S.; Almurayshid, A.; Park, M.Y.; Oh, S.H. Non-Ablative 1927 nm Fractional Thulium Fiber Laser: New, Promising Treatment Modality for Riehl’s Melanosis. Lasers Surg. Med. 2021, 53, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Ohn, J.; Suh, D.H.; Park, H.Y.; Choi, S.C.; Jung, J.Y.; Kwon, I.H.; Park, G.H. A pilot study for triple combination therapy with a low-fluence 1064 nm Q-switched Nd:YAG laser, hydroquinone cream and oral tranexamic acid for recalcitrant Riehl’s Melanosis. J. Dermatol. Treat. 2016, 28, 155–159. [Google Scholar] [CrossRef] [PubMed]
- On, H.R.; Hong, W.J.; Roh, M.R. Low-pulse energy Q-switched Nd:YAG laser treatment for hair-dye-induced Riehl’s melanosis. J. Cosmet. Laser Ther. 2015, 17, 135–138. [Google Scholar] [CrossRef]
- Xu, Z.; Xing, X.; Zhang, C.; Chen, L.; Flora Xiang, L. A pilot study of oral tranexamic acid and Glycyrrhizin compound in the treatment of recalcitrant Riehl’s melanosis. J. Cosmet. Dermatol. 2019, 18, 286–292. [Google Scholar] [CrossRef]
- Manfredini, M.; Liberati, S.; Ciardo, S.; Bonzano, L.; Guanti, M.; Chester, J.; Kaleci, S.; Pellacani, G. Microscopic and functional changes observed with dynamic optical coherence tomography for severe refractory atopic dermatitis treated with dupilumab. Ski. Res. Technol. 2020, 26, 779–787. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Y.J.; Wu, Y.H. In vivo characterization of extramammary Paget’s disease by ultra-high cellular resolution optical coherence tomography. Ski. Res. Technol. 2020, 27, 114–117. [Google Scholar] [CrossRef]

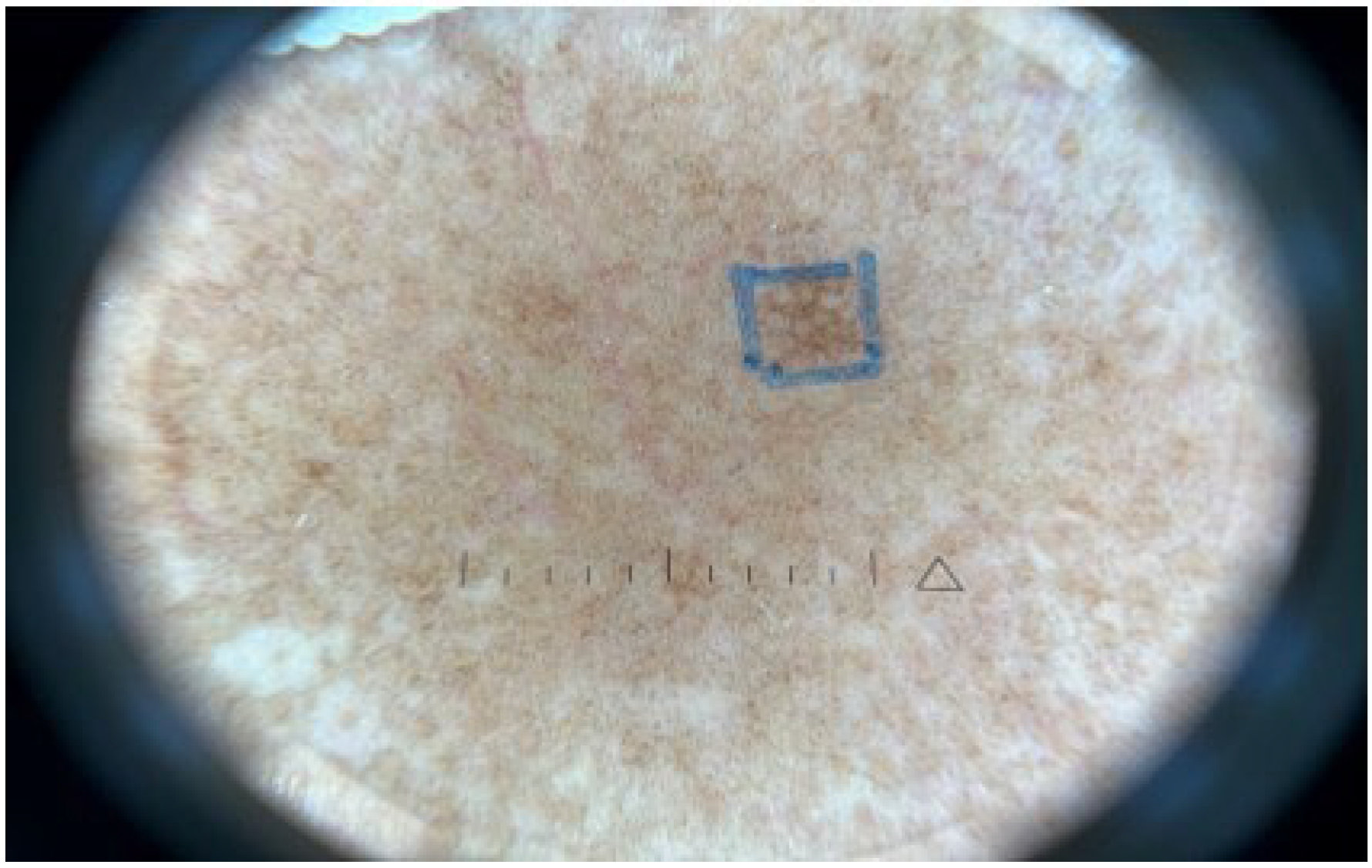
 ), and melanophages in the dermis (shown in arrow).
), and melanophages in the dermis (shown in arrow).
 ), and melanophages in the dermis (shown in arrow).
), and melanophages in the dermis (shown in arrow).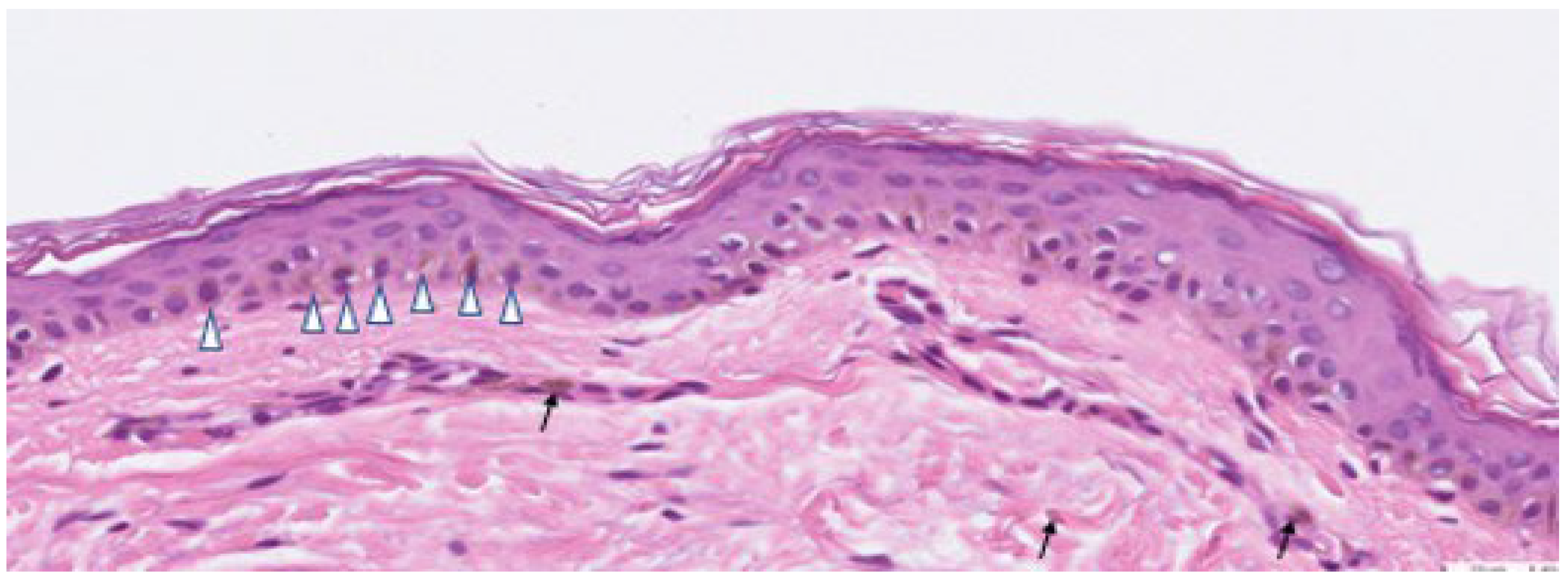

 ). Spotty donut shape and hyper-reflective melanophages can also be observed in the dermis (shown in the arrow). Blurred DEJ represents basal keratinocyte degeneration (shown in a circle). Irregular acanthosis and vessel dilatation with increased blood flow (shown in
). Spotty donut shape and hyper-reflective melanophages can also be observed in the dermis (shown in the arrow). Blurred DEJ represents basal keratinocyte degeneration (shown in a circle). Irregular acanthosis and vessel dilatation with increased blood flow (shown in  ) are the signs of dermal inflammation; (b) hypopigmented lesional skin of Riehl’s melanosis on cellular resolution OCT showed nearly no melanin capping nor dermal melanophages and resembled normal skin.
) are the signs of dermal inflammation; (b) hypopigmented lesional skin of Riehl’s melanosis on cellular resolution OCT showed nearly no melanin capping nor dermal melanophages and resembled normal skin.
 ). Spotty donut shape and hyper-reflective melanophages can also be observed in the dermis (shown in the arrow). Blurred DEJ represents basal keratinocyte degeneration (shown in a circle). Irregular acanthosis and vessel dilatation with increased blood flow (shown in
). Spotty donut shape and hyper-reflective melanophages can also be observed in the dermis (shown in the arrow). Blurred DEJ represents basal keratinocyte degeneration (shown in a circle). Irregular acanthosis and vessel dilatation with increased blood flow (shown in  ) are the signs of dermal inflammation; (b) hypopigmented lesional skin of Riehl’s melanosis on cellular resolution OCT showed nearly no melanin capping nor dermal melanophages and resembled normal skin.
) are the signs of dermal inflammation; (b) hypopigmented lesional skin of Riehl’s melanosis on cellular resolution OCT showed nearly no melanin capping nor dermal melanophages and resembled normal skin.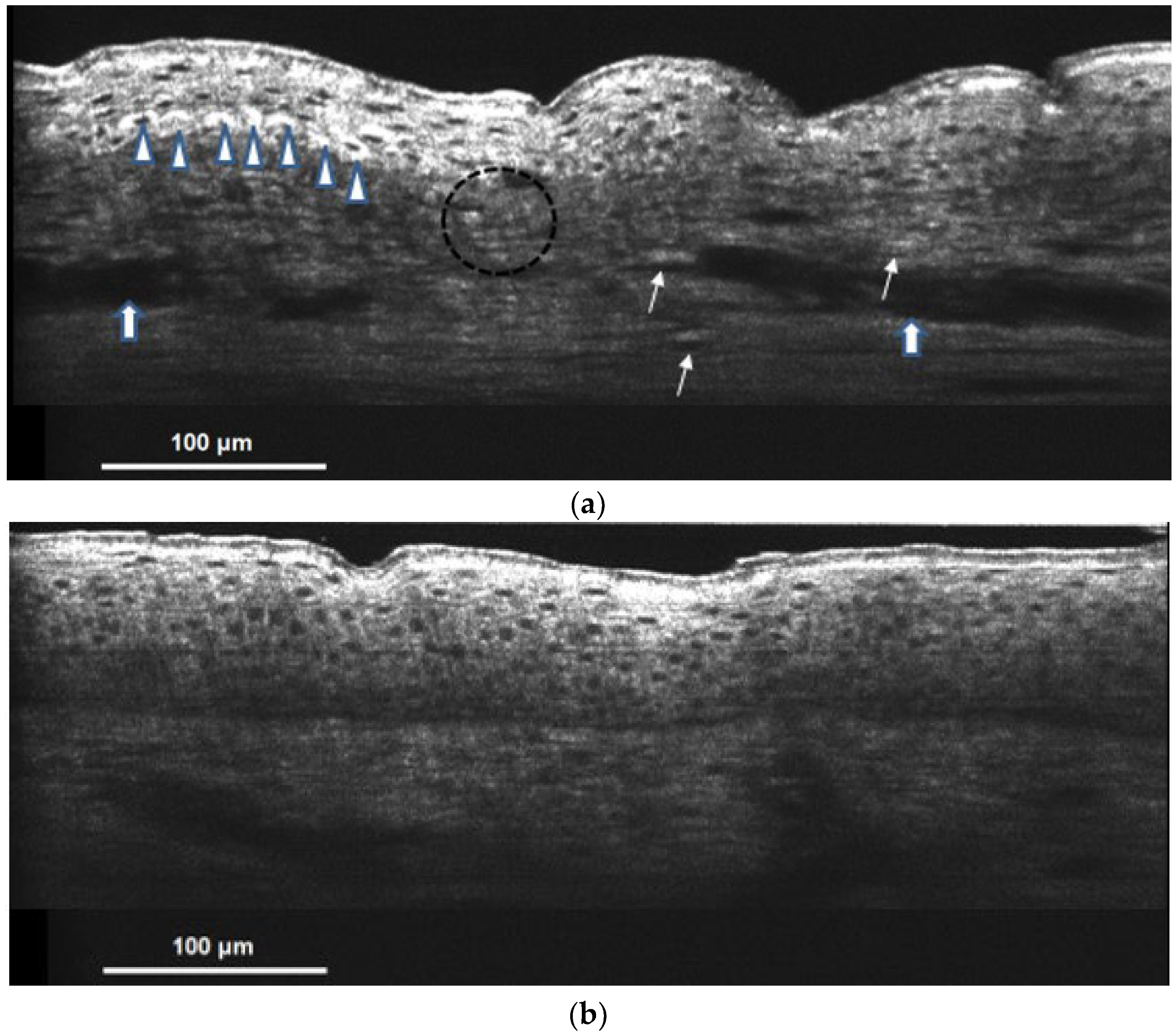
 ). The dermal area showed a heterogeneous pattern with dark linear blood vessels (shown in
). The dermal area showed a heterogeneous pattern with dark linear blood vessels (shown in  ) embedded.
) embedded.
 ). The dermal area showed a heterogeneous pattern with dark linear blood vessels (shown in
). The dermal area showed a heterogeneous pattern with dark linear blood vessels (shown in  ) embedded.
) embedded.
| Characteristics | Value |
|---|---|
| No. of cases | 30 |
| Sex, n (%) | |
| Male | 7 (23.3) |
| Female | 23 (76.7) |
| Age at onset (yrs), n (%) | 47.7, 12.3 (Mean, SD) |
| Disease duration (mo) | 19.6, 26.4 (Mean, SD) |
| Site of Riehl’s melanosis, n (%) | |
| Face | 27 (90.0) |
| Neck | 19 (63.3) |
| Associated symptoms, n (%) | |
| Inflammation | 5 (16.7) |
| Pruritus | 16 (53.3) |
| Erythema | 10 (33.3) |
| Scaling | 3 (10.0) |
| No. of cases with contact history (Causative factors), n (%) | 15 (50.0) |
| Cosmetics | 14 |
| Essential oil | 2 |
| Perfume | 2 |
| Sunscreens | 1 |
| Toner product | 1 |
| Treatment, n (%) | |
| Topical medications (including hydroquinone cream, retinoid cream, azelaic acid, and steroid cream) | 30 (100.0) |
| Oral medications (including tranexamic acid, antihistamine, and ascorbic acid) | 14 (46.7) |
| Laser and light device Nd-YAG | 8 (26.7) (including 2 Nd-YAG laser, 2 IPL, 3 Picolaser, and 1 Ruby laser) |
| Diagnostic Tools | Characteristics |
|---|---|
| Cellular resolution OCT | |
| Hyperpigmented Lesional Skin |
|
| Hypopigmented Lesional Skin |
|
| Skin Diagnosis System |
|
| Dermoscopy |
|
| Histology |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, P.-C.; Chan, Y.-P.; Huang, C.-H.; Ng, C.Y. Riehl’s Melanosis: A Multimodality, In Vivo, Real-Time Skin Imaging Study with Cellular Resolution Optical Coherence Tomography and Advanced Skin Diagnosis System in a Tertiary Medical Center. Bioengineering 2022, 9, 419. https://doi.org/10.3390/bioengineering9090419
Shen P-C, Chan Y-P, Huang C-H, Ng CY. Riehl’s Melanosis: A Multimodality, In Vivo, Real-Time Skin Imaging Study with Cellular Resolution Optical Coherence Tomography and Advanced Skin Diagnosis System in a Tertiary Medical Center. Bioengineering. 2022; 9(9):419. https://doi.org/10.3390/bioengineering9090419
Chicago/Turabian StyleShen, Peng-Chieh, Yu-Pei Chan, Chun-Hsien Huang, and Chau Yee Ng. 2022. "Riehl’s Melanosis: A Multimodality, In Vivo, Real-Time Skin Imaging Study with Cellular Resolution Optical Coherence Tomography and Advanced Skin Diagnosis System in a Tertiary Medical Center" Bioengineering 9, no. 9: 419. https://doi.org/10.3390/bioengineering9090419
APA StyleShen, P.-C., Chan, Y.-P., Huang, C.-H., & Ng, C. Y. (2022). Riehl’s Melanosis: A Multimodality, In Vivo, Real-Time Skin Imaging Study with Cellular Resolution Optical Coherence Tomography and Advanced Skin Diagnosis System in a Tertiary Medical Center. Bioengineering, 9(9), 419. https://doi.org/10.3390/bioengineering9090419






