A Thermosiphon Photobioreactor for Photofermentative Hydrogen Production by Rhodopseudomonas palustris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culturing
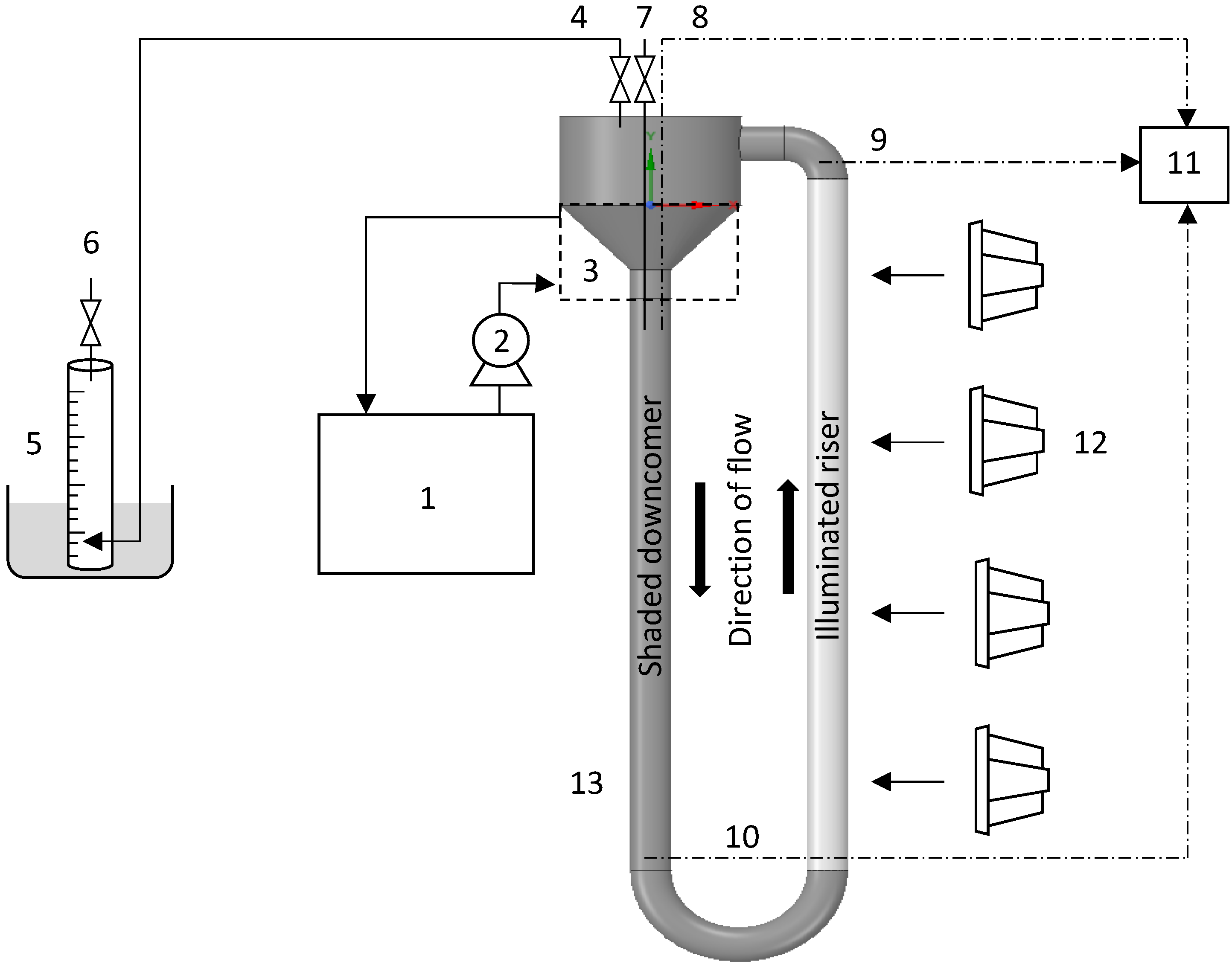
2.2. Photobioreactor Setup
2.3. Experimental Procedure
2.4. Analytical Methods
2.5. Theory and Calculations
3. Results & Discussion
3.1. Rate of Hydrogen Production
3.2. Glycerol Consumption
3.3. Hydrogen Yield
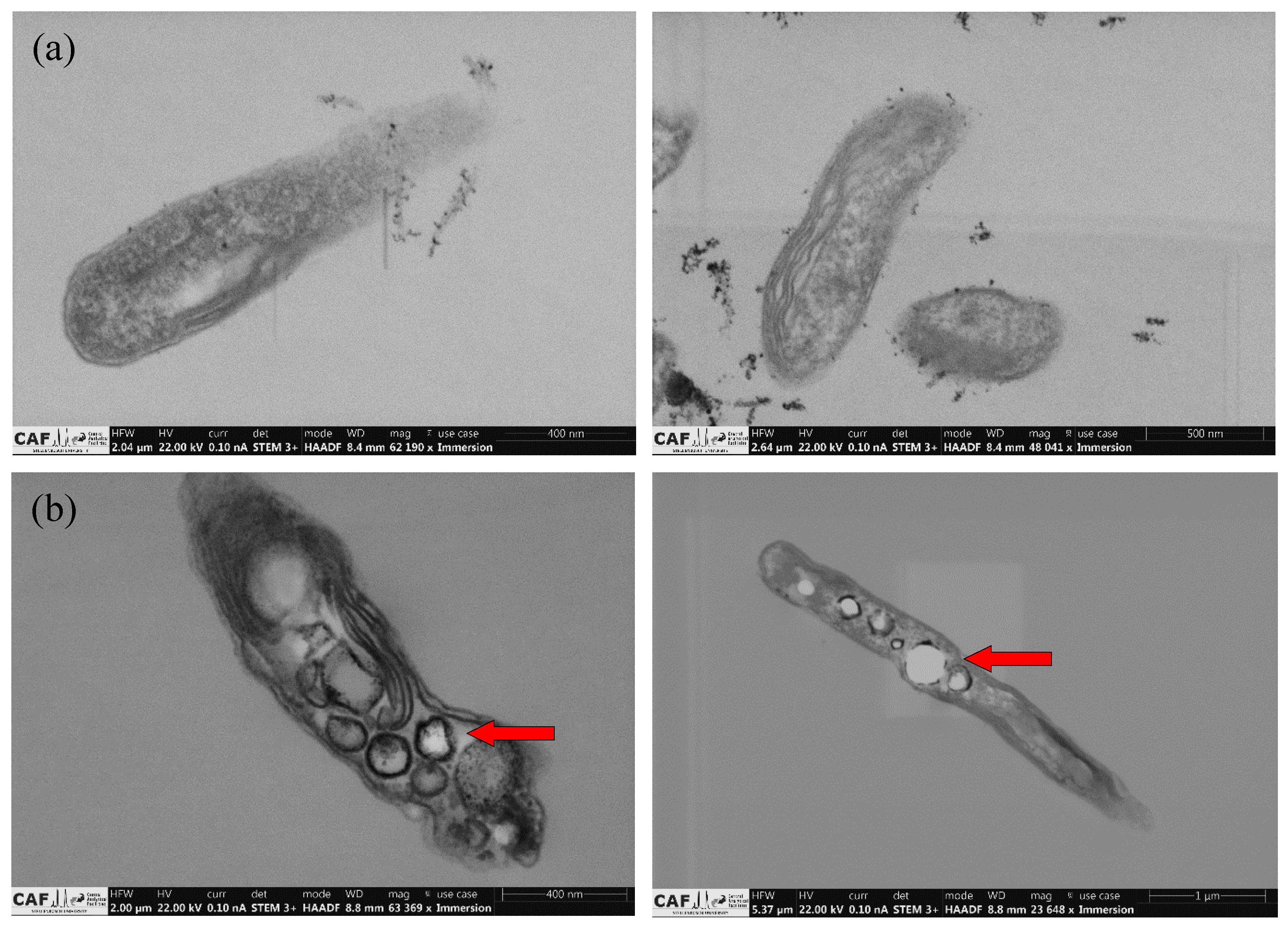
3.4. Biomass Suspension
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Pott, R.W.M.; Johnstone-Robertson, M.; Verster, B.; Rumjeet, S.; Nkadimeng, L.; Raper, T.; Rademeyer, S.; Harrison, S.T.L. Wastewater Biorefineries: Integrating Water Treatment and Value Recovery; Green Energy and Technology book series; Springer: Berlin/Heidelberg, Germany, 2018; pp. 289–302. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Hallenbeck, P.C. Fermentative Hydrogen Production: Principles, Progress, and Prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Ross, B.S. The Effect of Light Intensity and Reactor Configuration on Rhodopseudomonas palustris Growth and Hydrogen Production. Unpublished Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Pott, R.W.M.; Howe, C.J.; Dennis, J.S. Photofermentation of Crude Glycerol from Biodiesel Using Rhodopseudomonas palustris: Comparison with Organic Acids and the Identification of Inhibitory Compounds. Bioresour. Technol. 2013, 130, 725–730. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liao, Q.; Zhu, X.; Tian, X.; Zhang, C. Characteristics of Hydrogen Production and Substrate Consumption of Rhodopseudomonas palustris CQK 01 in an Immobilized-Cell Photobioreactor. Bioresour. Technol. 2010, 101, 4034–4041. [Google Scholar] [CrossRef]
- Uys, P.R.S. Photo-Fermentative Treatment of Wastewaters: Surveying Local Sources and Examining Their Treatment by Rhodopseudomonas palustris. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2019. [Google Scholar]
- Muzziotti, D.; Adessi, A.; Faraloni, C.; Torzillo, G.; De Philippis, R. Hydrogen Production in Rhodopseudomonas palustris as a Way to Cope with High Light Intensities. Res. Microbiol. 2016, 167, 350–356. [Google Scholar] [CrossRef]
- Muzziotti, D.; Adessi, A.; Faraloni, C.; Torzillo, G.; De Philippis, R. Acclimation Strategy of Rhodopseudomonas palustris to High Light Irradiance. Microbiol. Res. 2017, 197, 49–55. [Google Scholar] [CrossRef]
- Du Toit, J.P.; Pott, R.W.M. Heat-Acclimatised Strains of Rhodopseudomonas palustris Reveal Higher Temperature Optima with Concomitantly Enhanced Biohydrogen Production Rates. Int. J. Hydrogen Energy 2021, 46, 11564–11572. [Google Scholar] [CrossRef]
- Tandori, J.; Hideg, É.; Nagy, L.; Maróti, P.; Vass, I. Photoinhibition of Carotenoidless Reaction Centers from Rhodobacter sphaeroides by Visible Light. Effects on Protein Structure and Electron Transport. Photosynth. Res. 2001, 70, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, P. The Effect of Irradiance Growing on Hydrogen Photoevolution and on the Kinetic Growth in Rhodopseudomonas palustris, Strain 420L. Int. J. Hydrogen Energy 2009, 34, 7949–7958. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, J.S. Enhancing Phototropic Hydrogen Production by Solid-Carrier Assisted Fermentation and Internal Optical-Fiber Illumination. Process Biochem. 2006, 41, 2041–2049. [Google Scholar] [CrossRef]
- Adessi, A.; Torzillo, G.; Baccetti, E.; De Philippis, R. Sustained Outdoor H2 Production with Rhodopseudomonas palustris Cultures in a 50 L Tubular Photobioreactor. Int. J. Hydrogen Energy 2012, 37, 8840–8849. [Google Scholar] [CrossRef]
- Avcioglu, S.G.; Ozgur, E.; Eroǧlu, I.; Yücel, M.; Gündüz, U. Biohydrogen Production in an Outdoor Panel Photobioreactor on Dark Fermentation Effluent of Molasses. Int. J. Hydrogen Energy 2011, 36, 11360–11368. [Google Scholar] [CrossRef]
- Androga, D.D. Modeling and Simulation of Photobioreactors for Biological Hydrogen Production. Ph.D. Thesis, Middle East Technical University, Ankara, Turkey, 2014. [Google Scholar]
- Boran, E.; Özgür, E.; Van Der Burg, J.; Yücel, M.; Gündüz, U.; Eroǧlu, I. Biological Hydrogen Production by Rhodobacter capsulatus in Solar Tubular Photo Bioreactor. J. Clean. Prod. 2010, 18, S29–S35. [Google Scholar] [CrossRef]
- Boran, E.; Yücel, M.; Gündüz, U.; Eroǧlu, I. Biohydrogen Production by Rhodobacter capsulatus Hup-Mutant in Pilot Solar Tubular Photobioreactor. Int. J. Hydrogen Energy 2012, 37, 16437–16445. [Google Scholar] [CrossRef]
- Özgür, E.; Afsar, N.; De Vrije, T.; Yücel, M.; Gündüz, U. Potential Use of Thermophilic Dark Fermentation Effluents in Photofermentative Hydrogen Production by Rhodobacter capsulatus. J. Clean. Prod. 2010, 18, S23–S28. [Google Scholar] [CrossRef]
- Özgür, E.; Uyar, B.; Öztürk, Y.; Yücel, M.; Gündüz, U.; Eroǧlu, I. Biohydrogen Production by Rhodobacter capsulatus on Acetate at Fluctuating Temperatures. Resour. Conserv. Recycl. 2010, 54, 310–314. [Google Scholar] [CrossRef]
- Androga, D.D.; Özgür, E.; Gündüz, U.; Yücel, M.; Eroǧlu, I. Factors Affecting the Longterm Stability of Biomass and Hydrogen Productivity in Outdoor Photofermentation. Int. J. Hydrogen Energy 2011, 36, 11369–11378. [Google Scholar] [CrossRef]
- Ozkan, E.; Uyar, B.; Ozgur, E.; Yücel, M.; Eroǧlu, I.; Gündüz, U. Photofermentative Hydrogen Production Using Dark Fermentation Effluent of Sugar Beet Thick Juice in Outdoor Conditions. Int. J. Hydrogen Energy 2011, 37, 2044–2049. [Google Scholar] [CrossRef]
- Miyake, J.; Wakayama, T.; Schnackenberg, J.; Arai, T.; Asada, Y. Simulation of the Daily Sunlight Illumination Pattern for Bacterial Photo-Hydrogen Production. J. Biosci. Bioeng. 1999, 88, 659–663. [Google Scholar] [CrossRef]
- Eroǧlu, I.; Tabanog, A.; Gündüz, U.; Eroǧlu, E.; Yücel, M. Hydrogen Production by Rhodobacter Sphaeroides O.U. 001 in a Flat Plate Solar Bioreactor. Int. J. Hydrogen Energy 2008, 33, 531–541. [Google Scholar] [CrossRef]
- Kim, J.S.; Ito, K.; Takahashi, H. Agricultural and Biological Chemistry Production of Molecular Hydrogen in Outdoor Batch Cultures of Rhodopseudomonas sphaeroides. Agric. Biol. Chem. 1982, 46, 37–41. [Google Scholar] [CrossRef]
- Grabarczyk, R.; Urbaniec, K.; Wernik, J.; Trafczynski, M. Evaluation of the Two-Stage Fermentative Hydrogen Production from Sugar Beet Molasses. Energies 2019, 12, 4090. [Google Scholar] [CrossRef] [Green Version]
- Sönnichsen, N. Forecast Global Green Hydrogen Projects’ Retail Prices 2021. Available online: https://www.statista.com/statistics/1260117/projected-selling-prices-of-large-scale-hydrogen-green-projects/ (accessed on 21 January 2022).
- Anye Cho, B.; Pott, R.W.M. The Development of a Thermosiphon Photobioreactor and Analysis Using Computational Fluid Dynamics (CFD). Chem. Eng. J. 2019, 363, 141–154. [Google Scholar] [CrossRef]
- Zhu, C.; Chi, Z.; Bi, C.; Zhao, Y.; Cai, H. Biotechnology for Biofuels Hydrodynamic Performance of Floating Photobioreactors Driven by Wave Energy. Biotechnol. Biofuels 2019, 12, 54. [Google Scholar] [CrossRef]
- Carlozzi, P.; Sacchi, A. Biomass Production and Studies on Rhodopseudomonas palustris Grown in an Outdoor, Temperature Controlled, Underwater Tubular Photobioreactor. J. Biotechnol. 2001, 88, 239–249. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y.; Ramanan, R.N.; Jahim, J.M. Improvement of Biohydrogen Production through Combined Reuses of Palm Oil Mill Effluent Together with Pulp and Paper Mill Effluent in Photofermentation. Energy Fuels 2010, 29, 5816–5824. [Google Scholar] [CrossRef]
- Preethi; Mohamed Usman, T.M.; Rajesh Banu, J.; Gunasekaran, M.; Kumar, G. Biohydrogen Production from Industrial Wastewater: An Overview. Bioresour. Technol. Rep. 2019, 7, 100287. [Google Scholar] [CrossRef]
- Chou, Y.C.; Su, J.J. Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification. Animals 2019, 9, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sittijunda, S.; Sitthikitpanya, N.; Plangklang, P.; Reungsang, A. Two-Stage Anaerobic Codigestion of Crude Glycerol and Micro-Algal Biomass for Biohydrogen and Methane Production by Anaerobic Sludge Consortium. Fermentation 2021, 7, 175. [Google Scholar] [CrossRef]
- Borowski, S.; Cieciura-Włoch, W. Enzymatic Pretreatment of Byproducts from Soapstock Splitting and Glycerol Processing for Improvement of Biogas Production. Molecules 2021, 26, 6782. [Google Scholar] [CrossRef]
- Talbierz, S.; Dębowski, M.; Kujawska, N.; Kazimierowicz, J.; Zieliński, M. Optimization of Lipid Production by Schizochytrium limacinum Biomass Modified with Ethyl Methane Sulfonate and Grown on Waste Glycerol. Int. J. Environ. Res. Public Health 2022, 19, 3108. [Google Scholar] [CrossRef] [PubMed]
- Pott, R.W.M. The Development of Immobilization Matrices with Adjustable Density for Use in the Immobilization of Stationary-Phase Operating Microorganisms within Continuous Bioreactors. Int. J. Chem. Eng. Appl. 2016, 7, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Louisos, W.F.; Hitt, D.L.; Danforth, C.M. Chaotic Flow in a 2D Natural Convection Loop with Heat Flux Boundaries. Int. J. Heat Mass Transf. 2013, 61, 565–576. [Google Scholar] [CrossRef]
- Budihardjo, I.; Morrison, G.L.; Behnia, M. Natural Circulation Flow through Water-in-Glass Evacuated Tube Solar Collectors. Sol. Energy 2007, 81, 1460–1472. [Google Scholar] [CrossRef]
- Huang, J.; Ying, J.; Fan, F.; Yang, Q.; Wang, J.; Li, Y. Bioresource Technology Development of a Novel Multi-Column Airlift Photobioreactor with Easy Scalability by Means of a Computational Fluid Dynamics Simulations and Experiments. Bioresour. Technol. 2016, 222, 399–407. [Google Scholar] [CrossRef]
- Anye Cho, B. The Development and Characterization of a Thermosiphon Photobioreactor for the Cultivation of Photosynthetic Bacteria. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2018. [Google Scholar]
- Pott, R.W.M.; Howe, C.J.; Dennis, J.S. The Purification of Crude Glycerol Derived from Biodiesel Manufacture and Its Use as a Substrate by Rhodopseudomonas palustris to Produce Hydrogen. Bioresour. Technol. 2014, 152, 464–470. [Google Scholar] [CrossRef]
- Draper, N.R.; Lin, D.K.J. Small Response-Surface Designs. Technometrics 1990, 32, 187–194. [Google Scholar] [CrossRef]
- Shi, X.Y.; Yu, H.Q. Response Surface Analysis on the Effect of Cell Concentration and Light Intensity on Hydrogen Production by Rhodopseudomonas capsulata. Process Biochem. 2005, 40, 2475–2481. [Google Scholar] [CrossRef]
- Dean, A.; Voss, D.; Draguljic, D. Design and Analysis of Experiments, 2nd ed.; Springer Texts in Statistics; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319522487. [Google Scholar]
- Pott, R.W.M. The Bioconversion of Waste Glycerol into Hydrogen by Rhodopseudomonas palustris. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2013. [Google Scholar]
- Xiao, N. Use of a Purple Non-Sulphur Bacterium, Rhodopseudomonas palustris, as a Biocatalyst for Hydrogen Production from Glycerol. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2017. [Google Scholar]
- Ross, B.S.; Pott, R.W.M. Investigating and Modeling the Effect of Light Intensity on Rhodopseudomonas palustris Growth. Biotechnol. Bioeng. 2022, 119, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Schlee, H.; Kleber, H.P. Biotechnologie; Gustav Fischer Verlag: Jena, Germany, 1991. [Google Scholar]
- Guo, C.L.; Zhu, X.; Liao, Q.; Wang, Y.Z.; Chen, R.; Lee, D.J. Enhancement of Photo-Hydrogen Production in a Biofilm Photobioreactor Using Optical Fiber with Additional Rough Surface. Bioresour. Technol. 2011, 102, 8507–8513. [Google Scholar] [CrossRef]
- Hu, C.; Choy, S.Y.; Giannis, A. Evaluation of Lighting Systems, Carbon Sources, and Bacteria Cultures on Photofermentative Hydrogen Production. Appl. Biochem. Biotechnol. 2018, 185, 257–269. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wu, S.C.; Lee, C.M. Relationship between Cell Growth, Hydrogen Production and Poly-β-Hydroxybutyrate (PHB) Accumulation by Rhodopseudomonas palustris WP3-5. Int. J. Hydrogen Energy 2012, 37, 13887–13894. [Google Scholar] [CrossRef]
- Wu, S.C.; Liou, S.Z.; Lee, C.M. Correlation between Bio-Hydrogen Production and Polyhydroxybutyrate (PHB) Synthesis by Rhodopseudomonas palustris WP3-5. Bioresour. Technol. 2012, 113, 44–50. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Oda, Y.; Ruhl, M.; Posto, A.L.; Sauer, U.; Harwood, C.S. Non-Growing Rhodopseudomonas palustris Increases the Hydrogen Gas Yield from Acetate by Shifting from the Glyoxylate Shunt to the Tricarboxylic Acid Cycle. J. Biol. Chem. 2014, 289, 1960–1970. [Google Scholar] [CrossRef] [Green Version]
- Navid, A.; Jiao, Y.; Wong, S.E.; Pett-Ridge, J. System-Level Analysis of Metabolic Trade-Offs during Anaerobic Photoheterotrophic Growth in Rhodopseudomonas palustris. BMC Bioinform. 2019, 20, 233. [Google Scholar] [CrossRef] [Green Version]
- Whittenbury, R.; McLee, A.G. Rhodopseudomonas palustris and Rh. Viridis—Photosynthetic Budding Bacteria. Arch. Mikrobiol. 1967, 59, 324–334. [Google Scholar] [CrossRef]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T. Bergey’s Mannual of Systematic Bacteriology, 2nd ed.; Garrity, G.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Trunk, T.; Khalil, H.S.; Leo, J.C. Bacterial Autoaggregation. AIMS Microbiol. 2018, 4, 140–164. [Google Scholar] [CrossRef]



| Independent Variable | Symbol | Intervals | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Light Intensity (W m−2) | x1 | 400 | 500 | 600 |
| Cooling Water Inlet Temperature (°C) | x2 | 17 | 19 | 21 |
| Biomass Concentration (g L−1) | x3 | 0.40 | 0.82 | 1.25 |
| Run | Coded Values | Experimental Results | ||||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | Rate of H2 Production (mol m−3h−1) | Rate of H2 Production (mmol gCDW−1h−1) | % H2 Yield | % Glycerol Consumed | % Biomass in Suspension | |
| 1 | −1 | 0 | −0.927 | 0.063 | 0.147 | 48.7 | 9.45 | 74.36 |
| 2 | 1 | 0 | −0.984 | 0.076 | 0.185 | 57.6 | 8.48 | 73.72 |
| 3 | 0 | 0 | −0.189 | 0.128 | 0.171 | 60.7 | 12.8 | 53.33 |
| 4 | −1 | −1 | −0.259 | 0.123 | 0.178 | 48.1 | 14.4 | 42.49 |
| 5 | 1 | −1 | −0.184 | 0.145 | 0.194 | 45.2 | 18.8 | 48.08 |
| 6 | 0 | −1 | −1.00 | 0.081 | 0.201 | 50.5 | 9.83 | 58.14 |
| 7 | 1 | 0 | 1.00 | 0.134 | 0.107 | 53.4 | 13.7 | 47.80 |
| 8 | 0 | 0 | 0.009 | 0.128 | 0.154 | 58.2 | 12.1 | 47.64 |
| 9 | −1 | 0 | 0.960 | 0.149 | 0.121 | 60.5 | 12.9 | 56.46 |
| 10 | −1 | 1 | −0.075 | 0.132 | 0.166 | 64.3 | 12.2 | 51.80 |
| 11 | 1 | 1 | −0.085 | 0.142 | 0.180 | 77.1 | 17.3 | 49.46 |
| 12 | 0 | −1 | 0.968 | 0.148 | 0.119 | 48.9 | 18.5 | 45.96 |
| 13 | 0 | 0 | −0.111 | 0.140 | 0.179 | 65.7 | 13.0 | 50.95 |
| 14 | 0 | 1 | 0.921 | 0.156 | 0.128 | 45.9 | 18.5 | 43.46 |
| 15 | 0 | 1 | −0.979 | 0.088 | 0.215 | 53.3 | 9.09 | 67.37 |
| Reactor Type | Strain | H2 Production Rate (mol m−3h−1) | Reference |
|---|---|---|---|
| Biofilm PBR | R. palustris CQK01 | 1.74 | [8] |
| Biofilm PBR | R. palustris CQK01 | 1.75 | [52] |
| Optical fibre PBR | R. palustris WP 3-5 | 1.96 | [15] |
| Glass bottle PBR | R. palustris DSM 127 | 1.23 | [53] |
| Tubular PBR | R. palustris 420 L | 1.20 | [16] |
| Glass bottle PBR | R. palustris GCA009 | 0.72 | [12] |
| Glass bottle PBR | R. palustris ATH 2.1.37 | 0.98 | [12] |
| Thermosiphon PBR | R. palustris NMIB1774 | 0.16 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosman, C.E.; McClelland Pott, R.W.; Bradshaw, S.M. A Thermosiphon Photobioreactor for Photofermentative Hydrogen Production by Rhodopseudomonas palustris. Bioengineering 2022, 9, 344. https://doi.org/10.3390/bioengineering9080344
Bosman CE, McClelland Pott RW, Bradshaw SM. A Thermosiphon Photobioreactor for Photofermentative Hydrogen Production by Rhodopseudomonas palustris. Bioengineering. 2022; 9(8):344. https://doi.org/10.3390/bioengineering9080344
Chicago/Turabian StyleBosman, Catharine Elizabeth, Robert William McClelland Pott, and Steven Martin Bradshaw. 2022. "A Thermosiphon Photobioreactor for Photofermentative Hydrogen Production by Rhodopseudomonas palustris" Bioengineering 9, no. 8: 344. https://doi.org/10.3390/bioengineering9080344
APA StyleBosman, C. E., McClelland Pott, R. W., & Bradshaw, S. M. (2022). A Thermosiphon Photobioreactor for Photofermentative Hydrogen Production by Rhodopseudomonas palustris. Bioengineering, 9(8), 344. https://doi.org/10.3390/bioengineering9080344






