Selected Flavonoids to Target Melanoma: A Perspective in Nanoengineering Delivery Systems
Abstract
:1. Introduction
2. Cutaneous Melanoma
3. Natural Compounds against Melanoma In Vitro
3.1. Apigenin
3.2. Epigallocatechin-3-Gallate
3.3. Kaempferol
3.4. Naringenin
3.5. Silybin
4. Natural Compounds Function in Melanogenesis
5. Natural Compounds Function in Melanoma Prevention
6. Nanoengineering Delivery Systems for the Selected Flavonoids
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertrand, J.U.; Steingrimsson, E.; Jouenne, F.; Bressac-de Paillerets, B.; Larue, L. Melanoma Risk and Melanocyte Biology. Acta Derm. Venereol. 2020, 100, adv00139. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Olbryt, M. Molecular background of skin melanoma development and progression: Therapeutic implications. Postepy Derm. Alergol. 2019, 36, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; McCubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy. Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schvartsman, G.; Taranto, P.; Glitza, I.C.; Agarwala, S.S.; Atkins, M.B.; Buzaid, A.C. Management of metastatic cutaneous melanoma: Updates in clinical practice. Ther. Adv. Med. Oncol. 2019, 11, 1758835919851663. [Google Scholar] [CrossRef]
- Bellan, D.L.; Biscaia, S.M.P.; Rossi, G.R.; Cristal, A.M.; Gonçalves, J.P.; Oliveira, C.C.; Simas, F.F.; Sabry, D.A.; Rocha, H.A.O.; Franco, C.R.C.; et al. Green does not always mean go: A sulfated galactan from Codium isthmocladum green seaweed reduces melanoma metastasis through direct regulation of malignancy features. Carbohydr. Polym. 2020, 250, 116869. [Google Scholar] [CrossRef]
- Souto, E.B.; Sampaio, A.C.; Campos, J.R.; Martins-Gomes, C.; Aires, A.; Silva, A.M. Chapter 2—Polyphenols for skin cancer: Chemical properties, structure-related mechanisms of action and new delivery systems. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 21–42. [Google Scholar]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef]
- Sajid, M.; Channakesavula, C.N.; Stone, S.R.; Kaur, P. Synthetic Biology towards Improved Flavonoid Pharmacokinetics. Biomolecules 2021, 11, 754. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Meyskens, F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef] [Green Version]
- Fangueiro, J.F.; Souto, E.B.; Silva, A.M. 9—Encapsulation of nutraceuticals in novel delivery systems. In Nutraceuticals; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 305–342. [Google Scholar] [CrossRef]
- Heo, J.-R.; Lee, G.-A.; Kim, G.-S.; Hwang, K.-A.; Choi, K.-C. Phytochemical-induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis and differentiation in malignant melanoma cells. Phytomedicine 2018, 39, 100–110. [Google Scholar] [CrossRef]
- Vaid, M.; Singh, T.; Prasad, R.; Katiyar, S.K. Silymarin inhibits melanoma cell growth both in vitro and in vivo by targeting cell cycle regulators, angiogenic biomarkers and induction of apoptosis. Mol. Carcinog. 2015, 54, 1328–1339. [Google Scholar] [CrossRef]
- Woo, J.S.; Choo, G.S.; Yoo, E.S.; Kim, S.H.; Lee, J.H.; Han, S.H.; Kim, H.J.; Jung, S.H.; Park, Y.S.; Kim, B.S.; et al. Apigenin induces apoptosis by regulating Akt and MAPK pathways in human melanoma cell A375SM. Mol. Med. Rep. 2020, 22, 4877–4889. [Google Scholar] [CrossRef]
- Du, B.-X.; Lin, P.; Lin, J. EGCG and ECG induce apoptosis and decrease autophagy via the AMPK/mTOR and PI3K/AKT/mTOR pathway in human melanoma cells. Chin. J. Nat. Med. 2022, 20, 290–300. [Google Scholar] [CrossRef]
- Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
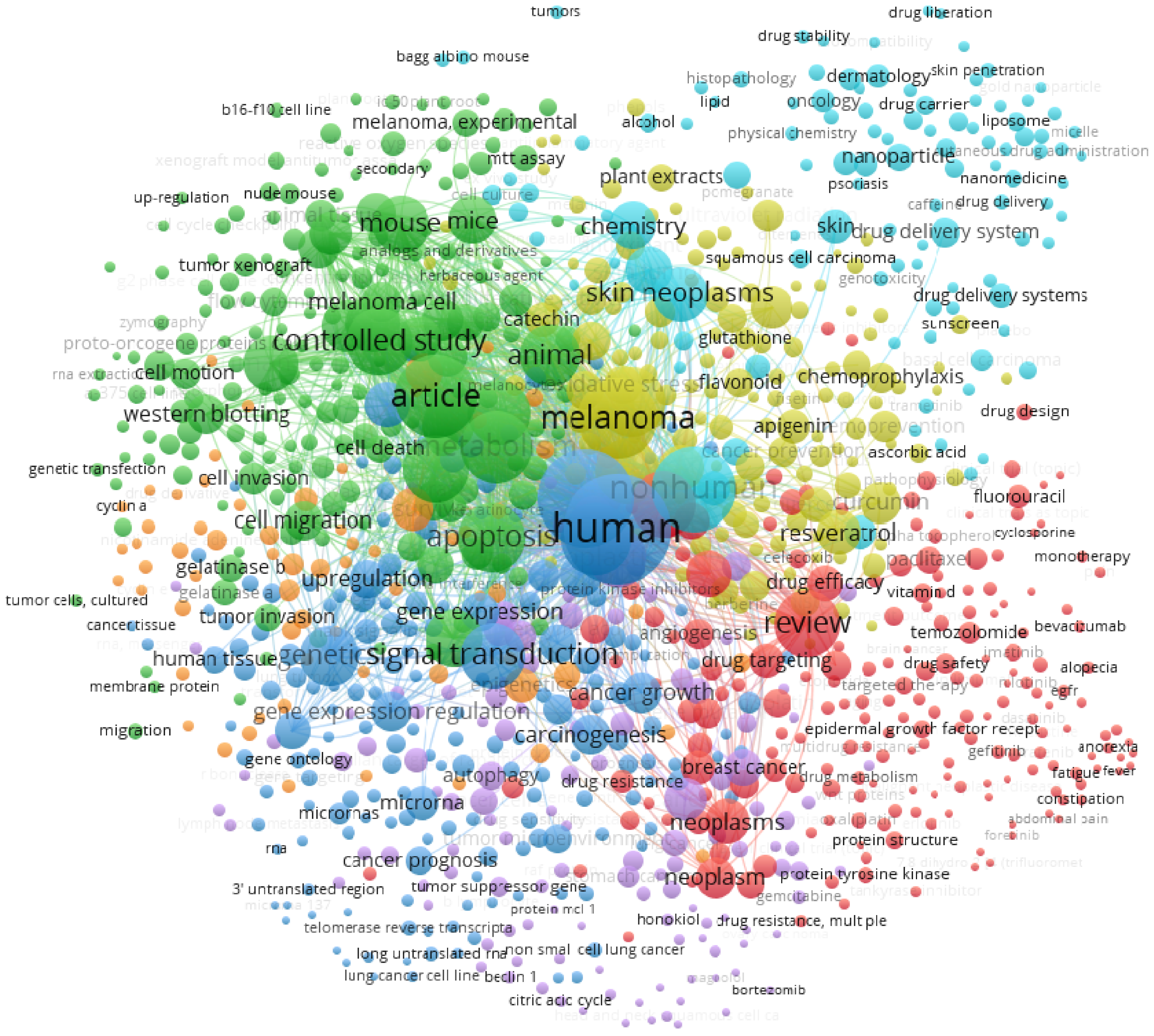
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, C.M.; Carroll, H.J.; Whiteman, D.C. Estimating the attributable fraction for melanoma: A meta-analysis of pigmentary characteristics and freckling. Int. J. Cancer 2010, 127, 2430–2445. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Porojnicu, A.C.; Dahlback, A. Ultraviolet radiation and malignant melanoma. Adv. Exp. Med. Biol 2008, 624, 104–116. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. Bras. Derm. 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Zielinska, A.; Luis, M.; Carbone, C.; Martins-Gomes, C.; Souto, S.B.; Silva, A.M. Uveal melanoma: Physiopathology and new in situ-specific therapies. Cancer Chemother. Pharmacol. 2019, 84, 15–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, V.K.; Lazar, A.J.F.; Warneke, C.L.; Redston, M.S.; Haluska, F.G. Examination of Mutations in BRAF, NRAS, and PTEN in Primary Cutaneous Melanoma. J. Investig. Dermatol. 2006, 126, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Volkovova, K.; Bilanicova, D.; Bartonova, A.; Letašiová, S.; Dusinska, M. Associations between environmental factors and incidence of cutaneous melanoma. Review. Environ. Health 2012, 11, S12. [Google Scholar] [CrossRef] [Green Version]
- Aasen, S.N.; Parajuli, H.; Hoang, T.; Feng, Z.; Stokke, K.; Wang, J.; Roy, K.; Bjerkvig, R.; Knappskog, S.; Thorsen, F. Effective Treatment of Metastatic Melanoma by Combining MAPK and PI3K Signaling Pathway Inhibitors. Int. J. Mol. Sci. 2019, 20, 4235. [Google Scholar] [CrossRef] [Green Version]
- Shtivelman, E.; Davies, M.Q.A.; Hwu, P.; Yang, J.; Lotem, M.; Oren, M.; Flaherty, K.T.; Fisher, D.E. Pathways and therapeutic targets in melanoma. Oncotarget 2014, 5, 1701–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seberg, H.E.; Van Otterloo, E.; Cornell, R.A. Beyond MITF: Multiple transcription factors directly regulate the cellular phenotype in melanocytes and melanoma. Pigment Cell Melanoma Res. 2017, 30, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Ross, K.; Huber, W.E.; Nishimura, E.K.; Golub, T.R.; Fisher, D.E. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 2004, 6, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, J.; Heine, M.; Arden, K.C.; Körner, B.; Pilch, H.; Herbst, R.A.; Jung, E.G. Mutation and Expression of TP53 in Malignant Melanomas. Recent Results Cancer Res. 1995, 139, 137–154. [Google Scholar] [CrossRef]
- Sargen, M.R.; Cloutier, J.M.; Sarin, K.Y.; Rieger, K.E.; Chu, P.; Swetter, S.M.; Novoa, R.A. Biomarker discovery analysis: Alterations in p14, p16, p53, and BAP1 expression in nevi, cutaneous melanoma, and metastatic melanoma. Pigment. Cell Melanoma Res. 2019, 32, 474–478. [Google Scholar] [CrossRef]
- Benedict, W.F.; Lerner, S.P.; Zhou, J.; Shen, X.; Tokunaga, H.; Czerniak, B. Level of retinoblastoma protein expression correlates with p16 (MTS-1/INK4A/CDKN2) status in bladder cancer. Oncogene 1999, 18, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Pellegrini, C.; Cardelli, L.; Ciciarelli, V.; Di Nardo, L.; Fargnoli, M.C. Familial Melanoma: Diagnostic and Management Implications. Dermatol. Pract. Concept. 2019, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, R.; Liu, D.; Yang, J.; Hu, Q.; Shan, C.; Li, X. Apigenin inhibits growth of melanoma by suppressing miR-512-3p and promoting the G1 phase of cell cycle involving the p27 Kip1 protein. Mol. Cell. Biochem. 2022, 477, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol. Lett. 2013, 223, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Pervin, M.; Lim, J.H.; Lim, B.O. Apigenin Attenuates Melanoma Cell Migration by Inducing Anoikis through Integrin and Focal Adhesion Kinase Inhibition. Molecules 2015, 20, 21157–21166. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, J.; Li, Y.; Liu, Z.; Lin, Y.; Huang, J.-A. Anti-melanogenic effects of epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG) and gallocatechin-3-gallate (GCG) via down-regulation of cAMP/CREB /MITF signaling pathway in B16F10 melanoma cells. Fitoterapia 2020, 145, 104634. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm. Dev. Technol. 2019, 24, 1243–1249. [Google Scholar] [CrossRef] [Green Version]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, P.; Sun, J.; Guo, L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J. BUON 2018, 23, 218–223. [Google Scholar]
- Qiang, D.; Ci, C.; Liu, W.; Wang, J.; He, C.; Ji, B.; Shao, X. Inhibitory effect of kaempferol on mouse melanoma cell line B16 in vivo and in vitro. Postepy Derm. Alergol. 2021, 38, 498–504. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.-H.; Jang, H.; Park, S.-Y.; Seol, J.-W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Yang, C.-H.; Chiou, Y.-L. Citrus flavanone naringenin enhances melanogenesis through the activation of Wnt/β-catenin signalling in mouse melanoma cells. Phytomedicine 2011, 18, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Nasr Bouzaiene, N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef]
- Lee, M.-H.; Huang, Z.; Kim, D.J.; Kim, S.-H.; Kim, M.O.; Lee, S.-Y.; Xie, H.; Park, S.J.; Kim, J.Y.; Kundu, J.K.; et al. Direct Targeting of MEK1/2 and RSK2 by Silybin Induces Cell-Cycle Arrest and Inhibits Melanoma Cell Growth. Cancer Prev. Res. 2013, 6, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaid, M.; Prasad, R.; Sun, Q.; Katiyar, S.K. Silymarin Targets β-Catenin Signaling in Blocking Migration/Invasion of Human Melanoma Cells. PLoS ONE 2011, 6, e23000. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Chou, G.-X.; Wang, H.; Chu, J.-H.; Yu, Z.-L. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine 2010, 18, 32–35. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Chu, J.-H.; Chou, G.-X.; Yu, Z.-L. Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp. Dermatol. 2011, 20, 755–757. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and Kaempferol Rhamnosides with Depigmenting and Anti-Inflammatory Properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [Green Version]
- Uto, T.; Ohta, T.; Katayama, K.; Shoyama, Y. Silibinin promotes melanogenesis through the PKA and p38 MAPK signaling pathways in melanoma cells. Biomed. Res. 2022, 43, 31–39. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Gabrielová, E.; Michaelides, L.; Kosina, P.; Ryšavá, A.; Ulrichová, J.; Zálešák, B.; Vostálová, J. UVA-photoprotective potential of silymarin and silybin. Arch. Dermatol. Res. 2018, 310, 413–424. [Google Scholar] [CrossRef]
- Britto, S.M.; Shanthakumari, D.; Agilan, B.; Radhiga, T.; Kanimozhi, G.; Prasad, N.R. Apigenin prevents ultraviolet-B radiation induced cyclobutane pyrimidine dimers formation in human dermal fibroblasts. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2017, 821, 28–35. [Google Scholar] [CrossRef]
- Spoerlein, C.; Mahal, K.; Schmidt, H.; Schobert, R. Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J. Inorg. Biochem. 2013, 127, 107–115. [Google Scholar] [CrossRef]
- Anwar, E.; Rizkamiarty, S. Formulation and Evaluation of Cosmetic Foundation Using Epigallocatechin Gallate as a Sun Protection. Int. J. Appl. Pharm. 2020, 12, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Hsieh, D.-S.; Huang, K.-J.; Chan, Y.-L.; Hong, P.-D.; Yeh, M.-K.; Wu, C.-J. Improving anticancer efficacy of (-)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells. Drug Des. Dev. Ther. 2014, 8, 459–474. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Kuramochi, H.; Takahashi, A.; Imai, K.; Katsuta, N.; Nakayama, T.; Fujiki, H.; Suganuma, M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (−)-epigallocatechin gallate-treated cells. J. Cancer Res. Clin. Oncol. 2012, 138, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Badea, G.; Badea, N.; Brasoveanu, L.I.; Mihaila, M.; Stan, R.; Istrati, D.; Balaci, T.; Lacatusu, I. Naringenin improves the sunscreen performance of vegetable nanocarriers. New J. Chem. 2017, 41, 480–492. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, D.; Sousa, Â. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Verma, R.; Salgotra, T.; Rahman, M.H.; Shah, M.; Akter, R.; Murad, W.; Mubin, S.; Bibi, P.; Qusti, S.; et al. Impacting the Remedial Potential of Nano Delivery-Based Flavonoids for Breast Cancer Treatment. Molecules 2021, 26, 5163. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Huang, C.-T.; Fu, L.-T.; Huang, Y.-B.; Tsai, Y.-H.; Wu, P.-C. The Effect of Submicron Emulsion Systems on Transdermal Delivery of Kaempferol. Chem. Pharm. Bull. 2012, 60, 1171–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrotriya, S.N.; Vidhate, B.V.; Shukla, M.S. Formulation and development of Silybin loaded solid lipid nanoparticle enriched gel for irritant contact dermatitis. J. Drug Deliv. Sci. Technol. 2017, 41, 164–173. [Google Scholar] [CrossRef]
- Panapisal, V.; Charoensri, S.; Tantituvanont, A. Formulation of Microemulsion Systems for Dermal Delivery of Silymarin. AAPS PharmSciTech 2012, 13, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013, 62, 670–680. [Google Scholar] [CrossRef]
- Lee, N.H.; Park, S.H.; Park, S.N. Preparation and characterization of novel pseudo ceramide-based nanostructured lipid carriers for transdermal delivery of apigenin. J. Drug Deliv. Sci. Technol. 2018, 48, 245–252. [Google Scholar] [CrossRef]
- Chou, T.-H.; Nugroho, D.S.; Chang, J.-Y.; Cheng, Y.-S.; Liang, C.-H.; Deng, M.-J. Encapsulation and Characterization of Nanoemulsions Based on an Anti-oxidative Polymeric Amphiphile for Topical Apigenin Delivery. Polymers 2021, 13, 1016. [Google Scholar] [CrossRef]
- Jangdey, M.S.; Gupta, A.; Saraf, S.; Saraf, S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: In vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1452–1462. [Google Scholar] [CrossRef] [Green Version]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release Off. J. Control. Release Soc. 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-based nanoformulated (−)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, P.K.; Manikkath, J.; Tupally, K.; Kokil, G.; Hegde, A.R.; Raut, S.Y.; Parekh, H.S.; Mutalik, S. Skin Delivery of EGCG and Silibinin: Potential of Peptide Dendrimers for Enhanced Skin Permeation and Deposition. AAPS PharmSciTech 2017, 18, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (−)-Epigallocatechin gallate (EGCG)-nanoethosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, R.P.; Novais, G.B.; Sangenito, L.S.; Santos, A.L.S.; Priefer, R.; Morsink, M.; Mendonça, M.C.; Souto, E.B.; Severino, P.; Cardoso, J.C. Naringenin-Functionalized Multi-Walled Carbon Nanotubes: A Potential Approach for Site-Specific Remote-Controlled Anticancer Delivery for the Treatment of Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4557. [Google Scholar] [CrossRef]

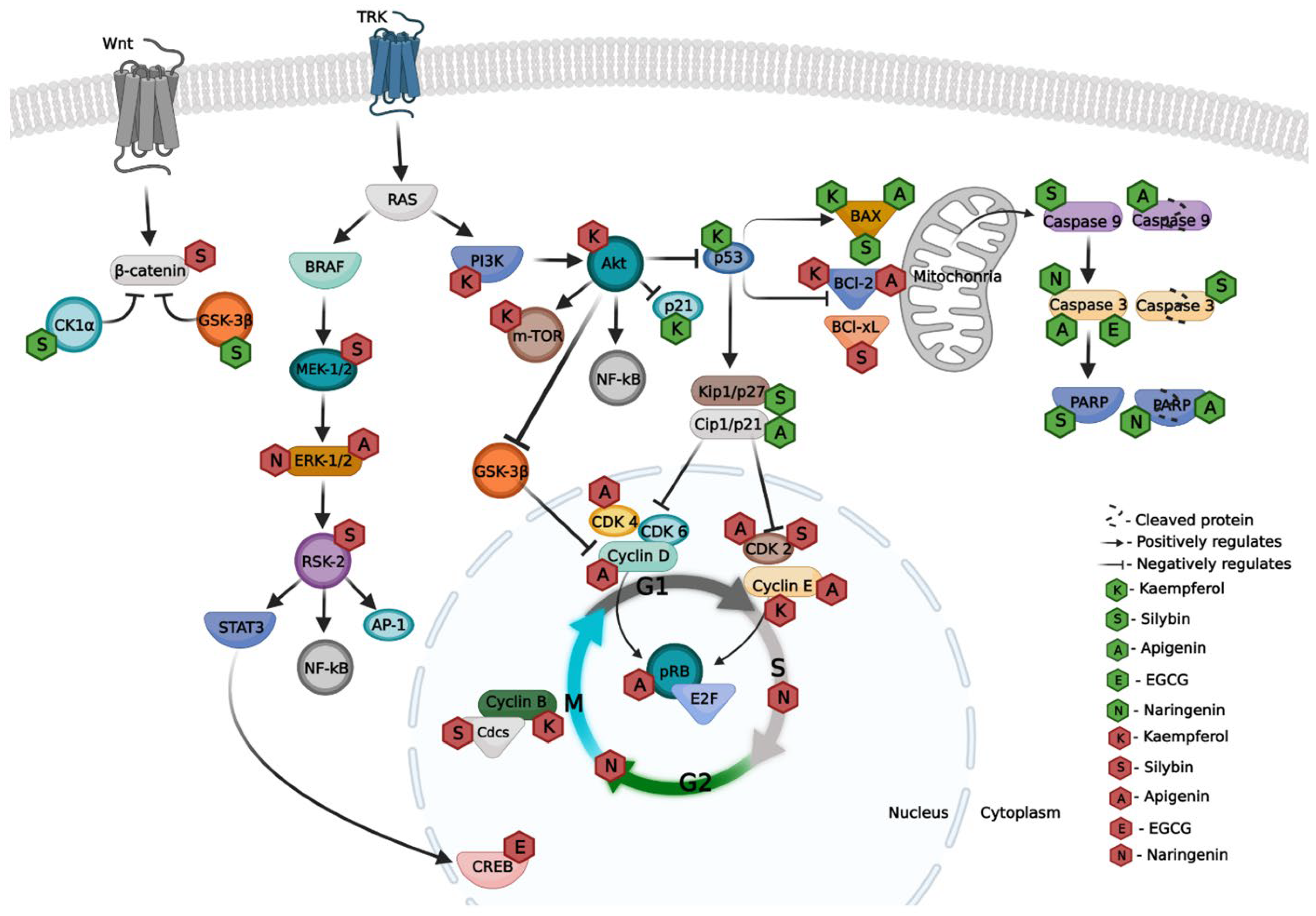
| Compound | Melanoma Cell Line | Effects | Ref. |
|---|---|---|---|
| Apigenin | A375P and A375SM | ↑ BAX ↑ p53 ↑ Cleaved caspase-9 ↑ Cleaved PARP ↓ BCl-2 | [15] |
| A375 and A2058 | ↓ p-FAK ↓ p-ERK-1/2 ↑ Caspase-3 ↑ Cleaved PARP | [36] | |
| A375 and C8161 | ↑ G2/M (cell cycle) ↑ Cleaved caspase 3 | [37] | |
| A375 | ↑ Caspase 3 ↑ Caspase 9 ↑ BAX ↓ BCl-2 | [35] | |
| WM1361B and WM983A | ↑ G0/G1 (cell cycle) ↓ Cyclin D1/2 ↓ Cyclin E ↓ CDK2/4/6 ↑ p27Kip1 ↓ pRB ↓ E2F | [34] | |
| EGCG | B16F10 | ↓ p-CREB ↓ CREB ↓ MITF | [38] |
| A375 | ↑ Caspase 3 ↓ BCl-2 ↑ p-PI3K ↑ p-AKT ↑ p-mTOR ↓ p-AMPK | [16] | |
| Kaempferol | A375SM | ↑ p21 ↓ Cyclin E and B ↑ p38 MAPK ↑ p53 ↓ BCl-2 ↑ BAX | [13] |
| A375 | ↑ G2/M (cell cycle) ↓ m-TOR↓ PI3K↓ Akt | [41] | |
| B16 | ↑ G2/M (cell cycle) | [42] | |
| Naringenin | B16F10 and SK-MEL-28 | ↓ p-ERK1/2 ↓ p-JNK ↑ Caspase 3 ↑ Cleaved PARP | [43] |
| B16F10 | ↑ subG0/G1; ↑ S; ↑ G2/M (cell cycle) ↓ G0/G1 (cell cycle) | [45] | |
| Silybin | A375 and HS294T | ↓ Nuclear β-catenin ↑ Cytosolic β-catenin ↓ MMP-2 and MMP-9 ↑ p-β-catenin ↑ CK1α ↑ GSK-3β ↓ BCl-2 and BCl-x ↑ BAX ↑ Caspase 9 ↑ Cleaved Caspase 3 ↑ PARP | [14,47] |
| Compound | Nanoparticle | In Vitro Model | Ref. |
|---|---|---|---|
| Apigenin | PLGA nanoparticles | Rat skin Cell culture | [35,68] |
| Nanostructured lipid carriers | Pig skin | [69] | |
| Nanoemulsion | Artificial skin | [70] | |
| EGCG | Cationic nanoparticles | Rat skin | [74] |
| Nanoethosomes | Rat skin | [76] | |
| Dendrimer | Rat skin | [75] | |
| Transferosomes | Rat skin | [77] | |
| Kaempferol | Submicron emulsion | Rat skin | [65] |
| Naringenin | Carbon nanotubes | Fibroblast cell line | [78] |
| Silybin | Solid lipid nanoparticles | Rat skin | [66] |
| Microemulsion | Pig skin | [67] | |
| Dendrimer | Rat skin | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutinho, T.E.; Souto, E.B.; Silva, A.M. Selected Flavonoids to Target Melanoma: A Perspective in Nanoengineering Delivery Systems. Bioengineering 2022, 9, 290. https://doi.org/10.3390/bioengineering9070290
Coutinho TE, Souto EB, Silva AM. Selected Flavonoids to Target Melanoma: A Perspective in Nanoengineering Delivery Systems. Bioengineering. 2022; 9(7):290. https://doi.org/10.3390/bioengineering9070290
Chicago/Turabian StyleCoutinho, Tiago E., Eliana B. Souto, and Amélia M. Silva. 2022. "Selected Flavonoids to Target Melanoma: A Perspective in Nanoengineering Delivery Systems" Bioengineering 9, no. 7: 290. https://doi.org/10.3390/bioengineering9070290
APA StyleCoutinho, T. E., Souto, E. B., & Silva, A. M. (2022). Selected Flavonoids to Target Melanoma: A Perspective in Nanoengineering Delivery Systems. Bioengineering, 9(7), 290. https://doi.org/10.3390/bioengineering9070290







