Effects on Tissue Integration of Collagen Scaffolds Used for Local Delivery of Gentamicin in a Rat Mandible Defect Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Description
2.2. Antimicrobial Selection
2.3. In Vitro Drug Loading and Elution
2.3.1. Hydrophilicity
2.3.2. Drug Loading
2.3.3. Drug Elution Protocol
2.3.4. Drug Concentration
2.4. In Vitro Cytocompatibility
2.4.1. Cell Culture Conditions
2.4.2. Cell Seeding to Scaffolds
2.4.3. Cell Adhesion and Proliferation
2.5. In Vivo Evaluation
2.5.1. Rodent Model
2.5.2. Surgical Preparation and Surgical Procedure
2.5.3. Computed Tomography Analysis
2.5.4. Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. Hydrophilicity
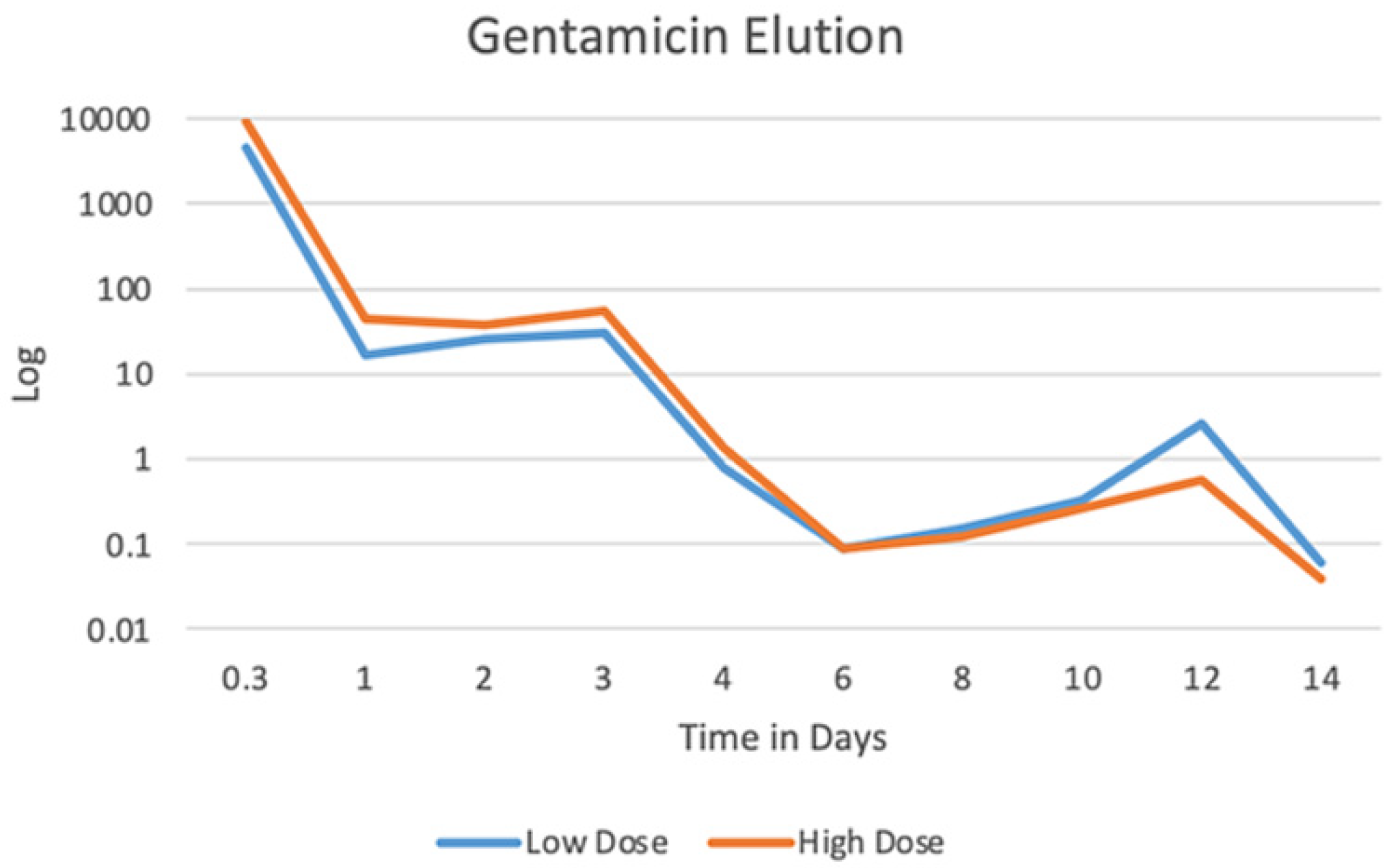
3.2. Gentamicin Elution
3.3. In Vitro Cytocompatibility
3.4. In Vivo Biocompatibility
3.4.1. Animal Observation and Care
3.4.2. CT Analysis
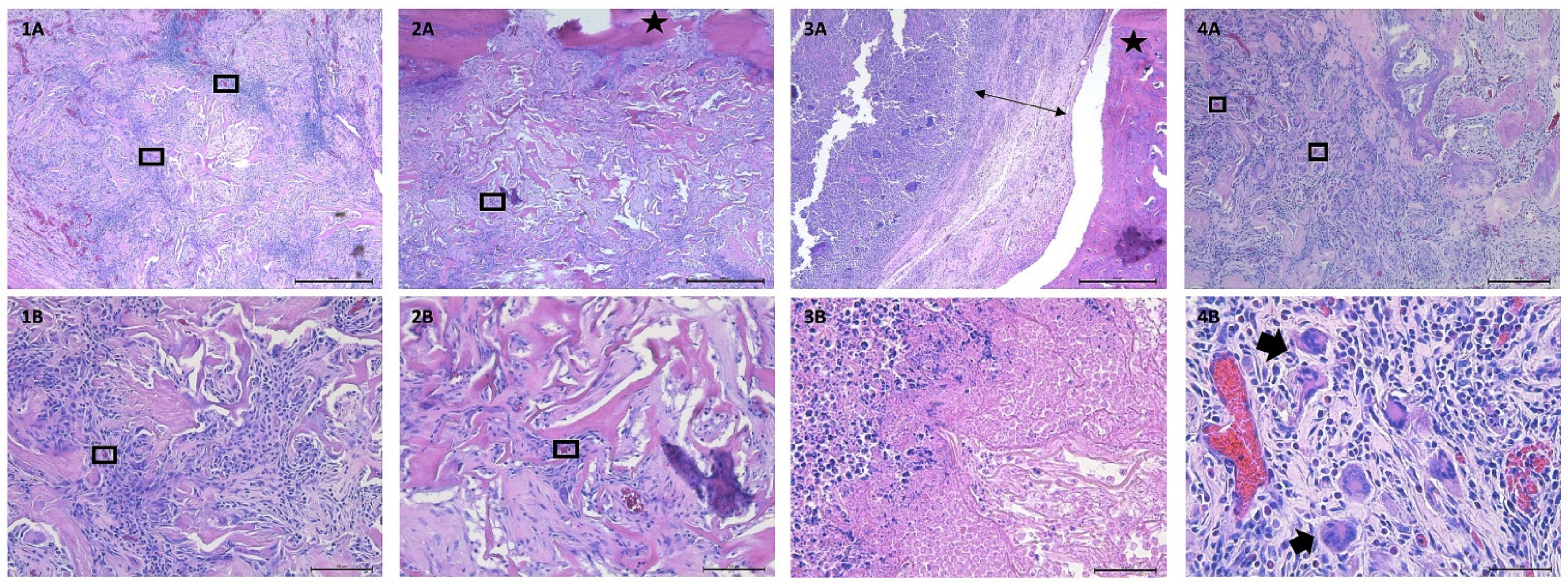
3.4.3. Histological Analysis
4. Discussion
5. Limitations
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onyekwelu, I.; Yakkanti, R.; Protzer, L.; Pinkston, C.M.; Tucker, C.; Seligson, D. Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2017, 1, e022. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.R. Guide to the elimination of orthopedic surgery surgical site infections: An executive summary of the Association for Professionals in Infection Control and Epidemiology elimination guide. Am. J. Infect. Control. 2012, 40, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pal, K.; Jain, S.; Chatterjee, S.S.; Konar, J. Surgical Site Infection by Methicillin Resistant Staphylococcus aureus- on Decline? J. Clin. Diagn. Res. 2016, 10, DC32–DC36. [Google Scholar] [CrossRef]
- Szczeblinska, J.; Fijalkowski, K.; Kohn, J.; El Fray, M. Antibiotic loaded microspheres as antimicrobial delivery systems for medical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 69–75. [Google Scholar] [CrossRef]
- Peterson, B.E.; Jiwanlal, A.; Della Rocca, G.J.; Crist, B.D. Orthopedic Trauma and Aging. Geriatr. Orthop. Surg. Rehabil. 2015, 6, 33–36. [Google Scholar] [CrossRef] [Green Version]
- National Healthcare Safety Network Patient Safety Component Manual. 2022. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf (accessed on 3 February 2022).
- Leong, H.N.; Kurup, A.; Tan, M.Y.; Kwa, A.L.H.; Liau, K.H.; Wilcox, M. Management of complicated skin and soft tissue infections with a special focus on the role of newer antibiotics. Infect. Drug Resist. 2018, 11, 1959–1974. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of infected wounds: Overcoming clinical challenges by advanced drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567. [Google Scholar] [CrossRef]
- Fish, D.N. Meropenem in the treatment of complicated skin and soft tissue infections. Ther. Clin. Risk Manag. 2006, 2, 401–415. [Google Scholar] [CrossRef] [Green Version]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2019, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C.T.; Boakye-Agyeman, F.; Brinkman, C.L.; Reid, J.M.; Patel, R.; Bajzer, Z.; Dadsetan, M.; Yaszemski, M.J. Controlled Delivery of Vancomycin via Charged Hydrogels. PLoS ONE 2016, 11, e0146401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beenken, K.E.; Campbell, M.J.; Ramirez, A.M.; Alghazali, K.; Walker, C.M.; Jackson, B.; Griffin, C.; King, W.; Bourdo, S.E.; Rifkin, R.; et al. Evaluation of a bone filler scaffold for local antibiotic delivery to prevent Staphylococcus aureus infection in a contaminated bone defect. Sci. Rep. 2021, 11, 10254. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef]
- Ferguson, J.; Diefenbeck, M.; McNally, M. Ceramic Biocomposites as Biodegradable Antibiotic Carriers in the Treatment of Bone Infections. J. Bone Jt. Infect. 2017, 2, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Barth, R.E.; Vogely, H.C.; Hoepelman, A.I.; Peters, E.J. ‘To bead or not to bead?’ Treatment of osteomyelitis and prosthetic joint-associated infections with gentamicin bead chains. Int. J. Antimicrob. Agents 2011, 38, 371–375. [Google Scholar] [CrossRef]
- Billings, C.; Anderson, D.E. Role of Implantable Drug Delivery Devices with Dual Platform Capabilities in the Prevention and Treatment of Bacterial Osteomyelitis. Bioengineering 2022, 9, 65. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Fragomen, A.T.; Moriarty, T.F.; Morgenstern, M.; Egol, K.A.; Zalavras, C.; Obremskey, W.T.; Raschke, M.; McNally, M.A.; Fracture-Related Infection consensus, g. Evidence-Based Recommendations for Local Antimicrobial Strategies and Dead Space Management in Fracture-Related Infection. J. Orthop. Trauma 2020, 34, 18–29. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Abou Neel, E.A.; Bozec, L.; Knowles, J.C.; Syed, O.; Mudera, V.; Day, R.; Hyun, J.K. Collagen—Emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev. 2013, 65, 429–456. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue Integration and Degradation of a Porous Collagen-Based Scaffold Used for Soft Tissue Augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Osorio, R. In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation. Polymers 2021, 13, 2633. [Google Scholar] [CrossRef]
- Chaves, B.J.; Tadi, P. Gentamicin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557550/?report=classic (accessed on 4 February 2022).
- Bennett-Guerrero, E. Effect of an Implantable Gentamicin-Collagen Sponge on Sternal Wound Infections Following Cardiac Surgery—A Randomized Trial. JAMA 2010, 304, 755. [Google Scholar] [CrossRef] [Green Version]
- Kasatpibal, N.; Nørgaard, M.; Sørensen, H.T.; Schønheyder, H.C.; Jamulitrat, S.; Chongsuvivatwong, V. Risk of surgical site infection and efficacy of antibiotic prophylaxis: A cohort study of appendectomy patients in Thailand. BMC Infect. Dis. 2006, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- ter Boo, G.J. Delivery of Gentamicin from Resorbable Polymeric Carriers as Anti-Infective Strategy for Implant-Associated Osteomyelitis; University of Twente: Enschede, The Netherlands, 2016. [Google Scholar]
- Kanellakopoulou, K.; Giamarellos-Bourboulis, E.J. Carrier Systems for the Local Delivery of Antibiotics in Bone Infections. Drugs 2000, 59, 1223–1232. [Google Scholar] [CrossRef]
- LeBrun, M.; Grenier, L.; Gourde, P.; Bergeron, M.G.; Labrecque, G.; Beauchamp, D. Effectiveness and Toxicity of Gentamicin in an Experimental Model of Pyelonephritis: Effect of the Time of Administration. Antimicrob. Agents Chemother. 1999, 43, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Udupa, V.; Prakash, V. Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol. Rep. 2019, 6, 91–99. [Google Scholar] [CrossRef]
- Jackson, B.K.; Bow, A.J.; Kannarpady, G.; Biris, A.S.; Anderson, D.E.; Dhar, M.; Bourdo, S.E. Polyurethane/nano-hydroxyapatite composite films as osteogenic platforms. J. Biomater. Sci. Polym. Ed. 2018, 29, 1426–1443. [Google Scholar] [CrossRef]
- Bow, A.; Newby, S.; Rifkin, R.; Jackson, B.K.; Matavosian, A.; Griffin, C.; King, W.; Alghazali, K.; Mhannawee, A.; Berryhill, S.B.; et al. Evaluation of a Polyurethane Platform for Delivery of Nanohydroxyapatite and Decellularized Bone Particles in a Porous Three-Dimensional Scaffold. ACS Appl. Bio Mater. 2019, 2, 1815–1829. [Google Scholar] [CrossRef]
- Elkhenany, H.; El-Badri, N.; Dhar, M. Green propolis extract promotes in vitro proliferation, differentiation, and migration of bone marrow stromal cells. Biomed. Pharmacother. 2019, 115, 108861. [Google Scholar] [CrossRef] [PubMed]
- Gielkens, P.F.; Schortinghuis, J.; de Jong, J.R.; Raghoebar, G.M.; Stegenga, B.; Bos, R.R. Vivosorb, Bio-Gide, and Gore-Tex as barrier membranes in rat mandibular defects: An evaluation by microradiography and micro-CT. Clin. Oral Implant. Res. 2008, 19, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Takayama, T.; Yamamoto, T.; Ozawa, Y.; Nagao, M.; Tanabe, N.; Nakajima, A.; Suzuki, N.; Maeno, M.; Yamano, S.; et al. A collagen membrane containing osteogenic protein-1 facilitates bone regeneration in a rat mandibular bone defect. Arch. Oral Biol. 2017, 84, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Lezcano, F.J.; Redondo-González, L.M.; Alberca-Zeballos, M.; Sánchez-García, A.M.; García-Sancho, J. Mandibular bone regeneration with autologous adipose-derived mesenchymal stem cells and coralline hydroxyapatite: Experimental study in rats. Br. J. Oral Maxillofac. Surg. 2021, YBJOM-6410, 1–8. [Google Scholar] [CrossRef]
- Dalu, A.; Blaydes, B.S.; Lomax, L.G.; Delclos, K.B. A comparison of the inflammatory response to a polydimethylsiloxane implant in male and female Balb/c mice. Biomaterials 2000, 21, 1947–1957. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Walker, P.D.; Barri, Y.; Shah, S.V. Oxidant Mechanisms in Gentamicin Nephrotoxicity. Ren. Fail. 1999, 21, 433–442. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatric Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Adusei, K.M.; Ngo, T.B.; Sadtler, K. T lymphocytes as critical mediators in tissue regeneration, fibrosis, and the foreign body response. Acta Biomater. 2021, 133, 17–33. [Google Scholar] [CrossRef]
- Kwee, B.J.; Budina, E.; Najibi, A.J.; Mooney, D.J. CD4 T-cells regulate angiogenesis and myogenesis. Biomaterials 2018, 178, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.F.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Tanneberger, A.M.; Al-Maawi, S.; Herrera-Vizcaíno, C.; Orlowska, A.; Kubesch, A.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells within the in vivo implantation bed of a collagen-based biomaterial determine its degradation pattern. Clin. Oral Investig. 2021, 25, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef]
- Meseguer-Olmo, L.; Ros-Nicolás, M.J.; Clavel-Sainz, M.; Vicente-Ortega, V.; Alcaraz-Baños, M.; Lax-Pérez, A.; Arcos, D.; Ragel, C.V.; Vallet-Regí, M. Biocompatibility and in vivo gentamicin release from bioactive sol–gel glass implants. J. Biomed. Mater. Res. 2002, 61, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Tal, H.; Kozlovsky, A.; Artzi, Z.; Nemcovsky, C.E.; Moses, O. Cross-linked and non-cross-linked collagen barrier membranes disintegrate following surgical exposure to the oral environment: A histological study in the cat. Clin. Oral Implant. Res. 2008, 19, 760–766. [Google Scholar] [CrossRef]
- Calciolari, E.; Ravanetti, F.; Strange, A.; Mardas, N.; Bozec, L.; Cacchioli, A.; Kostomitsopoulos, N.; Donos, N. Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. J. Periodontal Res. 2018, 53, 430–439. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billings, C.; Bow, A.J.; Newby, S.D.; Donnell, R.L.; Dhar, M.; Anderson, D.E. Effects on Tissue Integration of Collagen Scaffolds Used for Local Delivery of Gentamicin in a Rat Mandible Defect Model. Bioengineering 2022, 9, 275. https://doi.org/10.3390/bioengineering9070275
Billings C, Bow AJ, Newby SD, Donnell RL, Dhar M, Anderson DE. Effects on Tissue Integration of Collagen Scaffolds Used for Local Delivery of Gentamicin in a Rat Mandible Defect Model. Bioengineering. 2022; 9(7):275. https://doi.org/10.3390/bioengineering9070275
Chicago/Turabian StyleBillings, Caroline, Austin J. Bow, Steven D. Newby, Robert L. Donnell, Madhu Dhar, and David E. Anderson. 2022. "Effects on Tissue Integration of Collagen Scaffolds Used for Local Delivery of Gentamicin in a Rat Mandible Defect Model" Bioengineering 9, no. 7: 275. https://doi.org/10.3390/bioengineering9070275
APA StyleBillings, C., Bow, A. J., Newby, S. D., Donnell, R. L., Dhar, M., & Anderson, D. E. (2022). Effects on Tissue Integration of Collagen Scaffolds Used for Local Delivery of Gentamicin in a Rat Mandible Defect Model. Bioengineering, 9(7), 275. https://doi.org/10.3390/bioengineering9070275







