Gallium-Based Liquid Metal Materials for Antimicrobial Applications
Abstract
:1. Introduction
2. Antimicrobial Mechanisms of LM-Based Materials
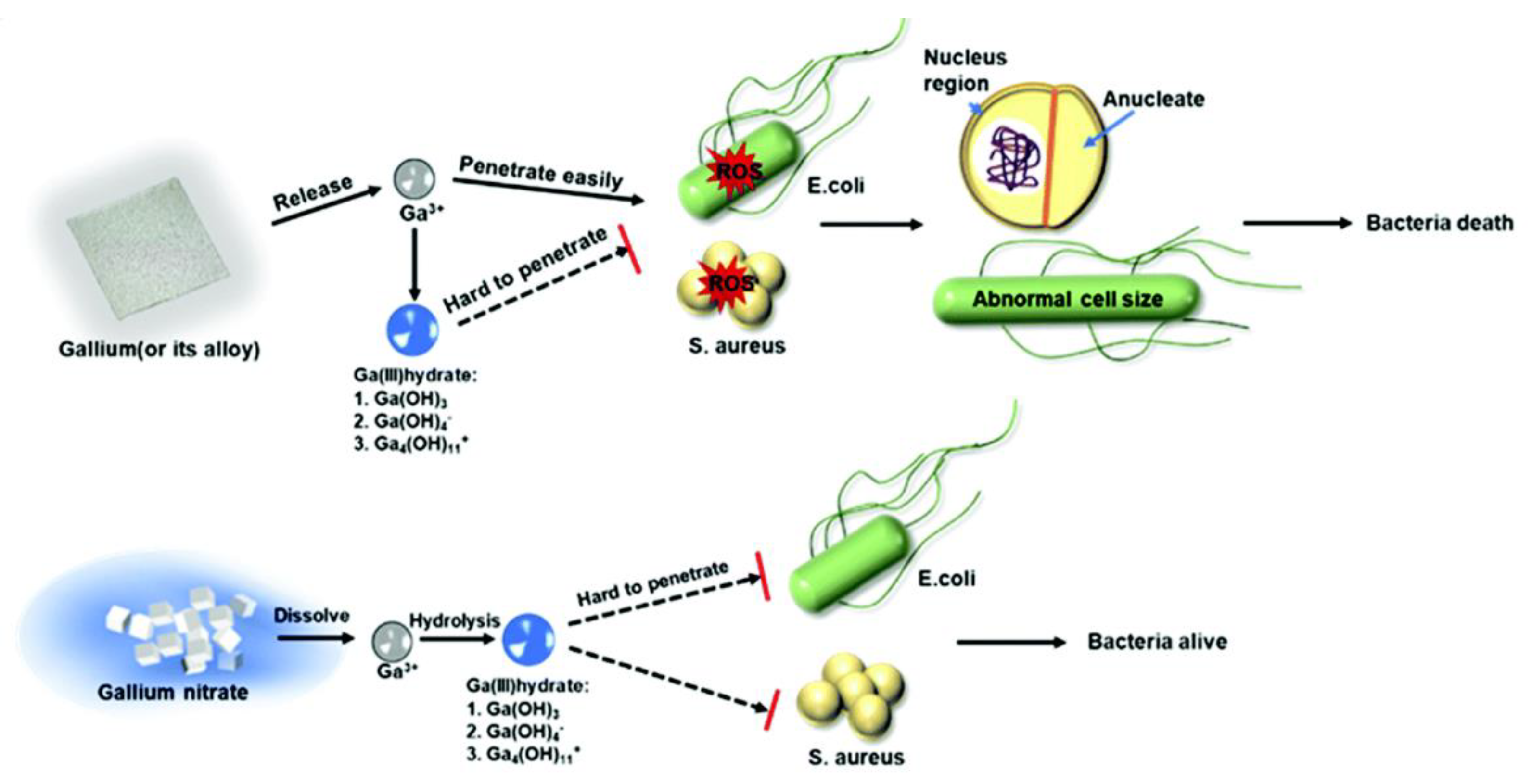
2.1. Iron Metabolism Disorder
2.2. Production of Reactive Oxygen Species
2.3. Thermal Injury
2.4. Mechanical Destruction
3. Antibacterial Application of LM-Based Materials
3.1. LM Motors
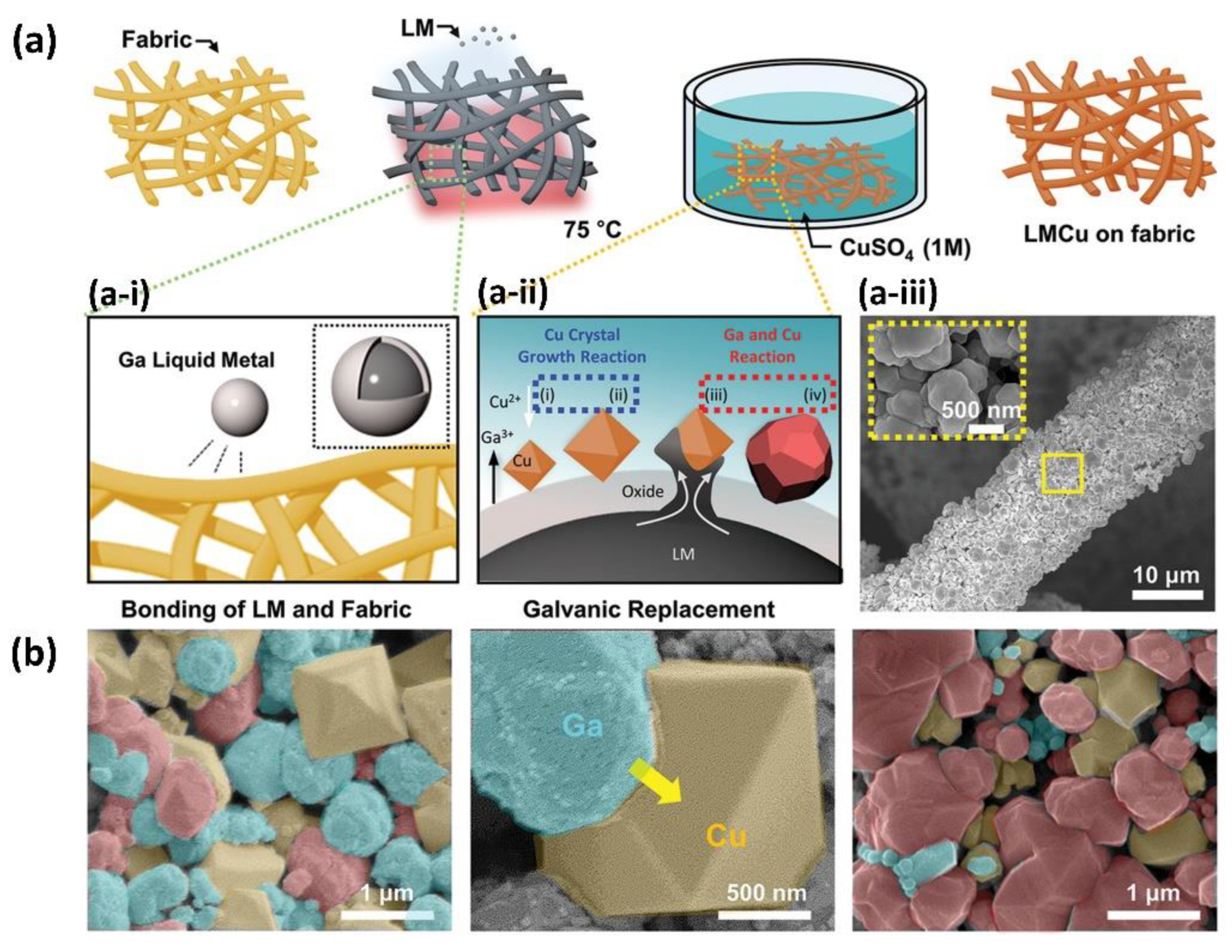
3.2. Antibacterial Fabrics
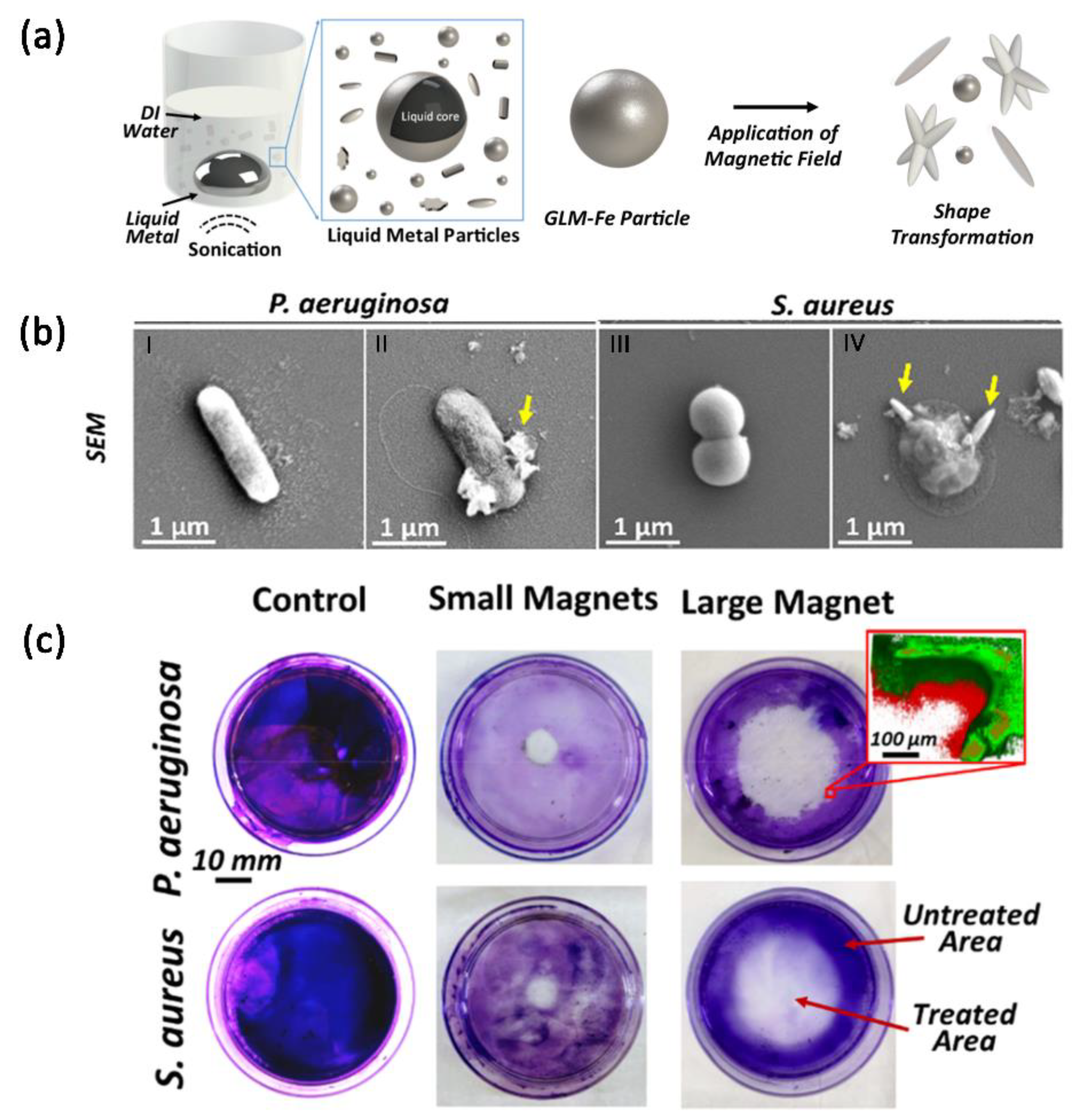
3.3. Magnetic-Field-Responsive Microparticles
3.4. LM Films
3.5. LM Polymer Composites
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mular, A.; Shanzer, A.; Kozłowski, H.; Hubmann, I.; Misslinger, M.; Krzywik, J.; Decristoforo, C.; Gumienna-Kontecka, E. Cyclic analogs of desferrioxamine e siderophore for 68Ga nuclear imaging: Coordination chemistry and biological activity in staphylococcus aureus. Inorg. Chem. 2021, 60, 17846–17857. [Google Scholar] [CrossRef]
- English, B.K.; Gaur, A.H. The use and abuse of antibiotics and the development of antibiotic resistance. In Hot Topics in Infection and Immunity in Children VI; Finn, A., Curtis, N., Pollard, A.J., Eds.; Springer: New York, NY, USA, 2010; pp. 73–82. [Google Scholar]
- Neill, J.O. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations the Review on Antimicrobial Resistance Chaired. Antimicrob. Resist. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 20 December 2014).
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Tang, J.; Baharfar, M.; Zhang, C.; Allioux, F.; Zhang, J.; Tajik, M.; Yang, J.; Biazik, J.; Centurion, F.; et al. Induction heating for the removal of liquid metal-based implant mimics: A proof-of-concept. Appl. Mater. Today 2022, 27, 101459. [Google Scholar] [CrossRef]
- Ma, Z.J.; Huang, Q.Y.; Xu, Q.; Zhuang, Q.N.; Zhao, X.; Yang, Y.H.; Qiu, H.; Yang, Z.L.; Wang, C.; Chai, Y.; et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 2021, 20, 859–868. [Google Scholar] [CrossRef]
- Andrews, J.B.; Mondal, K.; Neumann, T.V.; Cardenas, J.A.; Wang, J.; Parekh, D.P.; Lin, Y.; Ballentine, P.; Dickey, M.D.; Franklin, A.D. Patterned liquid metal contacts for printed carbon nanotube transistors. ACS Nano 2018, 12, 5482–5488. [Google Scholar] [CrossRef]
- Barbee, M.H.; Mondal, K.; Deng, J.Z.; Bharambe, V.; Neumann, T.V.; Adams, J.J.; Boechler, N.; Dickey, M.D.; Craig, S.L. Mechanochromic stretchable electronics. ACS Appl. Mater. Interfaces 2018, 10, 29918–29924. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Tang, S.Y.; Zhu, J.Y.; Schaefer, S.; Mitchell, A.; Kalantar-Zadeh, K.; Dickey, M.D. Liquid metal enabled microfluidics. Lab Chip 2017, 17, 974–993. [Google Scholar] [CrossRef]
- Dickey, M.D. Emerging applications of liquid metals featuring surface oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef]
- Cao, L.X.; Yu, D.H.; Xia, Z.S.; Wan, H.Y.; Liu, C.K.; Yin, T.; He, Z.Z. Ferromagnetic liquid metal putty-like material with transformed shape and reconfigurable polarity. Adv. Mater. 2020, 32, 2000827. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Tang, J.B.; Daeneke, T.; O’Mullane, A.P.; Stewart, L.A.; Liu, J.; Majidi, C.; Ruoff, R.S.; Weiss, P.S.; Dickey, M.D. Emergence of liquid metals in nanotechnology. ACS Nano 2019, 13, 7388–7395. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, B.; Sheng, L.; Rao, W.; Liu, J. Liquid metal enabled injectable biomedical technologies and applications. Appl. Mater. Today 2020, 20, 100722. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Yuan, B.; Liu, J. Liquid metal–enabled cybernetic electronics. Mater. Today Phys. 2020, 14, 100245. [Google Scholar] [CrossRef]
- Xie, W.J.; Allioux, F.M.; Ou, J.Z.; Miyako, E.; Tang, S.Y.; Kalantar-Zadeh, K. Gallium-based liquid metal particles for therapeutics. Trends Biotechnol. 2021, 39, 624–640. [Google Scholar] [CrossRef]
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 2018, 47, 2518–2533. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R. Gallium Complexes as Anticancer Drugs. Met. Ions Life Sci. 2018, 18, 281–301. [Google Scholar]
- Ha, S.S.; Xavierselvan, M.; Gokalp, S.; Labadini, D.; Barros, S.; Duong, J.; Foster, M.; Mallidi, S. Eutectic gallium-indium nanoparticles for photodynamic therapy of pancreatic cancer. ACS Appl. Nano Mater. 2022, 5, 6125–6139. [Google Scholar]
- Olakanmi, O.; Kesavalu, B.; Pasula, R.; Abdalla, M.Y.; Schlesinger, L.S.; Britigan, B.E. Gallium nitrate is efficacious in murine models of tuberculosis and inhibits key bacterial Fe-dependent enzymes. Antimicrob. Agents Chemother. 2013, 57, 6074–6080. [Google Scholar] [CrossRef]
- Olakanmi, O.; Gunn, J.S.; Su, S.; Soni, S.; Hassett, D.J.; Britigan, B.E. Gallium disrupts iron uptake by intracellular and extracellular francisella strains and exhibits therapeutic efficacy in a murine pulmonary infection model. Antimicrob. Agents Chemother. 2010, 54, 244–253. [Google Scholar] [CrossRef]
- Wang, L.; Lai, R.; Zhang, L.; Zeng, M.; Fu, L. Emerging liquid metal biomaterials: From design to application. Adv. Mater. 2022, 2201956. [Google Scholar] [CrossRef]
- Auger, C.; Lemire, J.; Appanna, V.; Appanna, V.D. Gallium in bacteria, metabolic and medical implications. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 800–807. [Google Scholar]
- Bernstein, L.R. Gallium, therapeutic effects. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 823–835. [Google Scholar]
- Choi, S.; Britigan, B.E.; Moran, D.M.; Narayanasamy, P. Gallium nanoparticles facilitate phagosome maturation and inhibit growth of virulent mycobacterium tuberculosis in macrophages. PLoS ONE 2017, 12, e177987. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium and its competing roles with iron in biological systems. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Britigan, B.E.; Narayanasamy, P. Iron/heme metabolism-targeted gallium(III) nanoparticles are active against extracellular and intracellular Pseudomonas aeruginosa and acinetobacter baumannii. Antimicrob. Agents Chemother. 2019, 63, e2618–e2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalska, K.; Rychłowski, M.; Krupińska, M.; Szewczyk, G.; Sarna, T.; Nakonieczna, J. Gallium mesoporphyrin IX-mediated photodestruction: A pharmacological trojan horse strategy to eliminate multidrug-resistant staphylococcus aureus. Mol. Pharm. 2022, 19, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.J.; Clagettcarr, K.; Hoth, D.; Leylandjones, B. Gallium nitrate—The 2nd metal with clinical activity. Cancer Treat. Rep. 1986, 70, 1311–1319. [Google Scholar] [PubMed]
- Xia, N.; Li, N.; Rao, W.; Yu, J.; Wu, Q.; Tan, L.; Li, H.; Gou, L.; Liang, P.; Li, L.; et al. Multifunctional and flexible ZrO2-coated EGain nanoparticles for photothermal therapy. Nanoscale 2019, 11, 10183–10189. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Chi, C.W.; Wang, S.H.; Wang, L.X.; Liang, P.; Liu, F.Y.; Shang, W.T.; Wang, W.W.; Zhang, F.R.; Li, S.; et al. A comparative study of clinical intervention and interventional photothermal therapy for pancreatic cancer. Adv. Mater. 2017, 29, 1700448. [Google Scholar] [CrossRef]
- Qi, Y.; Yu, Z.; Hu, K.; Wang, D.; Zhou, T.; Rao, W. Rigid metal/liquid metal nanoparticles: Synthesis and application for locally ablative therapy. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102535. [Google Scholar] [CrossRef]
- Kircheva, N.; Dudev, T. Competition between abiogenic and biogenic metal cations in biological systems: Mechanisms of gallium’s anticancer and antibacterial effect. J. Inorg. Biochem. 2021, 214, 111309. [Google Scholar] [CrossRef]
- Xie, T.; Qi, Y.; Li, Y.; Zhang, F.; Li, W.; Zhong, D.; Tang, Z.; Zhou, M. Ultrasmall Ga-ICG nanoparticles based gallium ion/photodynamic synergistic therapy to eradicate biofilms and against drug-resistant bacterial liver abscess. Bioact. Mater. 2021, 6, 3812–3823. [Google Scholar] [CrossRef]
- Yang, N.L.; Gong, F.; Zhou, Y.K.; Hao, Y.; Dong, Z.L.; Lei, H.L.; Zhong, L.P.; Yang, X.Y.; Wang, X.W.; Zhao, Y.X.; et al. A general in-situ reduction method to prepare core-shell liquid-metal/metal nanoparticles for photothermally enhanced catalytic cancer therapy. Biomaterials 2021, 277, 121125. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A liquid metal mediated metallic coating for antimicrobial and antiviral fabrics. Adv. Mater. 2021, 33, 2104298. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Gao, C.Y.; Wang, D.L.; He, Q. Bubble-propelled janus gallium/zinc micromotors for the active treatment of bacterial infections. Angew. Chem. Int. Ed. 2021, 60, 8750–8754. [Google Scholar] [CrossRef]
- Elbourne, A.; Cheeseman, S.; Atkin, P.; Truong, N.P.; Syed, N.; Zavabeti, A.; Mohiuddin, M.; Esrafilzadeh, D.; Cozzolino, D.; Mcconville, C.F.; et al. Antibacterial liquid metals: Biofilm treatment via magnetic activation. ACS Nano 2020, 14, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, H.; Yong, N.; Li, M.X.; Hou, Y.; Rao, W. Superior antibacterial activity of gallium based liquid metals due to ga3+ induced intracellular ros generation. J. Mater. Chem. B 2021, 9, 85–93. [Google Scholar] [CrossRef]
- Yang, J.J.; Wang, C.; Liu, X.L.; Yin, Y.; Ma, Y.H.; Gao, Y.F.; Wang, Y.Z.; Lu, Z.D.; Song, Y.J. Gallium-carbenicillin framework coated defect-rich hollow TiO2 as a photocatalyzed oxidative stress amplifier against complex infections. Adv. Funct. Mater. 2020, 30, 2004861. [Google Scholar] [CrossRef]
- Centola, G.; Xue, F.; Wilks, A. Metallotherapeutics development in the age of iron-clad bacteria. Metallomics 2020, 12, 1863–1877. [Google Scholar] [CrossRef]
- Choi, S.; Switzer, B.; Britigan, B.E.; Narayanasamy, P. Gallium porphyrin and gallium nitrate synergistically inhibit mycobacterial species by targeting different aspects of iron/heme metabolism. ACS Infect. Dis. 2020, 6, 2582–2591. [Google Scholar] [CrossRef]
- Choi, S.; Britigan, B.E.; Narayanasamy, P. Dual inhibition of klebsiella pneumoniae and Pseudomonas aeruginosa iron metabolism using gallium porphyrin and gallium nitrate. ACS Infect. Dis. 2019, 5, 1559–1569. [Google Scholar] [CrossRef]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in plant disease management: Dna-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef]
- Turek, D.; Simaeys, D.V.; Johnson, J.; Ocsoy, I.; Tan, W. Molecular recognition of live methicillin-resistant staphylococcus aureus cells using DNA aptamers. World J. Transl. Med. 2013, 2, 8. [Google Scholar] [CrossRef]
- Ungor, D.; Barbasz, A.; Czyżowska, A.; Csapó, E.; Oćwieja, M. Cytotoxicity studies of protein-stabilized fluorescent gold nanoclusters on human lymphocytes. Colloids Surf. B Biointerfaces 2021, 200, 111593. [Google Scholar] [CrossRef] [PubMed]
- Czyżowska, A.; Barbasz, A.; Szyk-Warszyńska, L.; Oćwieja, M.; Csapó, E.; Ungor, D. The surface-dependent biological effect of protein-gold nanoclusters on human immune system mimetic cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126569. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Some, S.; Bulut, O.; Biswas, K.; Kumar, A.; Roy, A.; Sen, I.K.; Mandal, A.; Franco, O.L.; İnce, İ.A.; Neog, K.; et al. Effect of feed supplementation with biosynthesized silver nanoparticles using leaf extract of Morus indica L. V1 on Bombyx mori L. (Lepidoptera: Bombycidae). Sci. Rep. 2019, 9, 14839. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Büyükbezirci, K.; Celik, C.; Kislakci, E.; Karaagac, Z.; Gokturk, E.; Kati, A.; Cimen, B.; Yilmaz, V.; Ocsoy, I. Synthesis of long-term stable gold nanoparticles benefiting from red raspberry (Rubus idaeus), strawberry (Fragaria ananassa), and blackberry (Rubus fruticosus) extracts–gold ion complexation and investigation of reaction conditions. ACS Omega 2019, 4, 18637–18644. [Google Scholar] [CrossRef]
- Chitambar, C.R.; Zivkovic, Z. Inhibition of hemoglobin production by transferrin-gallium. Blood 1987, 69, 144–149. [Google Scholar] [CrossRef]
- Gould, T.D.; Quiroz, J.A.; Singh, J.; Zarate, C.A.; Manji, H.K. Emerging experimental therapeutics for bipolar disorder: Insights from the molecular and cellular actions of current mood stabilizers. Mol. Psychiatry 2004, 9, 734–755. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mat. Sci. Eng. C-Mater. 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Lessa, J.A.; Parrilha, G.L.; Beraldo, H. Gallium complexes as new promising metallodrug candidates. Inorg. Chim. Acta 2012, 393, 53–63. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium-containing anticancer compounds. Future Med. Chem. 2012, 4, 1257–1272. [Google Scholar] [CrossRef]
- Banin, E.; Lozinski, A.; Brady, K.M.; Berenshtein, E.; Butterfield, P.W.; Moshe, M.; Chevion, M.; Greenberg, E.P.; Banin, E. The potential of desferrioxamine-gallium as an anti-pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. USA 2008, 105, 16761–16766. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.S.; Imperi, F.; Minandri, F.; Visca, P. In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5961–5970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolafrancesco, C.; Porcaro, F.; Pis, I.; Nappini, S.; Simonelli, L.; Marini, C.; Frangipani, E.; Visaggio, D.; Visca, P.; Mobilio, S.; et al. Gallium- and iron-pyoverdine coordination compounds investigated by X-ray photoelectron spectroscopy and X-ray absorption spectroscopy. Inorg. Chem. 2019, 58, 4935–4944. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, S.; Visca, P.; Frangipani, E. Gallium-protoporphyrin ix inhibits Pseudomonas aeruginosa growth by targeting cytochromes. Front. Cell. Infect. Microbiol. 2017, 7, 12. [Google Scholar] [CrossRef]
- Piatek, M.; Griffith, D.M.; Kavanagh, K. Quantitative proteomic reveals gallium maltolate induces an iron-limited stress response and reduced quorum-sensing in Pseudomonas aeruginosa. J. Biol. Inorg. Chem. 2020, 25, 1153–1165. [Google Scholar] [CrossRef]
- Nikolova, V.; Angelova, S.; Markova, N.; Dudev, T. Gallium as a therapeutic agent: A thermodynamic evaluation of the competition between Ga3+ and Fe3+ ions in metalloproteins. J. Phys. Chem. B 2016, 120, 2241–2248. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of gallium-based drugs for antibacterial therapy. Biofactors 2014, 40, 303–312. [Google Scholar] [CrossRef]
- Noujaim, A.A.; Lentle, B.C.; Hill, J.R.; Terner, U.K.; Wong, H. Role of transferrin in the uptake of gallium by tumor-cells. Int. J. Nucl. Med. Biol. 1979, 6, 193–199. [Google Scholar] [CrossRef]
- Larson, S.M.; Grunbaumi, Z.; Raseyz, J.S. The role of transferrins in gallium uptake. Int. J. Nucl. Med. Biol. 1981, 8, 257–266. [Google Scholar] [CrossRef]
- Harris, W.R.; Pecoraro, V.L. Thermodynamic binding constants for gallium transferrin. Biochemistry 1983, 22, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Min, Z.J.; Yu, B. Reactive oxygen species and immune regulation. Int. Rev. Immunol. 2020, 39, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Collins, J.J.; Walker, G.C. Unraveling the physiological complexities of antibiotic lethality. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H. Healthy immunity: It’s all about immune regulation. Int. Rev. Immunol. 2020, 39, 245–246. [Google Scholar] [CrossRef]
- Hong, Y.Z.; Zeng, J.; Wang, X.H.; Drlica, K.; Zhao, X.L. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Morales-De-Echegaray, A.V.; Lin, L.; Sivasubramaniam, B.; Yermembetova, A.; Wang, Q.; Abutaleb, N.S.; Seleem, M.N.; Wei, A. Antimicrobial photodynamic activity of gallium-substituted haemoglobin on silver nanoparticles. Nanoscale 2020, 12, 21734–21742. [Google Scholar] [CrossRef]
- Cheeseman, S.; Christofferson, A.J.; Kariuki, R.; Cozzolino, D.; Daeneke, T.; Crawford, R.J.; Truong, V.K.; Chapman, J.; Elbourne, A. Antimicrobial metal nanomaterials: From passive to stimuli-activated applications. Adv. Sci. 2020, 7, 1902913. [Google Scholar] [CrossRef]
- Dong, T.G.; Dong, S.; Catalano, C.; Moore, R.; Liang, X.; Mekalanos, J.J. Generation of reactive oxygen species by lethal attacks from competing microbes. Proc. Natl. Acad. Sci. USA 2015, 112, 2181–2186. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Shisaka, Y.; Iwai, Y.; Yamada, S.; Uehara, H.; Tosha, T.; Sugimoto, H.; Shiro, Y.; Stanfield, J.K.; Ogawa, K.; Watanabe, Y.; et al. Hijacking the heme acquisition system of Pseudomonas aeruginosa for the delivery of phthalocyanine as an antimicrobial. ACS Chem. Biol. 2019, 14, 1637–1642. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Q.; Wang, H.; Zhang, L.; Song, G.; Song, L.; Hu, J.; Wang, H.; Liu, J.; Zhu, M.; et al. Ultrathin pegylated W18O49 nanowires as a new 980 nm-laser-driven photothermal agent for efficient ablation of cancer cells in vivo. Adv. Mater. 2013, 25, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Cheng, Y.J.; Zhang, X.Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef]
- Xu, D.; Hu, J.; Pan, X.; Sánchez, S.; Yan, X.; Ma, X. Enzyme-powered liquid metal nanobots endowed with multiple biomedical functions. ACS Nano 2021, 15, 11543–11554. [Google Scholar] [CrossRef]
- Wang, D.; Xie, W.S.; Gao, Q.; Yan, H.; Zhang, J.X.; Lu, J.S.; Liaw, B.; Guo, Z.H.; Gao, F.; Yin, L.; et al. Non-magnetic injectable implant for magnetic field-driven thermochemotherapy and dual stimuli-responsive drug delivery: Transformable liquid metal hybrid platform for cancer theranostics. Small 2019, 15, 1900511. [Google Scholar] [CrossRef]
- Sun, X.Y.; Guo, R.; Yuan, B.; Wang, H.Z.; Duan, M.H.; Yang, Y.X.; Zhu, X.Y.; Wang, X.L.; Chen, S.; Cheng, J.S.; et al. Stiffness tunable implanted electrode enabled by magnetic liquid metal for wireless hyperthermia. Appl. Mater. Today 2022, 27, 101495. [Google Scholar] [CrossRef]
- Chechetka, S.A.; Yu, Y.; Zhen, X.; Pramanik, M.; Pu, K.; Miyako, E. Light-driven liquid metal nanotransformers for biomedical theranostics. Nat. Commun. 2017, 8, 15432. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Jin, T.; Yuan, K.; You, J.Y.; Shen, C.; Xie, K.Y. Chemically stable polypyrrole-modified liquid metal nanoparticles with the promising photothermal conversion capability. J. Mater. Sci. Technol. 2022, 127, 144–152. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Li, J.; Nickel, R.; Wu, J.; Lin, F.; van Lierop, J.; Liu, S. A new tool to attack biofilms: Driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale 2019, 11, 6905–6915. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhang, B.; Nie, Y.; Tang, X.; Yang, S.; Zhou, S. Enhanced antibacterial activity of V-doped ZnO@SiO2 composites. Appl. Surf. Sci. 2021, 546, 149127. [Google Scholar] [CrossRef]
- Cheeseman, S.; Elbourne, A.; Kariuki, R.; Ramarao, A.V.; Zavabeti, A.; Syed, N.; Christofferson, A.J.; Kwon, K.Y.; Jung, W.; Dickey, M.D.; et al. Broad-spectrum treatment of bacterial biofilms using magneto-responsive liquid metal particles. J. Mater. Chem. B 2020, 8, 10776–10787. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Sentandreu, R. Fungal cell wall synthesis and assembly. Curr. Top. Med. Mycol. 1989, 3, 168–217. [Google Scholar]
- Perez, P.; Ribas, J.C. Cell wall analysis. Methods 2004, 33, 245–251. [Google Scholar] [CrossRef]
- Verbancic, J.; Lunn, J.E.; Stitt, M.; Persson, S. Carbon supply and the regulation of cell wall synthesis. Mol. Plant 2018, 11, 75–94. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.Z.; Hoiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Lee, S.W.; Carnicelli, J.; Zhang, T.; Ren, D. Magnetically driven active topography for long-term biofilm control. Nat. Commun. 2020, 11, 2211. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.; Yuan, B.; Chen, S.; Wang, H.; Dou, M.; Liu, J.; He, Z. Low-temperature triggered shape transformation of liquid metal microdroplets. ACS Appl. Mater. Interfaces 2020, 12, 38386–38396. [Google Scholar] [CrossRef]
- Sun, X.; Cui, B.; Yuan, B.; Wang, X.; Fan, L.; Yu, D.; He, Z.; Sheng, L.; Liu, J.; Lu, J. Liquid metal microparticles phase change medicated mechanical destruction for enhanced tumor cryoablation and dual-mode imaging. Adv. Funct. Mater. 2020, 30, 2003359. [Google Scholar] [CrossRef]
- Hu, L.; Wang, H.; Wang, X.; Liu, X.; Guo, J.; Liu, J. Magnetic liquid metals manipulated in the three-dimensional free space. ACS Appl. Mater. Interfaces 2019, 11, 8685–8692. [Google Scholar] [CrossRef]
- Slate, A.J.; Karaky, N.; Crowther, G.S.; Butler, J.A.; Banks, C.E.; Mcbain, A.J.; Whitehead, K.A. Graphene matrices as carriers for metal ions against antibiotic susceptible and resistant bacterial pathogens. Coatings 2021, 11, 352. [Google Scholar] [CrossRef]
- Cheeseman, S.; Elbourne, A.; Gangadoo, S.; Shaw, Z.L.; Bryant, S.J.; Syed, N.; Dickey, M.D.; Higgins, M.J.; Vasilev, K.; Mcconville, C.F.; et al. Interactions between liquid metal droplets and bacterial, fungal, and mammalian cells. Adv. Mater. Interfaces 2022, 9, 2102113. [Google Scholar] [CrossRef]
- Levaditi, C.; Bardet, J.; Tchakirian, A.; Vaisman, A. Le gallium, propriétés thérapeutiques dans la syphilis et les trypanosomiases expérimentales. CR Hebd Seances Acad. Sci. Ser. D Sci. Nat. 1931, 192, 1142–1143. [Google Scholar]
- Stojiljkovic, I.; Kumar, V.; Srinivasan, N. Non-iron metalloporphyrins: Potent antibacterial compounds that exploit haem/HB uptake systems of pathogenic bacteria. Mol. Microbiol. 2010, 31, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Wang, W.; Bai, L.J.; Gentekos, D.T.; Hoyos, M.; Mallouk, T.E. Density and shape effects in the acoustic propulsion of bimetallic nanorod motors. ACS Nano 2016, 10, 4763–4769. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.F.; Lu, G.; He, Q.; Li, J.B. Highly flexible polyelectrolyte nanotubes. J. Am. Chem. Soc. 2003, 125, 11140–11141. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Guo, J.; Xu, X.; Fan, D.L. Recent progress on man-made inorganic nanomachines. Small 2015, 11, 4037–4057. [Google Scholar] [CrossRef]
- Gao, W.; Dong, R.; Thamphiwatana, S.; Li, J.; Gao, W.; Zhang, L.; Wang, J. Artificial micromotors in the mouse’s stomach: A step toward in vivo use of synthetic motors. ACS Nano 2014, 9, 117–123. [Google Scholar] [CrossRef]
- Wang, D.; Gao, C.; Wei, W.; Sun, M.; Guo, B. Suid metal nanomachine. ACS Nano 2018, 12, 10212–10220. [Google Scholar] [CrossRef]
- Li, F.; Kuang, S.; Li, X.; Shu, J.; Li, W.; Tang, S.; Zhang, S. Magnetically- and electrically-controllable functional liquid metal droplets. Adv. Mater. Technol. 2019, 4, 1800694. [Google Scholar] [CrossRef]
- Müller, A.; Fessele, C.; Zuber, F.; Rottmar, M.; Maniura-Weber, K.; Ren, Q.; Guex, A.G. Gallium complex-functionalized p4hb fibers: A trojan horse to fight bacterial infection. ACS Appl. Bio Mater. 2021, 4, 682–691. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; Vos, W.M.; Kamperman, M. Recent developments and practical feasibility of polymer-based antifouling coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A.; Higazy, S.A.; Elmarakbi, A. Progress in biomimetic leverages for marine antifouling using nanocomposite coatings. J. Mater. Chem. B 2020, 8, 3701–3732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Ye, Q.; Yu, Q.; Pei, X.; Yu, B.; Zhou, F. Novel anticorrosion property of organic coating based on liquid metal. Adv. Mater. Interfaces 2019, 6, 1900942. [Google Scholar] [CrossRef]
- Hohman, J.N.; Kim, M.; Wadsworth, G.A.; Bednar, H.R.; Jiang, J.; Lethai, M.A.; Weiss, P.S. Directing substrate morphology via self-assembly: Ligand-mediated scission of gallium-indium microspheres to the nanoscale. Nano Lett. 2011, 11, 5104–5110. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Du, Y.; Wang, B.; Zhao, X.; Liu, S.; Ye, Q.; Zhou, F. Self-healing polydimethylsiloxane antifouling coatings based on zwitterionic polyethylenimine-functionalized gallium nanodroplets. Chem. Eng. J. 2022, 427, 131019. [Google Scholar] [CrossRef]
- Centurion, F.; Namivandi-Zangeneh, R.; Flores, N.; Tajik, M.; Merhebi, S.; Abbasi, R.; Mayyas, M.; Allioux, F.M.; Tang, J.B.; Donald, W.A.; et al. Liquid metal-triggered assembly of phenolic nanocoatings with antioxidant and antibacterial properties. ACS Appl. Nano Mater. 2021, 4, 2987–2998. [Google Scholar] [CrossRef]
- Halwani, M.; Yebio, B.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Pajor, K.; Pajchel, L.; Zgadzaj, A.; Piotrowska, U.; Kolmas, J. Modifications of hydroxyapatite by gallium and silver ions-physicochemical characterization, cytotoxicity and antibacterial evaluation. Int. J. Mol. Sci. 2020, 21, 5006. [Google Scholar] [CrossRef]
- Best, M.G.; Cunha-Reis, C.; Ganin, A.Y.; Sousa, A.; Johnston, J.; Oliveira, A.L.; Smith, D.G.E.; Yiu, H.H.P.; Cooper, I.R. Antimicrobial properties of gallium(III)- and iron(III)-loaded polysaccharides affecting the growth of escherichia coli, staphylococcus aureus, and Pseudomonas aeruginosa, in vitro. ACS Appl. Bio Mater. 2020, 3, 7589–7597. [Google Scholar] [CrossRef]
- Ma, H.; Darmawan, E.T.; Zhang, M.; Zhang, L.; Bryers, J.D. Development of a poly(ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J. Control. Release 2013, 172, 1035–1044. [Google Scholar] [CrossRef]
- Valappil, S.P.; Yiu, H.H.P.; Bouffier, L.; Hope, C.K.; Evans, G.; Claridge, J.B.; Higham, S.M.; Rosseinsky, M.J. Effect of novel antibacterial gallium-carboxymethyl cellulose on Pseudomonas aeruginosa. Dalton Trans. 2013, 42, 1778–1786. [Google Scholar] [CrossRef]
- Young, M.; Ozcan, A.; Lee, B.; Maxwell, T.; Andl, T.; Rajasekaran, P.; Beazley, M.J.; Tetard, L.; Santra, S. N-acetyl cysteine coated gallium particles demonstrate high potency against Pseudomonas aeruginosa pao1. Pathogens 2019, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.A.; Hadzhieva, Z.; Ilyas, K.; Ali, M.S.; Peukert, W.; Boccaccini, A.R. Facile synthesis of gallium (III)-chitosan complexes as antibacterial biomaterial. Pharmaceutics 2021, 13, 1702. [Google Scholar] [CrossRef] [PubMed]
- Kurtjak, M.; Vukomanovic, M.; Kramer, L.; Suvorov, D. Biocompatible nano-gallium/hydroxyapatite nanocomposite with antimicrobial activity. J. Mater. Sci.-Mater. Med. 2016, 27, 170. [Google Scholar] [CrossRef]
- Khosravanihaghighi, A.; Koshy, P.; Yasir, M.; Romanazzo, S.; Lovric, V.; Kilian, K.A.; Willcox, M.D.; Walsh, W.R.; Sorrell, C.C. Production of antibacterial activity and bone cell proliferation by surface engineering of Ga- or Mn-doped ceria-coated biomedical titanium alloy. Adv. Eng. Mater. 2022, 2200077. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Chiesa, R.; Rimondini, L.; Gasik, M. Metallurgical gallium additions to titanium alloys demonstrate a strong time-increasing antibacterial activity without any cellular toxicity. ACS Biomater. Sci. Eng. 2019, 5, 2815–2820. [Google Scholar] [CrossRef]
- Shokri, M.; Kharaziha, M.; Tafti, H.A.; Eslaminejad, M.B.; Aghdam, R.M. Synergic role of zinc and gallium doping in hydroxyapatite nanoparticles to improve osteogenesis and antibacterial activity. Biomater. Adv. 2022, 134, 112684. [Google Scholar] [CrossRef] [PubMed]
- Agostino, A.D.; Tana, F.; Ettorre, A.; Pavarini, M.; Serafini, A.; Cochis, A.; Scalia, A.C.; Rimondini, L.; De Giglio, E.; Cometa, S.; et al. Mesoporous zirconia surfaces with anti-biofilm properties for dental implants. Biomed. Mater. 2021, 16, 45016. [Google Scholar] [CrossRef]
- Li, K.; Tian, H.; Guo, A.; Jin, L.; Chen, W.; Tao, B. Gallium (Ga)–strontium (Sr) layered double hydroxide composite coating on titanium substrates for enhanced osteogenic and antibacterial abilities. J. Biomed. Mater. Res. A 2022, 110, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Ekkelenkamp, M.B.; Cantón, R.; Díez-Aguilar, M.; Tunney, M.M.; Fluit, A. Susceptibility of Pseudomonas aeruginosa recovered from cystic fibrosis patients to murepavadin and thirteen comparator antibiotics. Antimicrob. Agents Chemother. 2019, 64, e1541. [Google Scholar]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of gallium and gallium-based compounds as antimicrobial agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, B.; Biazik, J.M.; Webster, R.F.; Xie, W.; Tang, J.; Allioux, F.; Abbasi, R.; Mousavi, M.; Goldys, E.M.; et al. Gallium nanodroplets are anti-inflammatory without interfering with iron homeostasis. ACS Nano 2022, 16, 8891–8903. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-transformable liquid metal nanoparticles in aqueous solution. Chem. Sci. 2017, 8, 3832–3837. [Google Scholar] [CrossRef]
- Li, H.; Qiao, R.; Davis, T.P.; Tang, S. Biomedical applications of liquid metal nanoparticles: A critical review. Biosensors 2020, 10, 196. [Google Scholar] [CrossRef]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, fabrication, and applications of gallium-based liquid metal particles. Adv. Sci. 2020, 7, 2000192. [Google Scholar] [CrossRef]
- Goerl, U.; Hunsche, A.; Mueller, A.; Koban, H.G. Investigations into the silica/silane reaction system. Rubber Chem. Technol. 1997, 70, 608–623. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, C.-C.; Liang, Y.-T.; Wang, X.-Q.; Gao, S.; He, Z.-Z.; Sun, X.-Y. Gallium-Based Liquid Metal Materials for Antimicrobial Applications. Bioengineering 2022, 9, 416. https://doi.org/10.3390/bioengineering9090416
Qu C-C, Liang Y-T, Wang X-Q, Gao S, He Z-Z, Sun X-Y. Gallium-Based Liquid Metal Materials for Antimicrobial Applications. Bioengineering. 2022; 9(9):416. https://doi.org/10.3390/bioengineering9090416
Chicago/Turabian StyleQu, Chun-Chun, Yu-Tong Liang, Xi-Qing Wang, Shang Gao, Zhi-Zhu He, and Xu-Yang Sun. 2022. "Gallium-Based Liquid Metal Materials for Antimicrobial Applications" Bioengineering 9, no. 9: 416. https://doi.org/10.3390/bioengineering9090416
APA StyleQu, C.-C., Liang, Y.-T., Wang, X.-Q., Gao, S., He, Z.-Z., & Sun, X.-Y. (2022). Gallium-Based Liquid Metal Materials for Antimicrobial Applications. Bioengineering, 9(9), 416. https://doi.org/10.3390/bioengineering9090416







