Scale-Up of Capsular Polysaccharide Production Process by Haemophilus influenzae Type b Using kLa Criterion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Medium Composition
2.3. Inoculum
2.4. Evaluation in Bioreactor Systems
2.4.1. Specifications of Bioreactor Systems
2.4.2. Determination of Volumetric Oxygen Mass Transfer Coefficient (kLa)
2.4.3. Batch Cultures in 1.5 L Bench-Scale Bioreactor System
2.4.4. Batch Cultures in 15 L Bench-Scale and 75 L Pilot-Scale Bioreactor Systems
2.4.5. Fed-Batch Culture in 75 L Pilot-Scale Bioreactor System
2.5. Analytical Methods
2.5.1. Biomass Measurement
2.5.2. Glucose and Organic Acids’ Determination
2.5.3. PRP Measurement and Molecular Weight (MW) Determination
2.6. Determination of Kinetic Parameters
2.6.1. Growth Specific Rate
2.6.2. Conversion Factors
2.6.3. Productivity of Biomass and Product
3. Results
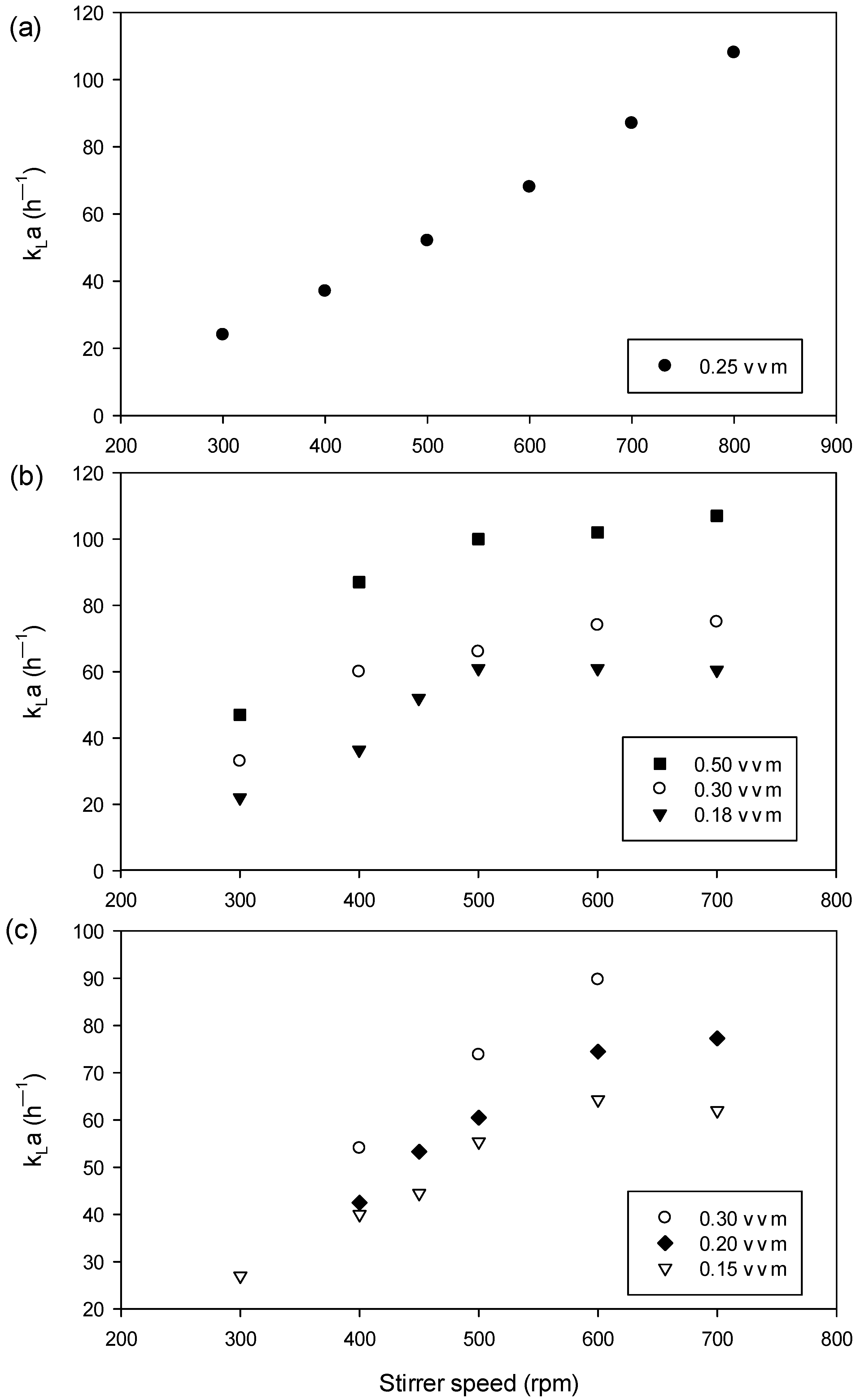
3.1. Batch Cultures in 1.5 L Bench-Scale Bioreactor System and Evaluation of kLa Values
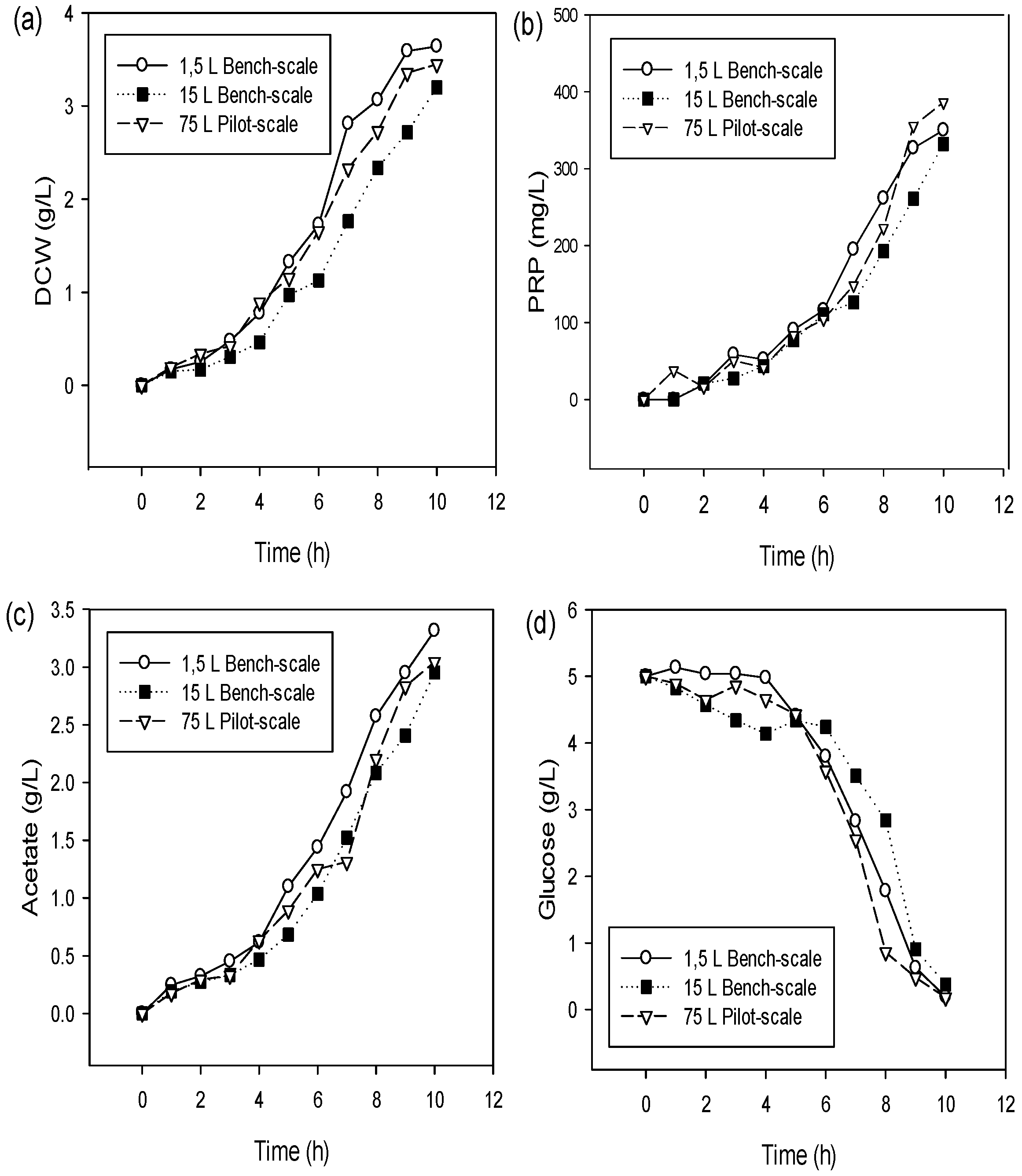
3.2. Scale-Up Batch Cultures in 15 L Bench-Scale and 75 L Pilot-Scale Bioreactor Systems
3.3. Fed-Batch Culture in 75 L Pilot-Scale Bioreactor System
3.4. Molecular Weight (MW) of Produced PRP
4. Discussion
4.1. Batch Cultures in 1.5 L bench-Scale Bioreactor and Evaluation of kLa Values
4.2. Scale-Up Batch Cultures in 15 L Bench-Scale and 75 L Pilot-Scale Bioreactor Systems
4.3. Fed-Batch Culture in 75 L Pilot-Scale Bioreactor System
4.4. Molecular Weight (MW) of Produced PRP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, E.; Terrade, A.; Denizon, M.; Aouiti-Trabelsi, M.; Falguières, M.; Taha, M.K.; Deghmane, A.E. Haemophilus influenzae type b (Hib) seroprevalence in France: Impact of vaccination schedules. BMC Infect. Dis. 2021, 21, 715. [Google Scholar] [CrossRef] [PubMed]
- WHO. Haemophilus influenzae Type b (Hib) Meningitis in the Pre-Vaccine Era: A Global Review of Incidence, Age Distributions, and Case-Fatality Rates; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Abdelhameed, A.S.; Adams, G.G.; Morris, G.A.; Almutairi, F.M.; Duvivier, P.; Conrath, K.; Harding, S.E. A glycoconjugate of Haemophilus influenzae Type b capsular polysaccharide with tetanus toxoid protein: Hydrodynamic properties mainly influenced by the carbohydrate. Sci. Rep. 2016, 6, 22208. [Google Scholar] [CrossRef]
- Arsang, A.; Tabatabaie, A.; Vaziri, F.; Nejati, M.; Zolfaghari, M.R.; Fateh, A.; Jamnani, F.R.; Bahrmand, A.R.; Siadat, S.D. Optimization of large scale production of Haemophilus influenzae type b polyribosyl-ribitol phosphate. Minerva Biotecnol. 2017, 29, 17–23. [Google Scholar] [CrossRef]
- Baek, J.Y.; Geissner, A.; Rathwell, D.C.K.; Meierhofer, D.; Pereira, C.L.; Seeberger, P.H. A modular synthetic route to size-defined immunogenic: Haemophilus influenzae b antigens is key to the identification of an octasaccharide lead vaccine candidate. Chem. Sci. 2018, 9, 1279–1288. [Google Scholar] [PubMed]
- WHO. Recommendations for the Production and Control of Haemophilus influenzae Type b Conjugate Vaccines; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. [Google Scholar] [PubMed]
- Linsley, C.S.; Sung, K.; White, C.; Abecunas, C.A.; Tawil, B.J.; Wu, B.M. Functionalizing Fibrin Hydrogels with Thermally Responsive Oligonucleotide Tethers for On-Demand Delivery. Bioengineering 2022, 9, 25. [Google Scholar] [CrossRef]
- Ada, G.; Isaacs, D. Carbohydrate–protein conjugate vaccines. Clin. Microbiol. Infect. 2003, 9, 79–85. [Google Scholar] [PubMed]
- Rappuoli, R. Glycoconjugate vaccines: Principles and mechanisms. Sci. Transl. Med. 2018, 10, eaat4615. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Gregorio, E.D. A sweet T cell response. Nat. Med. 2011, 17, 1551–1552. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.; Allard, G.; O’Toole, L.; Swartz, R.; Licari, P. Development and scale-up of a fed-batch process for the production of capsular polysaccharide from Haemophilus influenzae. J. Biotechnol. 2000, 81, 189–197. [Google Scholar] [CrossRef]
- Beurret, M.; Hamidi, A.; Kreeftenberg, H. Development and technology transfer of Haemophilus influenzae type b conjugate vaccines for developing countries. Vaccine 2012, 30, 4897–4906. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Mathematical model for scaling up bioprocesses using experiment design combined with buckingham Pi theorem. Appl. Sci. 2021, 11, 11338. [Google Scholar]
- Xu, S.; Hoshan, L.; Jiang, R.; Gupta, B.; Brodean, E.; O’Neill, K.; Seamans, T.C.; Bowers, J.; Chen, H. A practical approach in bioreactor scale-up and process transfer using a combination of constant P/V and vvm as the criterion. Biotechnol. Prog. 2017, 33, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Gameil, A.H.M.; Yusof, F.; Azmi, A.S.; Puad, N.I.M. Process scale-up criteria in production of recombinant proteins in E. coli: A systematic review. Biol. Nat. Resour. Eng. J. 2021, 5, 37–61. [Google Scholar]
- Allan, S.J.; De Bank, P.A.; Ellis, M.J. Bioprocess design considerations for cultured meat production with a focus on the expansion bioreactor. Front. Sustain. Food Syst. 2019, 3, 44. [Google Scholar]
- Xu, S.; Jiang, R.; Mueller, R.; Hoesli, N.; Kretz, T.; Bowers, J.; Chen, H. Probing lactate metabolism variations in large-scale bioreactors. Biotechnol. Prog. 2018, 34, 756–766. [Google Scholar] [PubMed]
- Takagi, M.; Cabrera-crespo, J.; Zangirolami, T.C.; Raw, I.; Massako, T.M. Improved cultivation conditions for polysaccharide production by H. influenzae type b. J. Chem. Technol. Biotechnol. 2006, 81, 182–188. [Google Scholar] [CrossRef]
- Salimova, E.; Kono, A.D.; Truhin, V.P.; Krasilnikov, I.V. Technology of obtain polyribosyl ribitol phosphate as an active ingredient for the production of polysaccharide vaccines. Pharm. Pharmacol. 2018, 6, 47–62. [Google Scholar]
- García-Salas, S.; Gómez-Montes, E.O.; Ramírez-Sotelo, M.G.; Oliver-Salvador, M.C. Shear rate as scale-up criterion of the protein production with enhanced proteolytic activity by phosphate addition in the Jacaratia mexicana cell culture. Biotechnol. Biotechnol. Equip. 2021, 35, 1031–1042. [Google Scholar] [CrossRef]
- Möller, J.; Hernández, T.; Müller, J.; Arndt, L.; Kuchemüller, K.B.; Frahm, B.; Eibl, R.; Eibl, D.; Pörtner, R. Model uncertainty-based evaluation of process strategies during scale-up of biopharmaceutical processes. Comput. Chem. Eng. 2020, 134, 106693. [Google Scholar]
- Shin, W.; Lee, D.; Kim, S.; Jeong, Y.; Chun, G. Application of scale-up criterion of constant oxygen mass transfer coefficient (kLa) for production of itaconic acid in 50 L pilot-scale fermentor by fungal cells of Aspergillus terreus. J. Microbiol. Biotechnol. 2013, 23, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Zhu, S.; Solsvik, J. Effects of geometric parameters on volumetric mass transfer coefficient of non-Newtonian fluids in stirred tanks. Int. J. Chem. React. Eng. 2021, 20, 1–15. [Google Scholar] [CrossRef]
- Michelin, M.; Oliveira, A.M.; Polizeli, M.; Silva, D.P.; Vicente, A.A.; Teixeira, J.A. Influence of volumetric oxygen transfer coefficient (kLa) on xylanases batch production by Aspergillus niger van Tieghem in stirred tank and internal-loop airlift bioreactors. Biochem. Eng. J. 2013, 80, 19–26. [Google Scholar] [CrossRef]
- Mahler, N.; Tschirren, S.; Pflügl, S.; Herwig, C. Optimized bioreactor setup for scale-up studies of extreme halophilic cultures. Biochem. Eng. J. 2018, 130, 39–46. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Gómez, E.; Santos, V.E. Oxygen transfer and uptake rates during xanthan gum production. Enzyme Microb. Technol. 2000, 27, 680–690. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Angenent, L.T.; Usack, J.G. The Measurement, application, and effect of oxygen in microbial fermentations: Focusing on methane and carboxylate production. Fermentation 2022, 8, 138. [Google Scholar] [CrossRef]
- Haan, A.D.; Put, R.M.F.; Beurret, M. HPAEC-PAD method for the analysis of alkaline hydrolyzates of Haemophilus influenzae type b capsular polysaccharide. Biomed. Chromatogr. 2013, 27, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Cintra, F.; Takagi, M. Comparison among different sample treatment methods for analysis of molecular weight and concentration of exopolysaccharide produced by Haemophilus influenzae type b F. Microb. Appl. Res. 2012, 513–517. [Google Scholar] [CrossRef]
- Pillaca-Pullo, O.; Rodrigues, D.; Sánchez-Moguel, I.; Lopes, A.; Pimenta, M.; Basi, T.; Feitosa, V.; Zavaleta, A.I.; Monteiro, G.; Pessoa, A.; et al. Recombinant L-asparaginase production using Pichia pastoris (MUTs strain): Establishment of conditions for growth and induction phases. J. Chem. Technol. Biotechnol. 2020, 96, 283–292. [Google Scholar] [CrossRef]
- Abdella, A.; Segato, F.; Wilkins, M.R. Optimization of process parameters and fermentation strategy for xylanase production in a stirred tank reactor using a mutant Aspergillus nidulans strain. Biotechnol. Rep. 2020, 26, e00457. [Google Scholar] [CrossRef] [PubMed]
- Averkina, A.S.; Kazakov, D.A.; Asnin, L.D.; Kaczmarski, K.; Krol, G.; Vol’khin, V.V. New insights on oxygen absorption in unsparged stirred vessels. Heat Mass Transf. 2017, 53, 1971–1982. [Google Scholar] [CrossRef]
- Tervasmäki, P.; Latva-Kokko, M.; Taskila, S.; Tanskanen, J. Effect of oxygen transfer on yeast growth—Growth kinetic and reactor model to estimate scale-up effects in bioreactors. Food Bioprod. Process 2018, 111, 129–140. [Google Scholar] [CrossRef]
- Karimi, A.; Golbabaei, F.; Mehrnia, M.R.; Neghab, M.; Mohammad, K.; Nikpey, A.; Pourmand, M.R. Oxygen mass transfer in a stirred tank bioreactor using different impeller configurations for environmental purposes. Iran. J. Environ. Health Sci. Eng. 2013, 10, 6. [Google Scholar] [CrossRef]
- Shukla, V.B.; Veera, U.P.; Kulkarni, P.R.; Pandit, A.B. Scale-up of biotransformation process in stirred tank reactor using dual impeller bioreactor. Biochem. Eng. J. 2001, 8, 19–29. [Google Scholar] [CrossRef]
- Rodrigues, D.; Pillaca-Pullo, O.; Torres-Obreque, K.; Flores-Santos, J.; Sánchez-Moguel, I.; Pimenta, M.V.; Basi, T.; Converti, A.; Lopes, A.M.; Monteiro, G.; et al. Fed-batch production of Saccharomyces cerevisiae L-Asparaginase II by recombinant Pichia pastoris MUTs strain. Front. Bioeng. Biotechnol. 2019, 7, 16. [Google Scholar] [CrossRef]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Xu, C.P.; Choi, J.W.; Yun, J.W. Effect of aeration and agitation on the production of mycelial biomass and exopolysaccharides in an enthomopathogenic fungus Paecilomyces sinclairii. Lett. Appl. Microbiol. 2003, 36, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, Z.; Ong, S.L.; Hu, J.; Zhang, Z.; Ng, W.J. Optimization of agitation, aeration, and temperature conditions for maximum β-mannanase production. Enzyme Microb. Technol. 2003, 32, 282–289. [Google Scholar] [CrossRef]
- Kadri, T.; Miri, S.; Robert, T.; Kaur, S.; Rouissi, T.; Laxman, V.; Lauzon, J.M. Pilot-scale production and in-situ application of petroleum-degrading enzyme cocktail from Alcanivorax borkumensis. Chemosphere 2022, 295, 133840. [Google Scholar] [CrossRef] [PubMed]
- Herbst, H.; Schumpe, A.; Deckwer, W. Xanthan production in stirred tank fermenters: Oxygen transfer and scale-up. Chem. Eng. Technol. 1992, 15, 425–434. [Google Scholar] [CrossRef]
- Hoiseth, S.K.; Connelly, C.J.; Moxont, E.R. Genetics of spontaneous, high-frequency loss of b capsule expression in Haemophilus influenzae. Infect. Immun. 1985, 49, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Jun Lv, P.; Qiang, S.; Liu, L.; Hu, C.Y.; Meng, Y.H. Dissolved-oxygen feedback control fermentation for enhancing β-carotene in engineered Yarrowia lipolytica. Sci. Rep. 2020, 10, 17114. [Google Scholar] [CrossRef]
- Nor, Z.M.; Tamer, M.I.; Scharer, J.M.; Moo-Young, M.; Jervis, E.J. Automated fed-batch culture of Kluyveromyces fragilis based on a novel method for on-line estimation of cell specific growth rate. Biochem. Eng. J. 2001, 9, 221–231. [Google Scholar] [CrossRef]
- Barberis, S.E.; Segovia, R.F. Dissolved oxygen concentration-controlled feeding of substrate into Kluyveromyces fragilis culture. Biotechnol. Tech. 1997, 11, 797–799. [Google Scholar] [CrossRef]
- Zheng, R.; Pan, F. On-line tendency control of dissolved oxygen concentration during aerobic fed-batch fermentations. Appl. Sci. 2019, 9, 5232. [Google Scholar] [CrossRef]
- Da Silva, M.; Freixo Portela, A.; Ferreira Albani, S.; Rizzo de Paiva, P.; Massako Tanizaki, M.; Zangirolami, T. Experimental design and metabolic flux analysis tools to optimize industrially relevant Haemophilus influenzae type b growth medium. Appl. Cell Physiol. Metab. Eng. 2017, 33, 1508–1519. [Google Scholar]
- Chong, H.; Yeow, J.; Wang, I.; Song, H.; Jiang, R. Improving Acetate Tolerance of Escherichia coli by Rewiring Its Global Regulator cAMP Receptor Protein (CRP). PLoS ONE 2013, 8, e77422. [Google Scholar] [CrossRef]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; Jong, H. Acetate metabolism and the inhibition of bacterial growth by acetate. J. Bacteriol. 2019, 201, e00147-19. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Ferreira, S.M.; Cabrera-crespo, J.; Zangirolami, T.C.; Takagi, M.; Da Cruz, J.G. Production of capsular polysaccharide in batch and fed-batch cultivation by Haemophilus influenzae type b. In Proceedings of the Symposium Brazil-Japan in Economy, Science and Technological Innovation, São Paulo, Brazil, 14–16 June 2008; pp. 1–4. [Google Scholar]
- Takagi, M.; Barbosa, R.; Ferreira, S.M.; Zangirolami, T.C.; Massako, M.; Cabrera-Crespo, J. Purification of capsular polysaccharide produced by Haemophilus influenzae type b through a simple, efficient and suitable method for scale-up. J. Ind. Microbiol. Biotechnol. 2008, 35, 1217–1222. [Google Scholar] [CrossRef]
- Cunha, B.L.C.; Bahú, J.O.; Xavier, L.F.; Crivellin, S.; de Souza, S.D.A.; Lodi, L. Lactide: Production Routes, Properties, and Applications. Bioengineering 2022, 9, 164. [Google Scholar] [CrossRef]
- Rana, R.; Dalal, J.; Singh, D.; Kumar, N.; Hanif, S.; Joshi, N.; Chhikara, M.K. Development and characterization of Haemophilus influenzae type b conjugate vaccine prepared using different polysaccharide chain lengths. Vaccine 2015, 33, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Anish, C.; Beurret, M.; Poolman, J. Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines. NPJ Vaccines 2021, 6, 150. [Google Scholar] [CrossRef] [PubMed]

| Specifications | Bioreactors—Nominal Size | ||
|---|---|---|---|
| 1.5-L | 15-L | 75-L | |
| Brand | Infors-HT | Bioengineering | Bioengineering |
| Impeller | Rushton 2 impellers with 6 blades each. Diameters: 38 mm | Rushton 2 impellers with 6 blades each Diameter: 80 mm | Rushton 2 impellers with 6 blades each Diameter: 163.5 mm |
| Drive | Magnetic | Bottom drive with belt shaft | Bottom drive with belt shaft |
| Tank diameter—ID (mm) | 90 | 200 | 400 |
| Type of sparge | Ring sparger | Ring sparger | Ring sparger |
| Working volume | 0.8-L | 10-L | 35-L |
| kLa (h−1) | Stirring Speed (rpm) | Aeration Rate (vvm) | Time (h) | Productivity | μ (h−1) | |
|---|---|---|---|---|---|---|
| DCW (g/L.h) | PRP (g/L.h) | |||||
| 24 (Low) | 300 | 0.25 | 12 | 0.22 | 0.01 | 0.31 |
| 52 (Intermediate) | 500 | 0.25 | 10 | 0.38 | 0.04 | 0.44 |
| 52 (Intermediate)’ | 700 | 0.15 | 10 | 0.36 | 0.04 | 0.43 |
| 80 (High) | 700 | 0.25 | 9 | 0.40 | 0.04 | 0.46 |
| Total Volume (L) | Working Volume (L) | DCW (g/L) | PRP (g/L) | DCW (g/L.h) | PRP (g/L.h) | YDCW/Glu (g/g) | YPRP/Glu (g/g) | YPRP/DCW (g/g) | μ (h−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 0.8 | 3.8 | 0.36 | 0.38 | 0.04 | 0.8 | 0.08 | 0.10 | 0.44 |
| 15 | 10 | 3.2 | 0.33 | 0.32 | 0.03 | 0.7 | 0.07 | 0.10 | 0.46 |
| 75 | 35 | 3.4 | 0.38 | 0.34 | 0.04 | 0.7 | 0.08 | 0.11 | 0.49 |
| Bioreator Size (L) | Operational Mode | Conditions | Time (h) | MW (kDa) |
|---|---|---|---|---|
| 1.5 | Batch | 0.25 vvm- 500 rpm | 10 | 355.5 |
| 15 | Batch | 0.15 vvm -450 rpm | 10 | 337.4 |
| 75 | * Batch | 0.18 vvm -450 rpm | 10 | 348.6 |
| 75 | * Fed-batch | pO2 30% | 9 | 375.0 |
| Volume | Medium Composition (g/L) | Culture Conditions | DCW (g/L) | PRP (g/L) | Ref. | |
|---|---|---|---|---|---|---|
| Batch | Fed-Batch | |||||
| FV: 50 L WV: ND FM: ND | glucose 6.0; yeast extract 2.5; casamino acids 10; NaH2PO4 0.1 M; hemin 0.03; NAD 0.015. | Inoculum 0.5%, pH 7.3, 36.5 °C, 30% (pO2) [0.6–0.8 vvm, 400–900 rpm], 22 h | 5.2 | 1.16 | [4] | |
| FV: 500 L WV: 370 L FM: 40 L | glucose 10.0; yeast extract; casamino acids 10; Na2HPO4 12.4; NaH2PO4.H2O 1.8; hemine chloride 0.04; NAD 0.02 | pH 7.3, 36.5 °C, 50% pO2 [0.6–0.8 vvm, 400–900 rpm], 14.5 h | 6.0 | 1.3 | [12] | |
| FV: 75 L WV: 35 L FM: 4 L | described on item 2.2 | pH 7.0, 37 °C, kLa 52 h−1, [0.18 vvm, 450 rpm], 10 h | 30% (pO2) [0.18–0.20 vvm, 400–900 rpm], 9 h | 9.0 | 1.4 | This article |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillaca-Pullo, O.; Vieira, L.D.; Takagi, M. Scale-Up of Capsular Polysaccharide Production Process by Haemophilus influenzae Type b Using kLa Criterion. Bioengineering 2022, 9, 415. https://doi.org/10.3390/bioengineering9090415
Pillaca-Pullo O, Vieira LD, Takagi M. Scale-Up of Capsular Polysaccharide Production Process by Haemophilus influenzae Type b Using kLa Criterion. Bioengineering. 2022; 9(9):415. https://doi.org/10.3390/bioengineering9090415
Chicago/Turabian StylePillaca-Pullo, Omar, Lucas Dias Vieira, and Mickie Takagi. 2022. "Scale-Up of Capsular Polysaccharide Production Process by Haemophilus influenzae Type b Using kLa Criterion" Bioengineering 9, no. 9: 415. https://doi.org/10.3390/bioengineering9090415
APA StylePillaca-Pullo, O., Vieira, L. D., & Takagi, M. (2022). Scale-Up of Capsular Polysaccharide Production Process by Haemophilus influenzae Type b Using kLa Criterion. Bioengineering, 9(9), 415. https://doi.org/10.3390/bioengineering9090415







