Effect of Pulse Frequency on the Microstructure and the Degradation of Pulse Electroformed Zinc for Fabricating the Shell of Biodegradable Dosing Pump
Abstract
1. Introduction
2. Materials and Methods
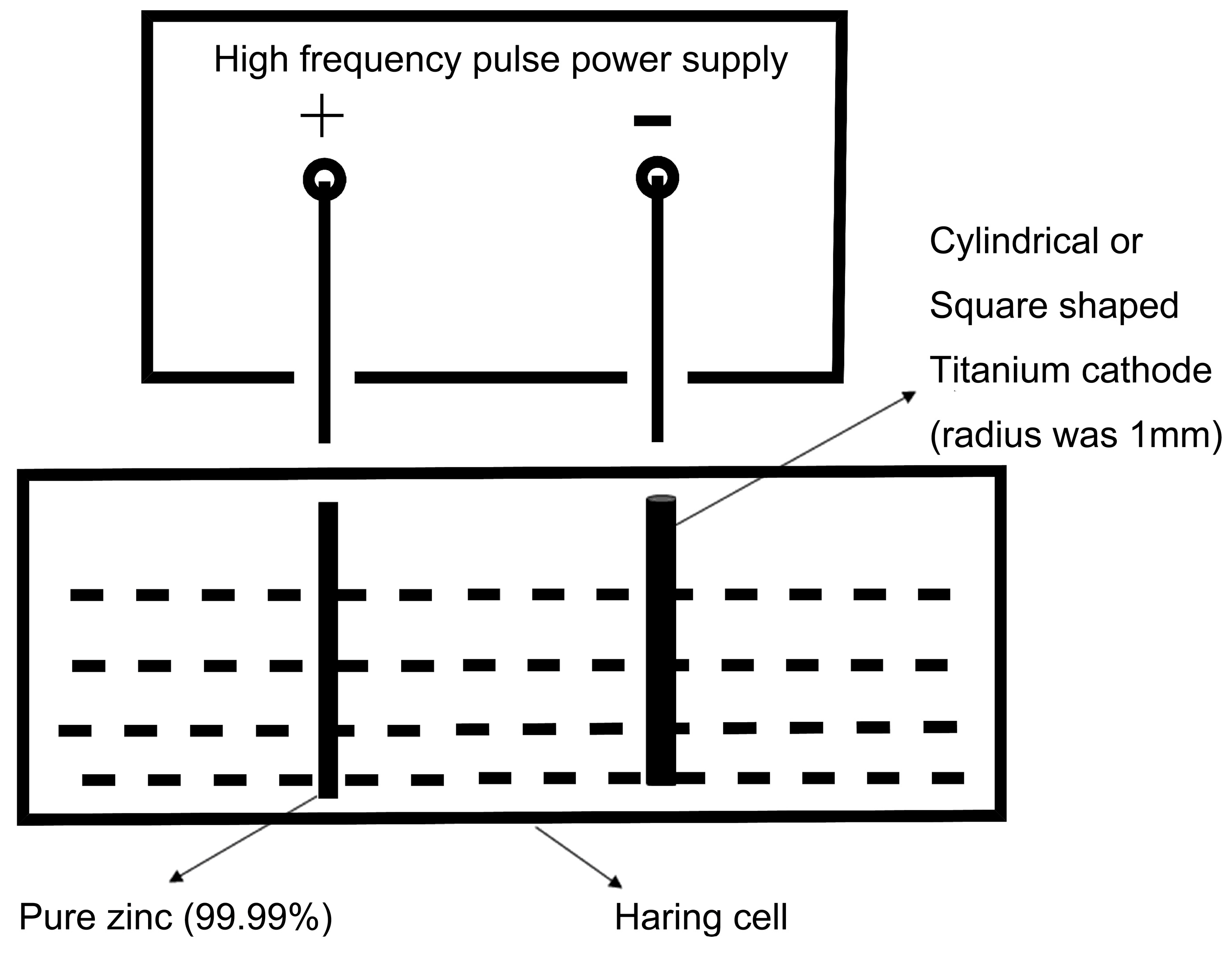
2.1. Materials Preparation
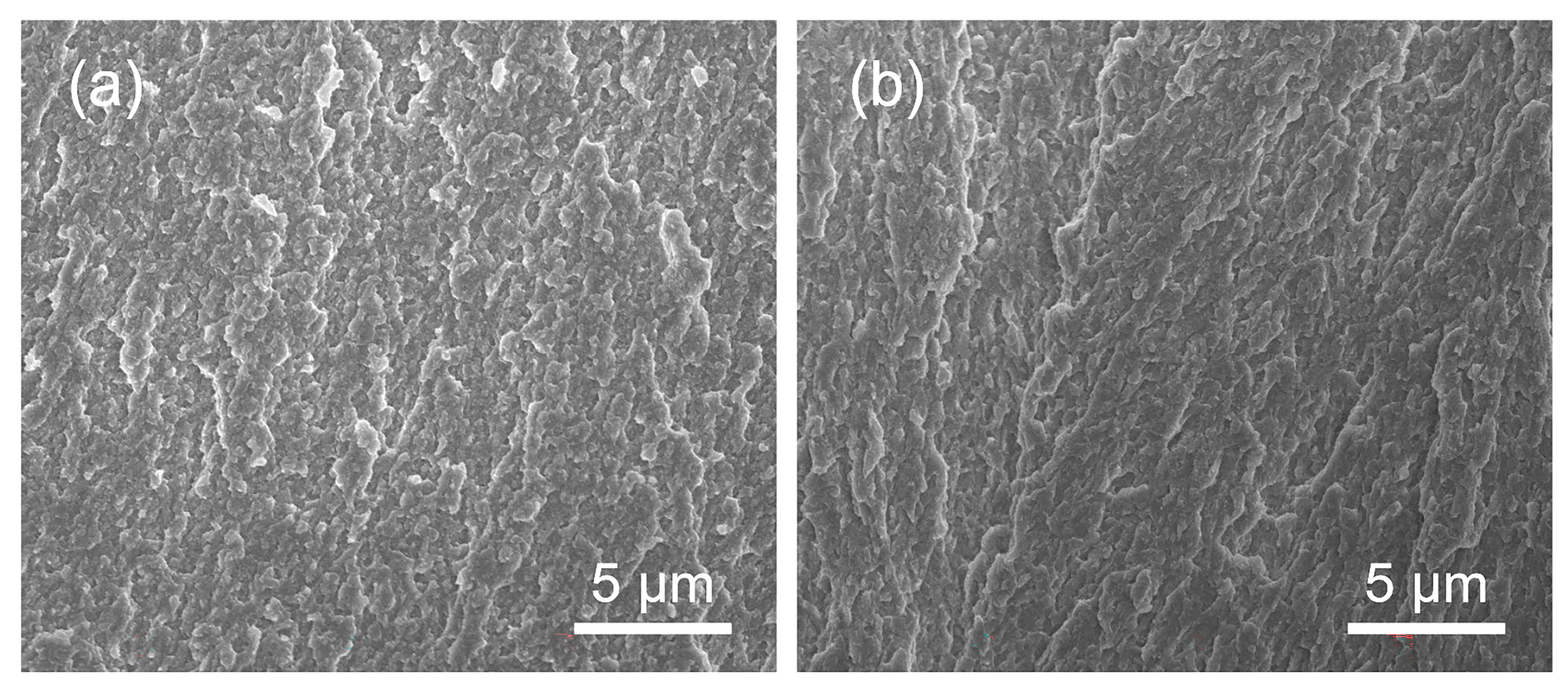
2.2. Microstructure Characterization
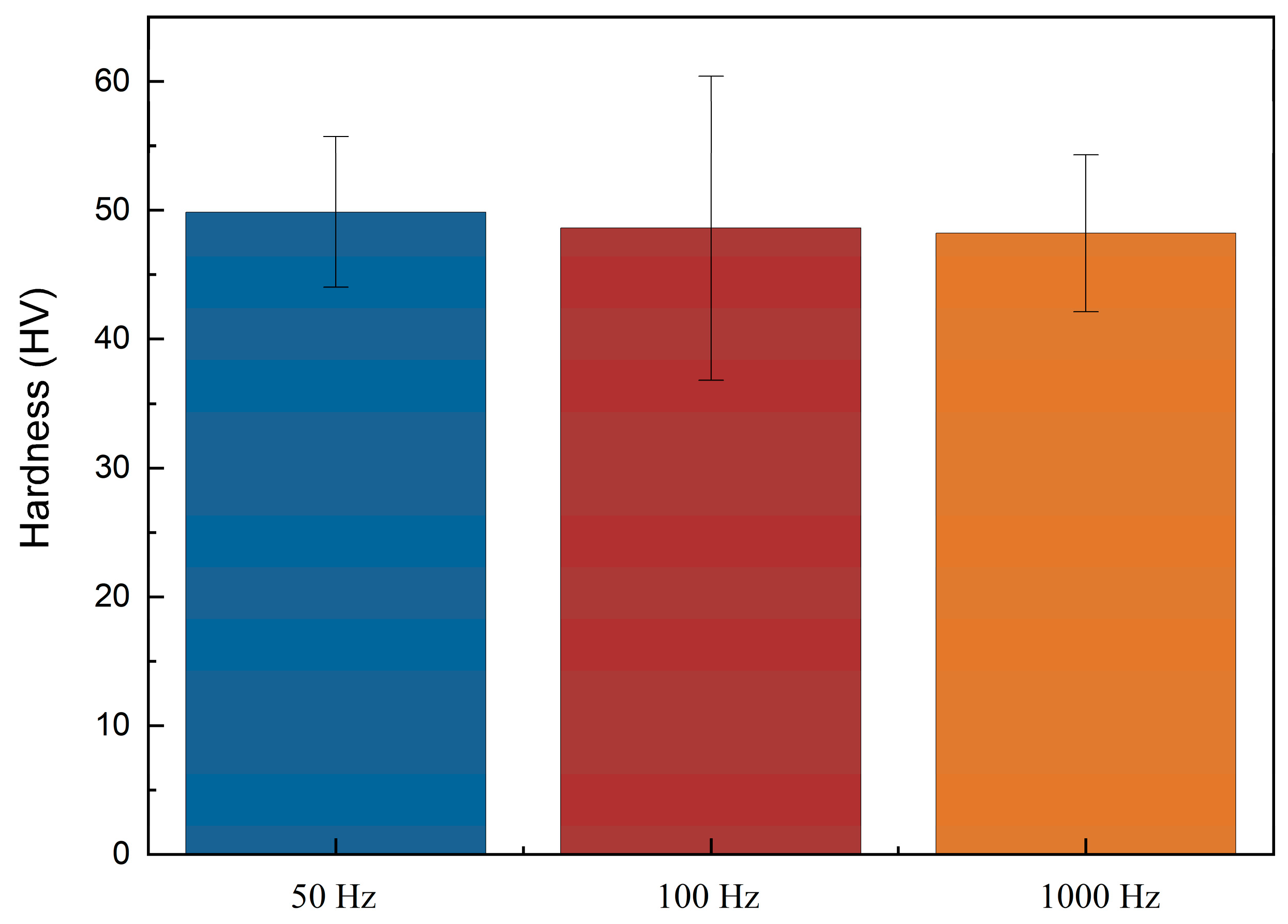
2.3. Mechanical Test
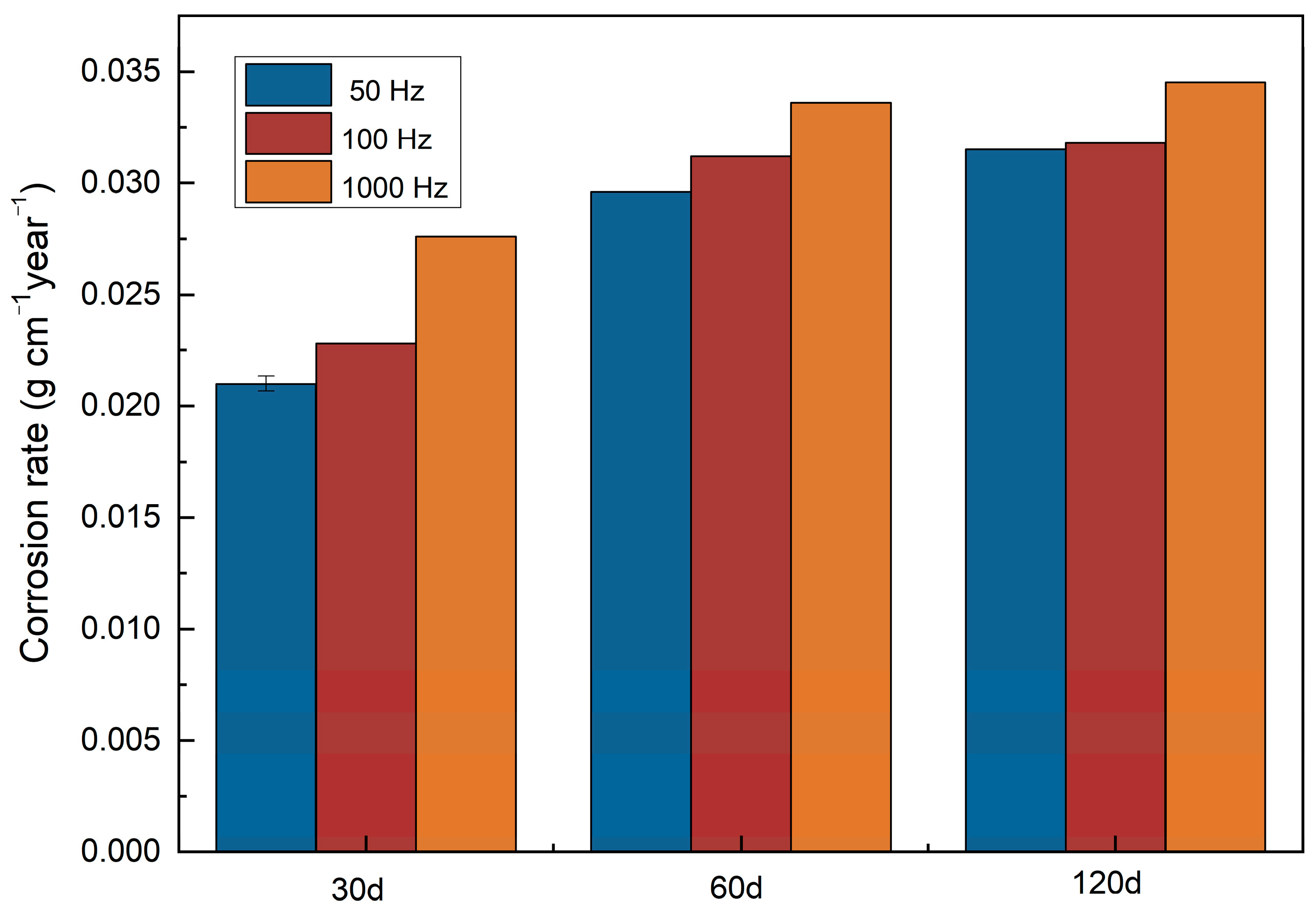
2.4. Static Immersion Test
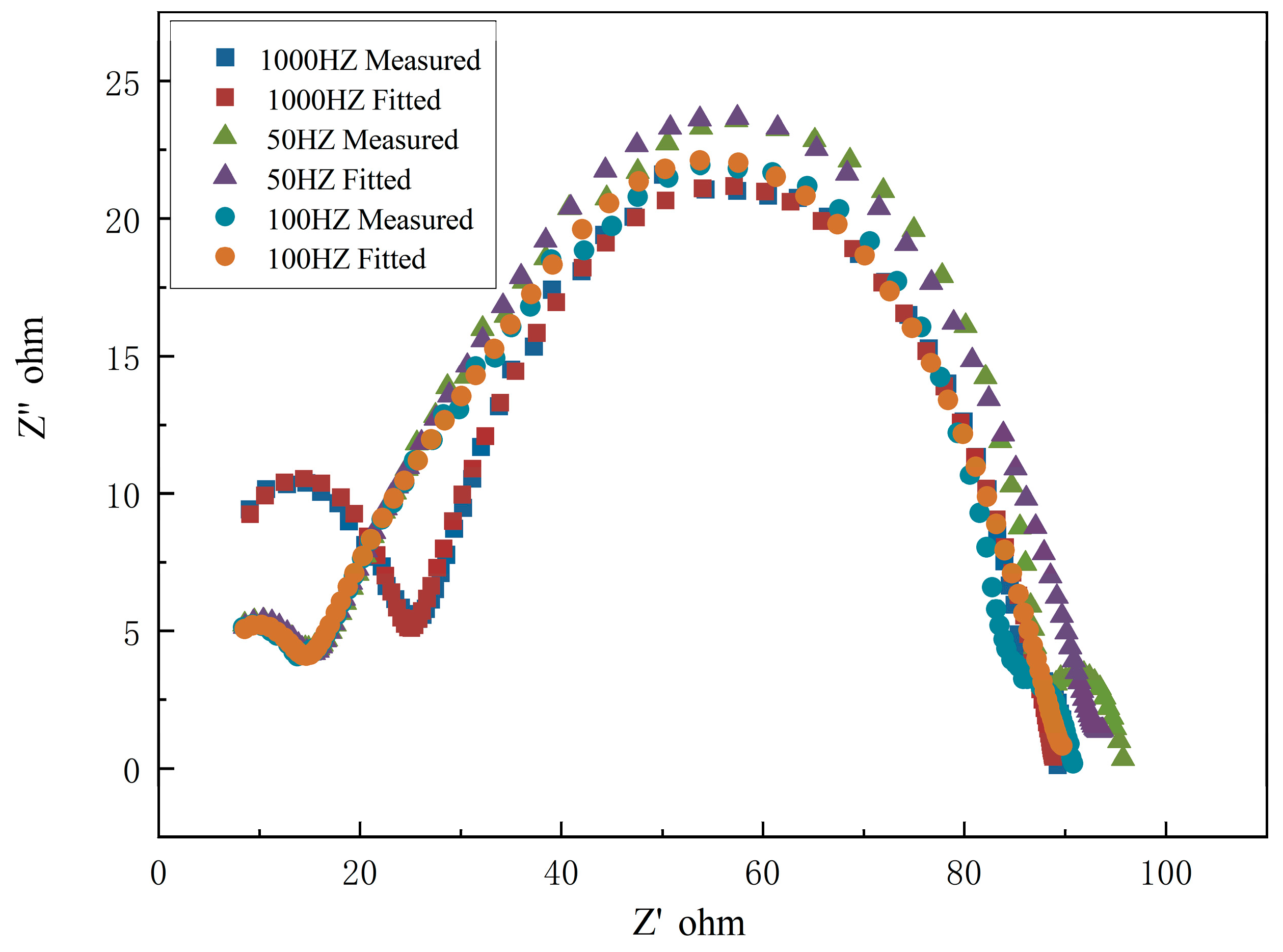
2.5. Electrochemical Measurements
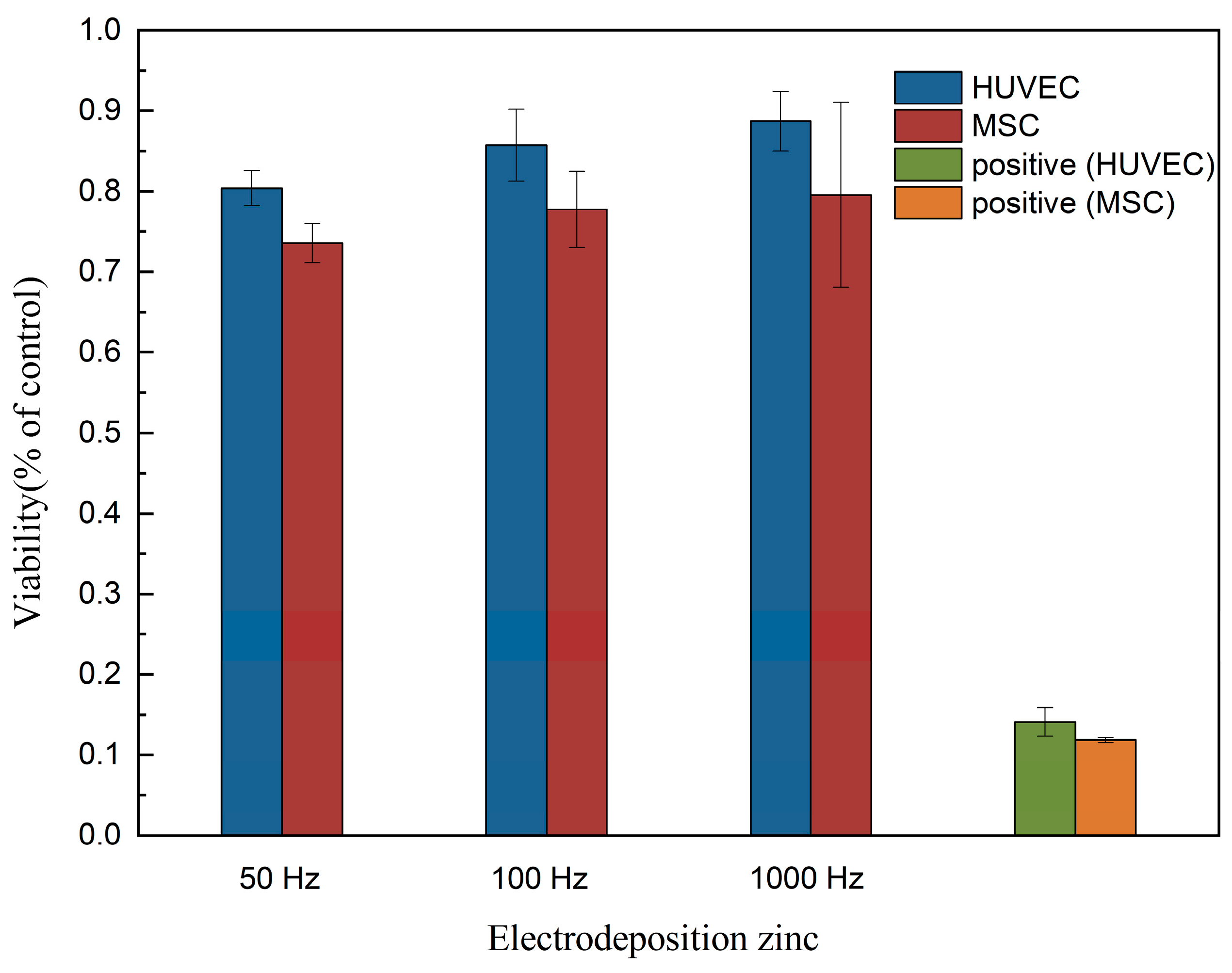
2.6. Cytocompatibility Evaluation
2.7. Hemolysis Evaluation
2.8. Statistic Analysis
3. Results and Discussion
4. Conclusions
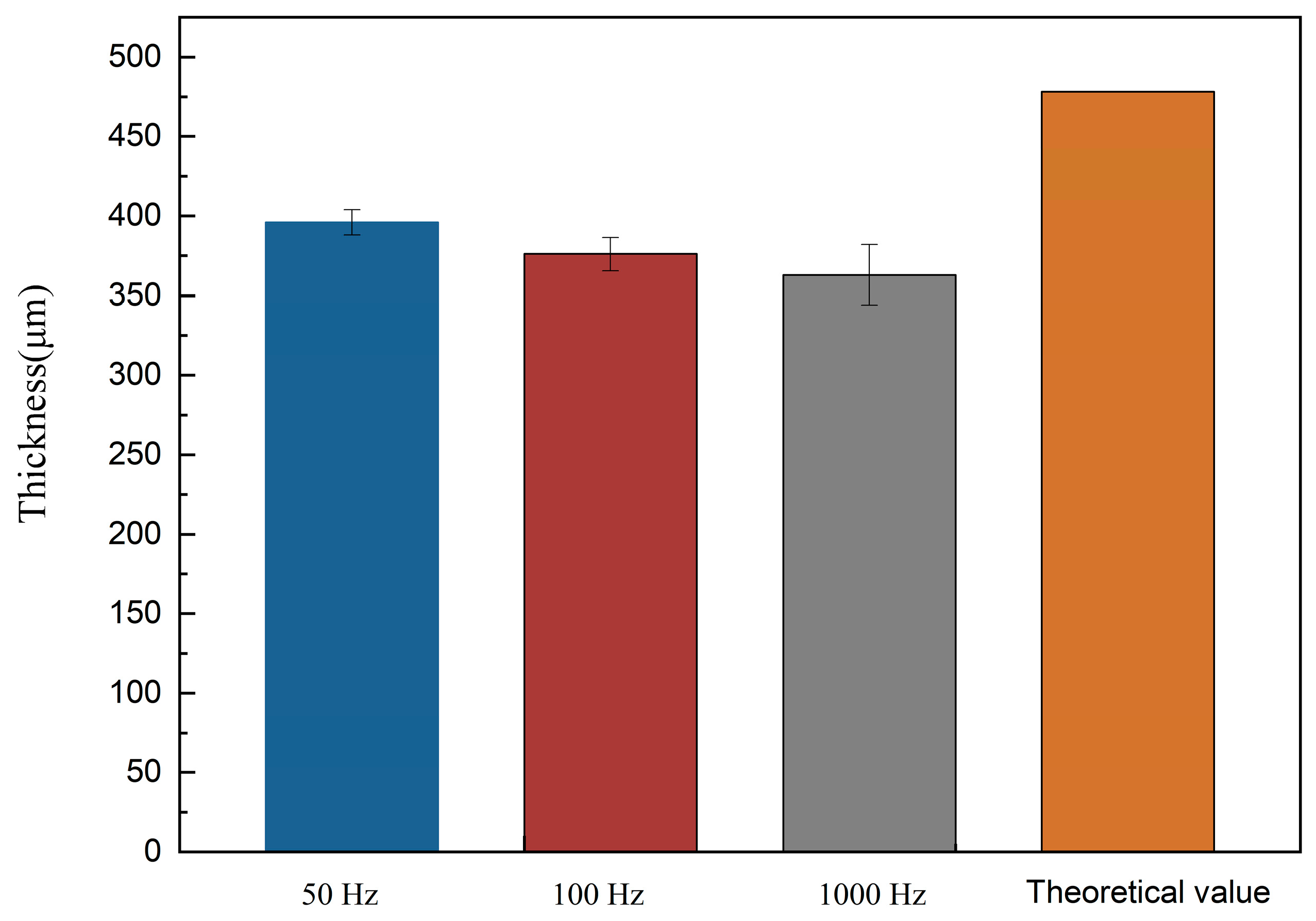
- The thickness can be adjusted by electrodeposition, which is convenient and controllable, and the product has no cracking phenomenon.
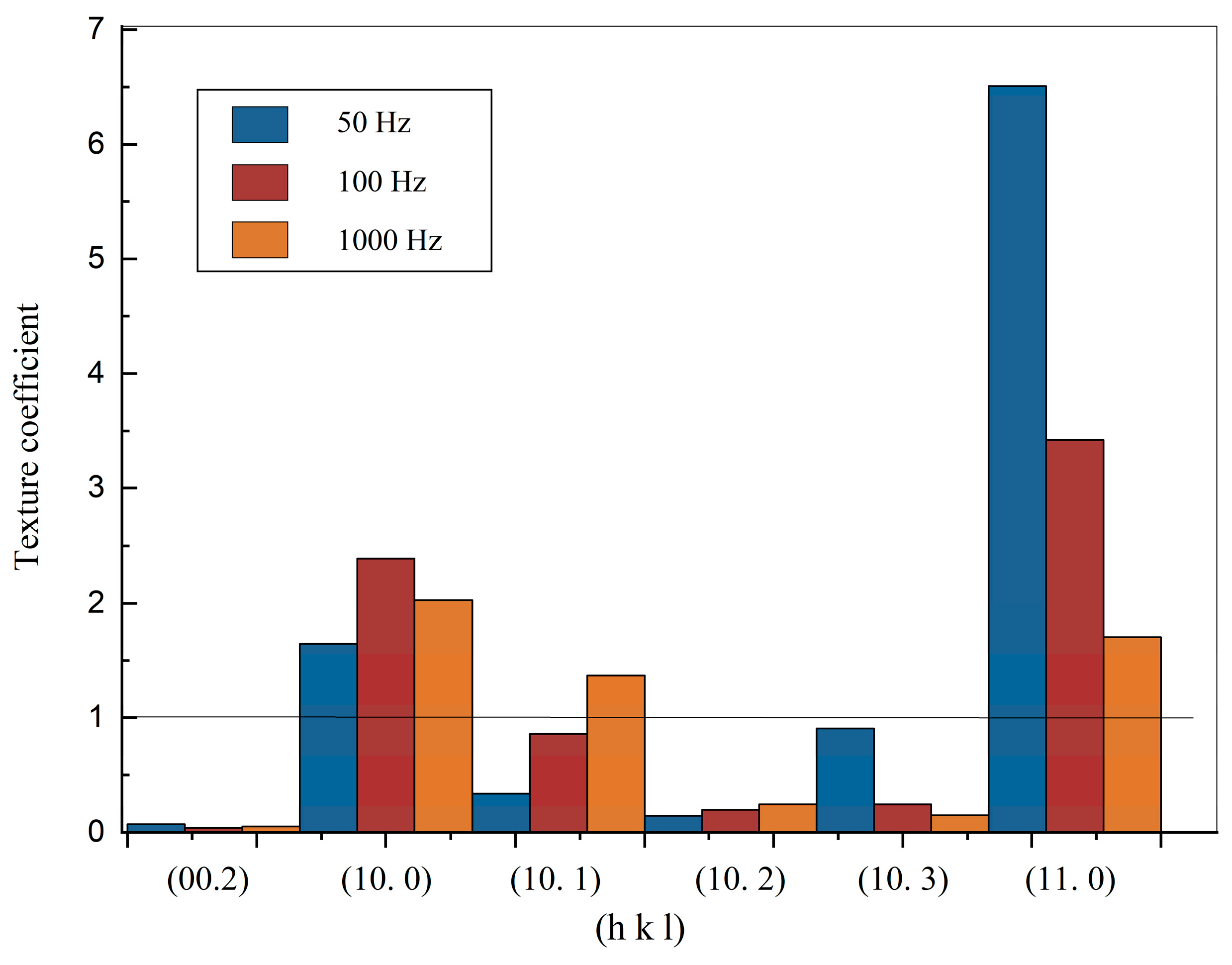
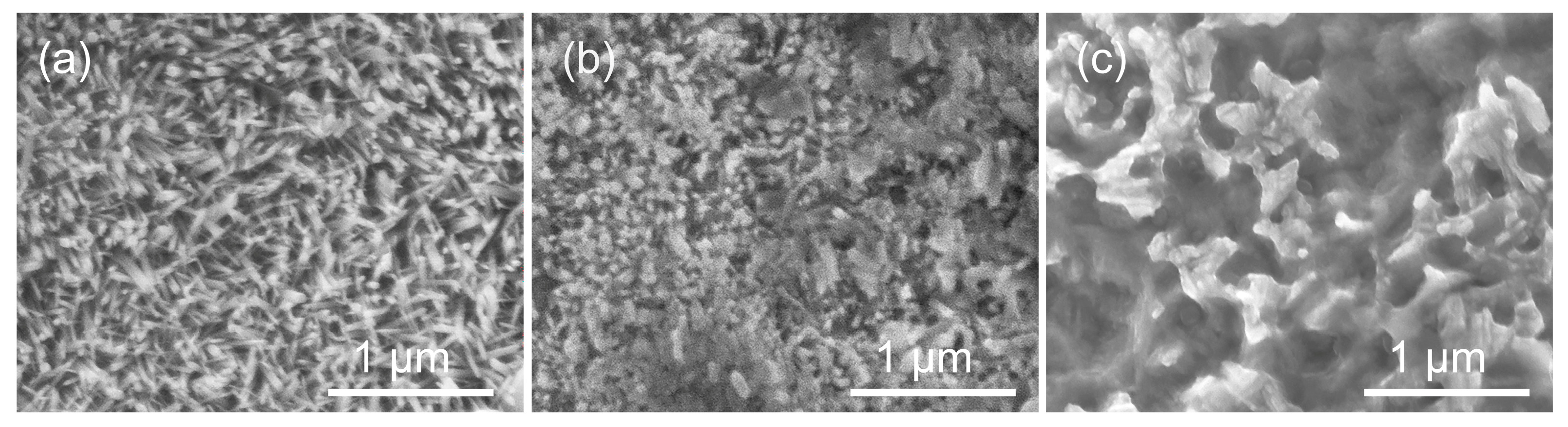
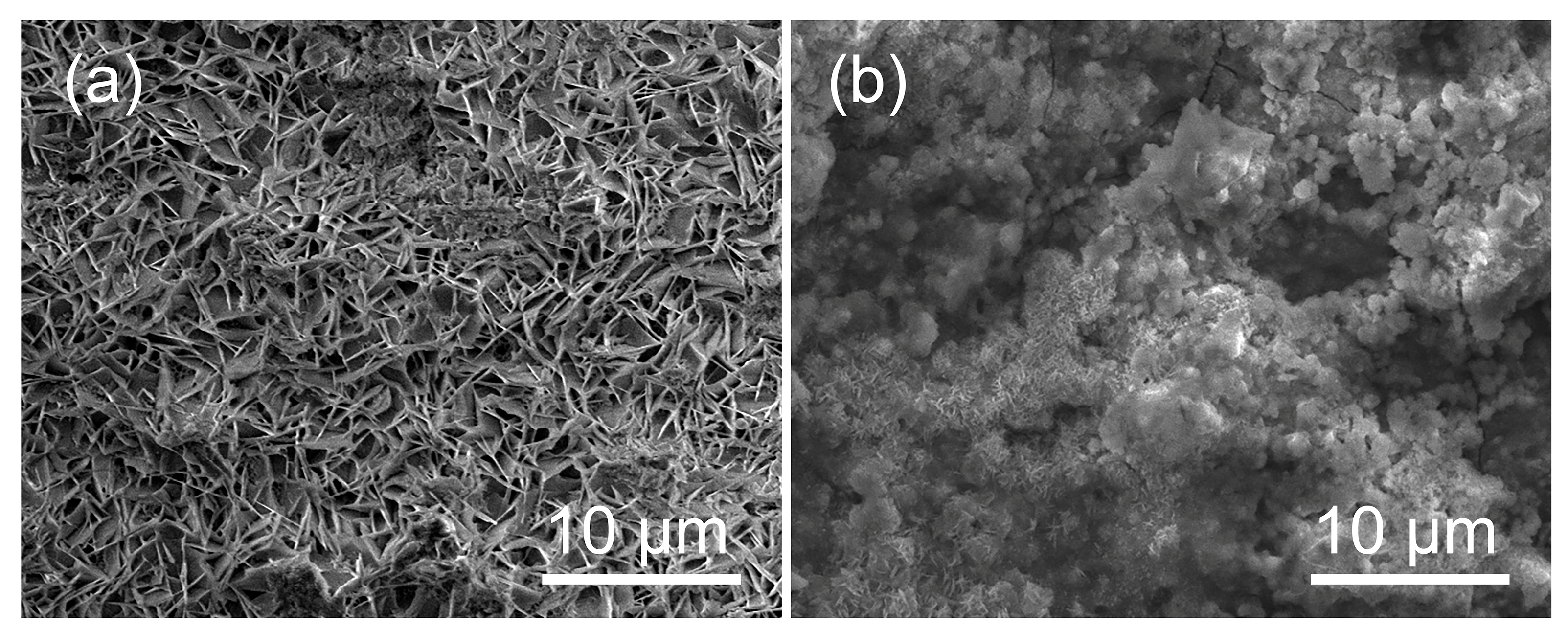
- The test results also imply that pulse frequency will affect the grain orientation, and thus the corrosion properties. It is shown that the 50 Hz produced zinc film possesses strong (11.0) grain orientation, 100 Hz produced zinc film possesses clear (11.0) and (10.0) grain orientations, yet 1000 Hz produced zinc film shows more random grain orientations of (10.0), (10.1) and (11.0).
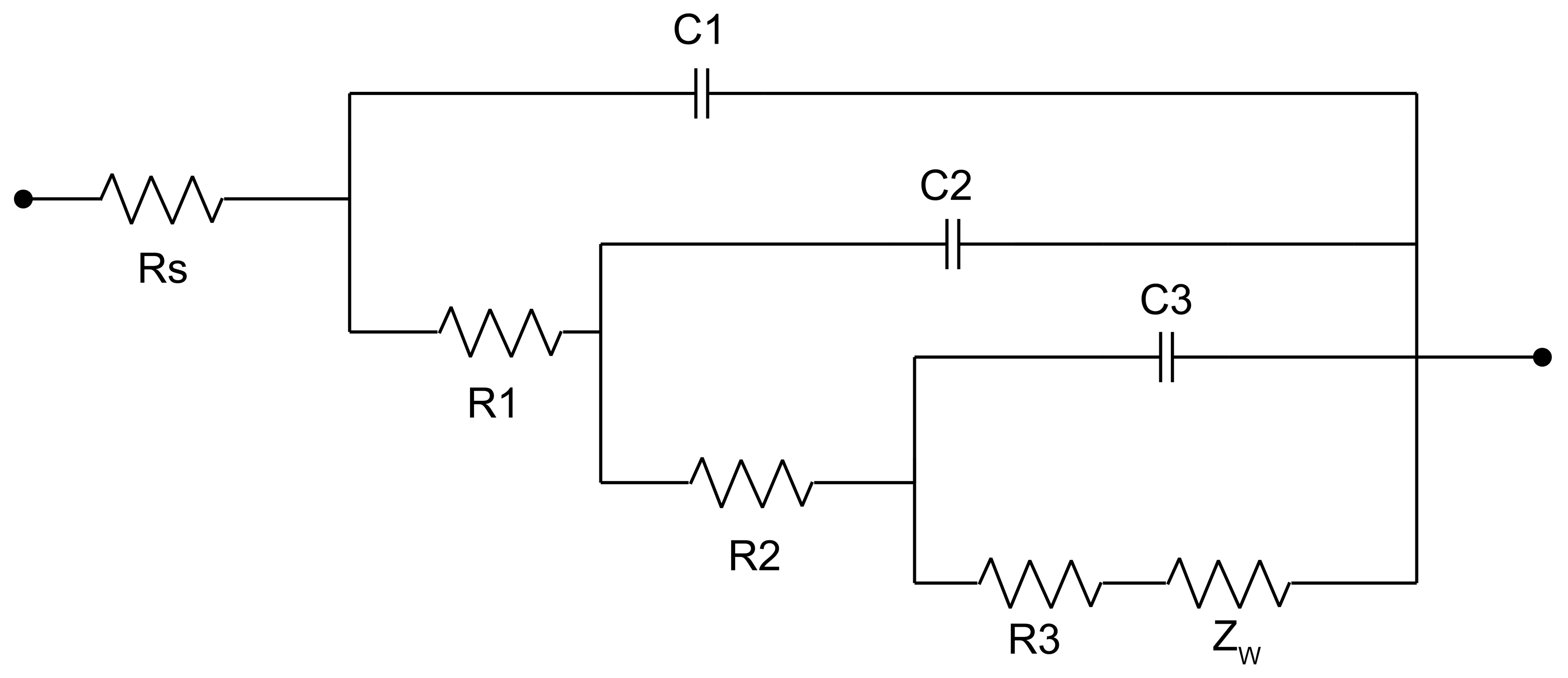
- The effect of the pulse frequency resulting from microstructures was clarified by electrochemical tests. Although thermodynamic degradation tendency implied from open current corrosion voltage (Ecorr) were similar, the kinetic corrosion rate showed a clear increasing trend as pulse frequency increased from 50 Hz to 1000 Hz, which corresponded with the EIS test and long-term soaking test in hanks solution. This tendency is probably attributed to the refined grain that increased the structural stability of the PC-formed zinc. It provides a possible way to design a controllable nanometer surface microtopography by adjusting PC frequency.
- Our results also indicated that appropriate pulse frequency can improve the blood compatibility of the material. According to ISO 10,993-5:2009 and ISO 10993-4:2002, electrodeposited zinc materials produced in this study showed a benign hemolysis ratio and no toxicity for cell growth. Zinc prepared under 50 Hz shows the best blood compatibility. Electrodeposited zinc materials are expected to be used for the shell of a degradable dosing pump.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, H.; Seo, M.H.; Rogers, J.A. Bioresorbable Metals for Biomedical Applications: From Mechanical Components to Electronic Devices. Adv. Healthc Mater. 2021, 10, e2002236. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Meng, Y.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.-N. Initial formation of corrosion products on pure zinc in simulated body fluid. J. Mater. Sci. Technol. 2018, 34, 2271–2282. [Google Scholar] [CrossRef]
- Sawidis, T.; Yurukova, L.; Askitis, T. Chios mastic, a natural supplement for zinc to enhance male sexuality and prostate function. Pharm. Biol. 2010, 48, 48–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dardenne, M. Zinc and immune function. Eur. J. Clin. Nutr. 2002, 56, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Moonga, B.S.; Dempster, D.W. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J. Bone Miner. Res. 1995, 10, 453–457. [Google Scholar] [CrossRef]
- Tenaud, I.; Saiagh, I.; Dreno, B. Addition of zinc and manganese to a biological dressing. J. Dermatol. Treat. 2009, 20, 90–93. [Google Scholar] [CrossRef]
- Su, Y.C.; Wang, K.; Gao, J.L.; Yang, Y.; Qin, Y.X.; Zheng, Y.F.; Zhu, D.H. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.W.; Yang, Y.W.; Yang, M.L.; Zheng, H.Z.; Xie, D.Q.; Wang, D.S.; Shen, L.D. Laser Additive Manufacturing of Zinc Targeting for Biomedical Application. Int. J. Bioprinting 2022, 8, 501. [Google Scholar] [CrossRef]
- Betancourt, T.; Brannon-Peppas, L. Micro- and nanofabrication methods in nanotechnological medical and pharmaceutical devices. Int. J. Nanomed. 2006, 1, 483–495. [Google Scholar] [CrossRef]
- Yuan, Z.; He, Y.; Lin, C.; Liu, P.; Cai, K. Antibacterial surface design of biomedical titanium materials for orthopedic applications. J. Mater. Sci. Technol. 2021, 78, 51–67. [Google Scholar] [CrossRef]
- Badv, M.; Bayat, F.; Weitz, J.I.; Didar, T.F. Single and multi-functional coating strategies for enhancing the biocompatibility and tissue integration of blood-contacting medical implants. Biomaterials 2020, 258, 25. [Google Scholar] [CrossRef] [PubMed]
- del Olmo, J.A.; Perez-Alvarez, L.; Pacha-Olivenza, M.A.; Ruiz-Rubio, L.; Gartziandia, O.; Vilas-Vilela, J.L.; Alonso, J.M. Antibacterial catechol-based hyaluronic acid, chitosan and poly (N-vinyl pyrrolidone) coatings onto Ti6Al4V surfaces for application as biomedical implant. Int. J. Biol. Macromol. 2021, 183, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Mantovani, D. Biodegradable metals for cardiovascular stent application: Interests and new opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef]
- Flejszar, M.; Chmielarz, P. Surface Modifications of Poly(Ether Ether Ketone) via Polymerization Methods—Current Status and Future Prospects. Materials 2020, 13, 999. [Google Scholar] [CrossRef]
- Fu, M.; Liang, Y.; Lv, X.; Li, C.; Yang, Y.Y.; Yuan, P.; Ding, X. Recent advances in hydrogel-based anti-infective coatings. J. Mater. Sci. Technol. 2021, 85, 169–183. [Google Scholar] [CrossRef]
- Song, Y.; Tang, J.; Hu, J.; Yang, H.; Gu, W.; Fu, Y.; Ji, X. Interfacial assistant role of amine additives on zinc electrodeposition from deep eutectic solvents: An in situ X-ray imaging investigation. Electrochim. Acta 2017, 240, 90–97. [Google Scholar] [CrossRef]
- Moravej, M.; Prima, F.; Fiset, M.; Mantovani, D. Electroformed iron as new biomaterial for degradable stents: Development process and structure–properties relationship. Acta Biomater. 2010, 6, 1726–1735. [Google Scholar] [CrossRef]
- Wu, G.; Li, N.; Zhou, D.; Mitsuo, K. Mitsuo Microstructure of Co-Ni-Al2O3 Composite Coatings by Electroforming. J. Mater. Sci. Technol. 2003, 19, 133–134. [Google Scholar]
- Kumar, C.M.P.; Lakshmikanthan, A.; Chandrashekarappa, M.P.G.; Pimenov, D.Y.; Giasin, K. Electrodeposition Based Preparation of Zn-Ni Alloy and Zn-Ni-WC Nano-Composite Coatings for Corrosion-Resistant Applications. Coatings 2021, 11, 17. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Chandrashekarappa, M.P.G.; Kulkarni, R.M.; Pimenov, D.Y.; Giasin, K. The Effect of Zn and Zn-WO3 Composites Nano-Coatings Deposition on Hardness and Corrosion Resistance in Steel Substrate. Materials 2021, 14, 2253. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.M.S.; Koch, C.C.; Fedkiw, P.S. Improved corrosion behavior of nanocrystalline zinc produced by pulse-current electrodeposition. Corros. Sci. 2004, 46, 51–64. [Google Scholar] [CrossRef]
- Muresan, L.; Oniciu, L.; Froment, M.; Maurin, G. Inhibition of lead electrocrystallization by organic additives. Electrochim. Acta 1992, 37, 2249–2254. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Hanks, J.H.; Wallace, R.E. Relation of Oxygen and Temperature in the Preservation of Tissues by Refrigeration. Proc. Soc. Exp. Biol. Med. 1949, 71, 196–200. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Yang, Y.; Pu, Z.; Zheng, Y. In vitro investigation of ultra-pure Zn and its mini-tube as potential bioabsorbable stent material. Mater. Lett. 2015, 161, 53–56. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Huang, W.; Yang, W.Z.; Yin, X.S.; Liu, Y. In vitro degradation, cytocompatibility and hemolysis tests of CaF2 doped TiO2-SiO2 composite coating on AZ31 alloy. Appl. Surf. Sci. 2016, 382, 268–279. [Google Scholar] [CrossRef]
- Yılmaz, G.; Hapçı, G.; Orhan, G. Properties of Ni/Nano-TiO2 Composite Coatings Prepared by Direct and Pulse Current Electroplating. J. Mater. Eng. Perform. 2015, 24, 709–720. [Google Scholar] [CrossRef]
- Fustes, J.; Gomes, A.; Pereira, M.I.D. Electrodeposition of Zn-TiO2 nanocomposite films-effect of bath composition. J. Solid State Electrochem. 2008, 12, 1435–1443. [Google Scholar] [CrossRef]
- Girin, O.; Panasenko, S. Influence of the texture of electrolytic zinc coatings on their corrosion resistance. Prot. Met. 1989, 25, 480–482. [Google Scholar]
- Chen, L.; Wang, L.; Zeng, Z.; Xu, T. Influence of pulse frequency on the microstructure and wear resistance of electrodeposited Ni-Al2O3 composite coatings. Surf. Coat. Technol. 2006, 201, 599–605. [Google Scholar] [CrossRef]
- Kurdowski, W.; Bochenek, A. Three principles of concrete corrosion prevention. Cem. Wapno Beton 2012, 17, 434–442. [Google Scholar]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.W.; Jain, H.; Kang, S.K.; Su, Y. Dissolvable metals for transient electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Li, M.C.; Jiang, L.L.; Zhang, W.Q.; Qian, Y.H.; Luo, S.Z.; Shen, J.N. Electrochemical corrosion behavior of nanocrystalline zinc coatings in 3.5% NaCl solutions. J. Solid State Electrochem. 2007, 11, 1319–1325. [Google Scholar] [CrossRef]
- Jantaping, N.; Schuh, C.A.; Boonyongmaneerat, Y. Influences of crystallographic texture and nanostructural features on corrosion properties of electrogalvanized and chromate conversion coatings. Surf. Coat. Technol. 2017, 329, 120–130. [Google Scholar] [CrossRef]
- Park, H.; Szpunar, J.A. The role of texture and morphology in optimizing the corrosion resistance of zinc-based electrogalvanized coatings. Corros. Sci. 1998, 40, 525–545. [Google Scholar] [CrossRef]
- Mouanga, M.; Ricq, L.; Douglade, J.; Berçot, P. Effects of some additives on the corrosion behaviour and preferred orientations of zinc obtained by continuous current deposition. J. Appl. Electrochem. 2007, 37, 283–289. [Google Scholar] [CrossRef]
- Otani, T.; Nagata, M.; Fukunaka, Y.; Homma, T. Morphological evolution of mossy structures during the electrodeposition of zinc from an alkaline zincate solution. Electrochim. Acta 2016, 206, 366–373. [Google Scholar] [CrossRef]
- Zhang, A.X.; Lv, D.L.; Zhong, W.; Cheng, W.Z.; Du, Q.G. Blood Compatibility of Biomaterials. Prog. Biomed. Eng. 2004, 25, 53–58. [Google Scholar]


| Bath Composition | Range | Operation Conditions |
|---|---|---|
| ZnSO4·7H2O | 300–450 g/L | 300 g/L γ = 30% |
| H3BO3 | 25–35 g/L | 25 g/L t = 7 h |
| Brightening agent (S-Z95) | 18–20 mg/L | 19 mg/L |
| pH | 3.5–5.5 | 4 |
| Temperature | 283–323 K | 313 K |
| Cathode-current density | 1–4 A/dm2 | 4 A/dm2 |
| Ton/Toff = 2/1 |
| Samples (Hz) | βa (mV/decade) | βb (mV/decade) | Icorr (10−6 A/cm2) | Ecorr (V) | C (mm∙year−1∙cm2) |
|---|---|---|---|---|---|
| 50 | 15.148 | 8.118 | 7.993 | −1.0427 | 0.11182 |
| 100 | 16.155 | 8.334 | 8.134 | −1.0497 | 0.12092 |
| 1000 | 16.388 | 8.445 | 8.596 | −1.0474 | 0.12779 |
| F (Hz) | Rs (Ω·cm2) | C1 (μF·cm−2) | R1 (Ω·cm2) | C2 (μF·cm−2) | R2 (Ω·cm2) | C3 (μF·cm−2) | R3 (Ω·cm2) | Rp * (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| 50 | 4.664 | 0.1916 | 10.22 | 184.1 | 49.65 | 41.89 | 28.45 | 88.32 |
| 100 | 4.425 | 0.1849 | 9.857 | 21.84 | 48.21 | 57.28 | 27.12 | 85.19 |
| 1000 | 4.582 | 0.1301 | 8.82 | 135.7 | 47.57 | 82.59 | 27.8 | 83.65 |
| 50 Hz | 100 Hz | 1000 Hz | Positive Control | Blank Control | |
|---|---|---|---|---|---|
| OD (A) | 0.043 | 0.056 | 0.047 | 0.036 | 1.498 |
| T (%) | 90.7 | 87.7 | 89.7 | 91.8 | 3.1 |
| Hemolysis rate | 0.478 | 1.368 | 0.752 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Luo, Y.; Hu, W.; Chen, Y.; Huang, Z. Effect of Pulse Frequency on the Microstructure and the Degradation of Pulse Electroformed Zinc for Fabricating the Shell of Biodegradable Dosing Pump. Bioengineering 2022, 9, 289. https://doi.org/10.3390/bioengineering9070289
Wu S, Luo Y, Hu W, Chen Y, Huang Z. Effect of Pulse Frequency on the Microstructure and the Degradation of Pulse Electroformed Zinc for Fabricating the Shell of Biodegradable Dosing Pump. Bioengineering. 2022; 9(7):289. https://doi.org/10.3390/bioengineering9070289
Chicago/Turabian StyleWu, Shuhui, Yizhuo Luo, Wei Hu, Yonghong Chen, and Zhi Huang. 2022. "Effect of Pulse Frequency on the Microstructure and the Degradation of Pulse Electroformed Zinc for Fabricating the Shell of Biodegradable Dosing Pump" Bioengineering 9, no. 7: 289. https://doi.org/10.3390/bioengineering9070289
APA StyleWu, S., Luo, Y., Hu, W., Chen, Y., & Huang, Z. (2022). Effect of Pulse Frequency on the Microstructure and the Degradation of Pulse Electroformed Zinc for Fabricating the Shell of Biodegradable Dosing Pump. Bioengineering, 9(7), 289. https://doi.org/10.3390/bioengineering9070289






