Application of Precision-Cut Lung Slices as an In Vitro Model for Research of Inflammatory Respiratory Diseases
Abstract
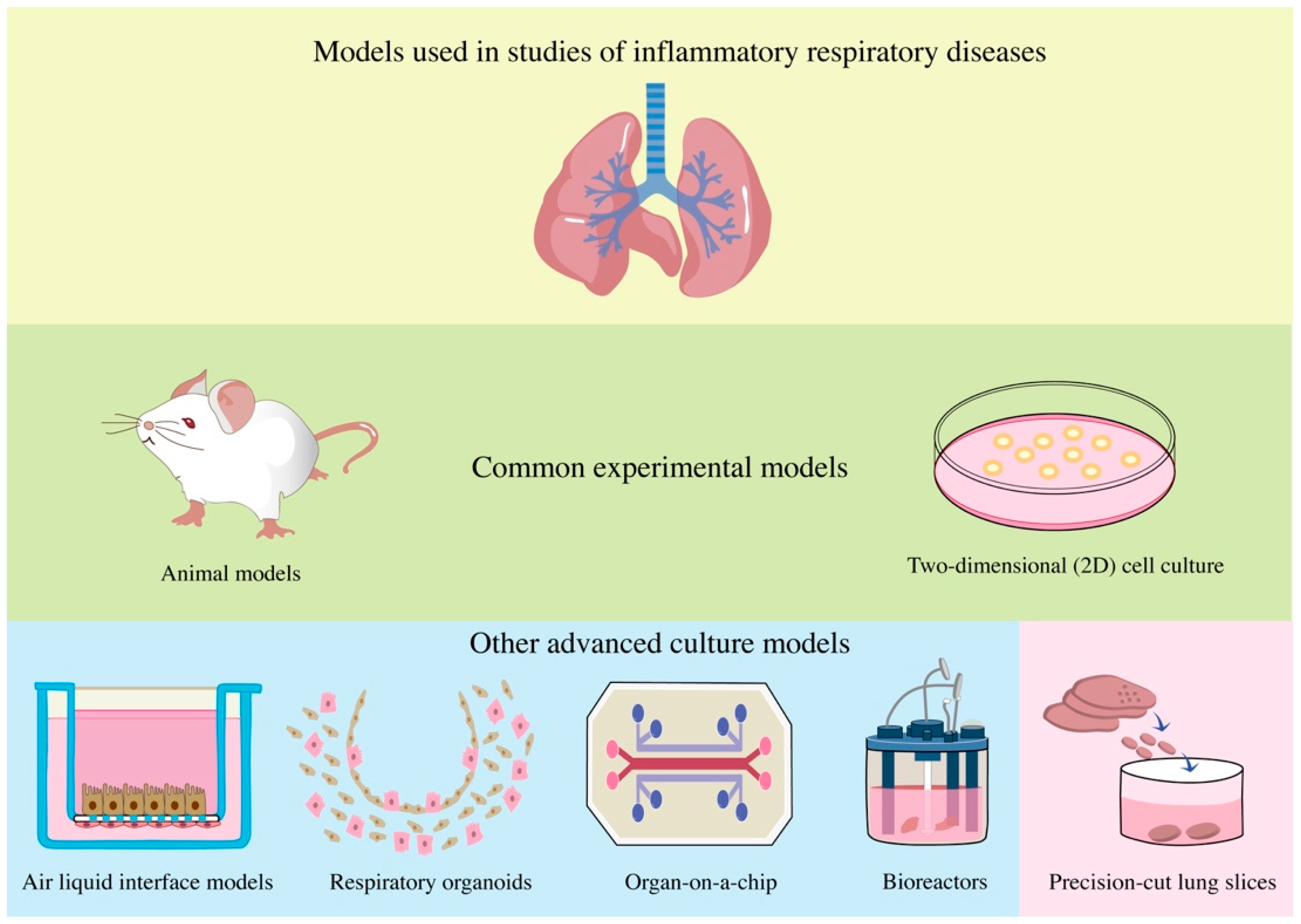
1. Introduction
2. Preparation and Storage of PCLSs
3. Combined Application of PCLSs and Modern Technology
4. Application of PCLS Technology in Various Respiratory Inflammatory Diseases
4.1. PCLSs Can Be Used as a Model of Bacterial Infectious Inflammation and Injury
4.2. PCLS as a Research Tool for Respiratory Viral Infectious Inflammation
4.3. Application of PCLS in Classical Chronic Inflammatory Diseases
4.3.1. Chronic Obstructive Pulmonary Disease
4.3.2. Asthma
4.3.3. Other Chronic Inflammatory Lung Diseases
5. Optimization and Limitations of PCLS Technology
6. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cagnina, R.E.; Duvall, M.G.; Nijmeh, J.; Levy, B.D. Specialized pro-resolving mediators in respiratory diseases. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Selvakumar, A.; See, K.C. Treatable Traits in Chronic Respiratory Disease: A Comprehensive Review. Cells 2021, 10, 3263. [Google Scholar] [CrossRef] [PubMed]
- Bonniaud, P.; Fabre, A.; Frossard, N.; Guignabert, C.; Inman, M.; Kuebler, W.M.; Maes, T.; Shi, W.; Stampfli, M.; Uhlig, S.; et al. Optimising experimental research in respiratory diseases: An ERS statement. Eur. Respir. J. 2018, 51, 1702133. [Google Scholar] [CrossRef]
- Sanderson, M.J. Exploring lung physiology in health and disease with lung slices. Pulm. Pharmacol. Ther. 2011, 24, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Coyle, J.P.; Xiong, R.; Wang, Y.; Heflich, R.H.; Ren, B.; Gwinn, W.M.; Hayden, P.; Rojanasakul, L. Invited review: Human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells—overview and perspectives. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Tetley, T.D.; Janes, S.M. Airway and alveolar epithelial cells in culture. Eur. Respir. J. 2019, 54, 1900742. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Chung, M.I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L. Lung organoids: Current uses and future promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef]
- Liu, G.; Betts, C.; Cunoosamy, D.M.; Åberg, P.M.; Hornberg, J.J.; Sivars, K.B.; Cohen, T.S. Use of precision cut lung slices as a translational model for the study of lung biology. Respir. Res. 2019, 20, 162. [Google Scholar] [CrossRef]
- Kızılkurtlu, A.A.; Polat, T.; Aydın, G.B.; Akpek, A. Lung on a Chip for Drug Screening and Design. Curr. Pharm. Des. 2019, 24, 5386–5396. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, H.; Wang, X. Microfluidic-Chip-Integrated Biosensors for Lung Disease Models. Biosensors 2021, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Le, A.V.; Baevova, P.; Niklason, L.E. Controlled gas exchange in whole lung bioreactors. J. Tissue Eng. Regen. Med. 2018, 12, e119–e129. [Google Scholar] [CrossRef]
- Nossa, R.; Costa, J.; Cacopardo, L.; Ahluwalia, A. Breathing in vitro: Designs and applications of engineered lung models. J. Tissue Eng. 2021, 12, 20417314211008696. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef]
- Krumdieck, C.L.; dos Santos, J.; Ho, K.-J. A new instrument for the rapid preparation of tissue slices. Anal. Biochem. 1980, 104, 118–123. [Google Scholar] [CrossRef]
- Placke, M.E.; Fisher, G.L. Adult peripheral lung organ culture—a model for respiratory tract toxicology. Toxicol. Appl. Pharmacol. 1987, 90, 284–298. [Google Scholar] [CrossRef]
- Hesse, C.; Beneke, V.; Konzok, S.; Diefenbach, C.; Sand, J.M.B.; Rønnow, S.R.; Karsdal, M.A.; Jonigk, D.; Sewald, K.; Braun, A.; et al. Nintedanib modulates type III collagen turnover in viable precision-cut lung slices from bleomycin-treated rats and patients with pulmonary fibrosis. Respir. Res. 2022, 23, 201. [Google Scholar] [CrossRef]
- Alsafadi, H.N.; Staab-Weijnitz, C.A.; Lehmann, M.; Lindner, M.; Peschel, B.; Königshoff, M.; Wagner, D.E. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L896–L902. [Google Scholar] [CrossRef]
- Liu, G.; Särén, L.; Douglasson, H.; Zhou, X.-H.; Åberg, P.M.; Ollerstam, A.; Betts, C.J.; Sivars, K.B. Precision cut lung slices: An ex vivo model for assessing the impact of immunomodulatory therapeutics on lung immune responses. Arch. Toxicol. 2021, 95, 2871–2877. [Google Scholar] [CrossRef]
- Alsafadi, H.N.; Uhl, F.E.; Pineda, R.H.; Bailey, K.E.; Rojas, M.; Wagner, D.E.; Königshoff, M. Applications and Approaches for Three-Dimensional Precision-Cut Lung Slices. Disease Modeling and Drug Discovery. Am. J. Respir. Cell Mol. Biol. 2020, 62, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, N.; Chhour, P.; Margulies, S.S. Uses of Remnant Human Lung Tissue for Mechanical Stretch Studies. Cell. Mol. Bioeng. 2013, 6, 175–182. [Google Scholar] [CrossRef]
- Switalla, S.; Lauenstein, L.; Prenzler, F.; Knothe, S.; Förster, C.; Fieguth, H.-G.; Pfennig, O.; Schaumann, F.; Martin, C.; Guzman, C.; et al. Natural innate cytokine response to immunomodulators and adjuvants in human precision-cut lung slices. Toxicol. Appl. Pharmacol. 2010, 246, 107–115. [Google Scholar] [CrossRef]
- Khan, M.M.; Poeckel, D.; Halavatyi, A.; Zukowska-Kasprzyk, J.; Stein, F.; Vappiani, J.; Sevin, D.C.; Tischer, C.; Zinn, N.; Eley, J.D.; et al. An integrated multiomic and quantitative label-free microscopy-based approach to study pro-fibrotic signalling in ex vivo human precision-cut lung slices. Eur. Respir. J. 2021, 58, 2000221. [Google Scholar] [CrossRef] [PubMed]
- Gerckens, M.; Alsafadi, H.N.; Wagner, D.E.; Lindner, M.; Burgstaller, G.; Königshoff, M. Generation of Human 3D Lung Tissue Cultures (3D-LTCs) for Disease Modeling. J. Vis. Exp. 2019, 144, e58437. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.Y.; Damiani, F.; Ram-Mohan, S.; Rodrigues, S.; Queiroz, P.D.M.; Donaghey, T.C.; Lichtenstein, J.H.R.; Brain, J.D.; Krishnan, R.; Molina, R.M. Screening for Chemical Toxicity Using Cryopreserved Precision Cut Lung Slices. Toxicol. Sci. 2015, 150, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Krishnamoorthy, N.; Patel, K.R.; Rosas, I.; Sanderson, M.J.; Ai, X. Cryopreserved Human Precision-Cut Lung Slices as a Bioassay for Live Tissue Banking. A Viability Study of Bronchodilation with Bitter-Taste Receptor Agonists. Am. J. Respir. Cell Mol. Biol. 2016, 54, 656–663. [Google Scholar] [CrossRef]
- Rosner, S.R.; Ram-Mohan, S.; Paez-Cortez, J.R.; Lavoie, T.L.; Dowell, M.L.; Yuan, L.; Ai, X.; Fine, A.; Aird, W.C.; Solway, J.; et al. Airway Contractility in the Precision-Cut Lung Slice after Cryopreservation. Am. J. Respir. Cell Mol. Biol. 2014, 50, 876–881. [Google Scholar] [CrossRef]
- Tigges, J.; Eggerbauer, F.; Worek, F.; Thiermann, H.; Rauen, U.; Wille, T. Optimization of long-term cold storage of rat precision-cut lung slices with a tissue preservation solution. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L1023–L1035. [Google Scholar] [CrossRef]
- Preuß, E.B.; Schubert, S.; Werlein, C.; Stark, H.; Braubach, P.; Höfer, A.; Plucinski, E.K.; Shah, H.R.; Geffers, R.; Sewald, K.; et al. The Challenge of Long-Term Cultivation of Human Precision-Cut Lung Slices. Am. J. Pathol. 2021, 192, 239–253. [Google Scholar] [CrossRef]
- Nußbaum, S.M.; Krabbe, J.; Böll, S.; Babendreyer, A.; Martin, C. Functional changes in long-term incubated rat precision-cut lung slices. Respir. Res. 2022, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.E.; Pino, C.; Lennon, M.L.; Lyons, A.; Jacot, J.G.; Lammers, S.R.; Königshoff, M.; Magin, C.M. Embedding of Precision-Cut Lung Slices in Engineered Hydrogel Biomaterials Supports Extended Ex Vivo Culture. Am. J. Respir. Cell Mol. Biol. 2020, 62, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef] [PubMed]
- Urso, A.; D’Ovidio, F.; Xu, D.; Emala, C.W.; Bunnett, N.W.; Perez-Zoghbi, J.F. Bile acids inhibit cholinergic constriction in proximal and peripheral airways from humans and rodents. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L264–L275. [Google Scholar] [CrossRef]
- Akram, K.M.; Yates, L.L.; Mongey, R.; Rothery, S.; Gaboriau, D.C.A.; Sanderson, J.; Hind, M.; Griffiths, M.; Dean, C.H. Live imaging of alveologenesis in precision-cut lung slices reveals dynamic epithelial cell behaviour. Nat. Commun. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Branchfield, K.; Li, R.; Lungova, V.; Verheyden, J.M.; McCulley, D.; Sun, X. A three-dimensional study of alveologenesis in mouse lung. Dev. Biol. 2016, 409, 429–441. [Google Scholar] [CrossRef]
- Hirani, D.; Alvira, C.M.; Danopoulos, S.; Milla, C.; Donato, M.; Tian, L.; Mohr, J.; Dinger, K.; Vohlen, C.; Selle, J.; et al. Macrophage-derived IL-6 trans-signalling as a novel target in the pathogenesis of bronchopulmonary dysplasia. Eur. Respir. J. 2021, 59, 2002248. [Google Scholar] [CrossRef]
- Viana, F.; O’Kane, C.M.; Schroeder, G.N. Precision-cut lung slices: A powerful ex vivo model to investigate respiratory infectious diseases. Mol. Microbiol. 2022, 117, 578–588. [Google Scholar] [CrossRef]
- Ebsen, M.; Mogilevski, G.; Anhenn, O.; Maiworm, V.; Theegarten, D.; Schwarze, J.; Morgenroth, K. Infection of murine precision cut lung slices (PCLS) with respiratory syncytial virus (RSV) and chlamydophila pneumoniae using the Krumdieck technique. Pathol Res. Pract. 2002, 198, 747–753. [Google Scholar] [CrossRef]
- Martin, C.; Uhlig, S.; Ullrich, V. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur. Respir. J. 1996, 9, 2479–2487. [Google Scholar] [CrossRef]
- Löfdahl, A.; Jern, A.; Flyman, S.; Kåredal, M.; Karlsson, H.L.; Larsson-Callerfelt, A.-K. Silver Nanoparticles Alter Cell Viability Ex Vivo and In Vitro and Induce Proinflammatory Effects in Human Lung Fibroblasts. Nanomaterials 2020, 10, 1868. [Google Scholar] [CrossRef] [PubMed]
- Dassow, C.; Wiechert, L.; Martin, C.; Schumann, S.; Müller-Newen, G.; Pack, O.; Guttmann, J.; Wall, W.A.; Uhlig, S. Biaxial distension of precision-cut lung slices. J. Appl. Physiol. 2010, 108, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Mathyssen, C.; Gayan-Ramirez, G.; Bouillon, R.; Janssens, W. Vitamin D supplementation in respiratory diseases: Evidence from randomized controlled trials. Pol. Arch. Intern. Med. 2017, 127, 775–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calvert, B.A. Application of iPSC to Modelling of Respiratory Diseases. Cell Biol. Transl. Med. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Brave, H.; MacLoughlin, R. State of the Art Review of Cell Therapy in the Treatment of Lung Disease, and the Potential for Aerosol Delivery. Int. J. Mol. Sci. 2020, 21, 6435. [Google Scholar] [CrossRef]
- Uzzaman, N.; Chan, S.C.; Shunmugam, R.H.; Engkasan, J.P.; Agarwal, D.; Habib, G.M.M.; Hanafi, N.S.; Jackson, T.; Jebaraj, P.; Khoo, E.M.; et al. Clinical effectiveness and components of Home-pulmonary rehabilitation for people with chronic respiratory diseases: A systematic review protocol. BMJ Open 2021, 11, e050362. [Google Scholar] [CrossRef]
- Kolbe, U.; Yi, B.; Poth, T.; Saunders, A.; Boutin, S.; Dalpke, A.H. Early Cytokine Induction Upon Pseudomonas aeruginosa Infection in Murine Precision Cut Lung Slices Depends on Sensing of Bacterial Viability. Front. Immunol. 2020, 11, 598636. [Google Scholar] [CrossRef]
- Ordway, D.; Henao-Tamayo, M.; Smith, E.; Shanley, C.; Harton, M.; Troudt, J.; Bai, X.; Basaraba, R.J.; Orme, I.M.; Chan, E.D. Animal model of Mycobacterium abscessus lung infection. J. Leukoc. Biol. 2008, 83, 1502–1511. [Google Scholar] [CrossRef]
- Brann, K.R.; Fullerton, M.S.; Onyilagha, F.I.; Prince, A.A.; Kurten, R.C.; Rom, J.S.; Blevins, J.S.; Smeltzer, M.S.; Voth, D.E. Infection of Primary Human Alveolar Macrophages Alters Staphylococcus aureus Toxin Production and Activity. Infect. Immun. 2019, 87, e00167-9. [Google Scholar] [CrossRef]
- Banerjee, S.; Huckuntod, S.D.; Mills, S.D.; Kurten, R.C.; Pechous, R.D. Modeling Pneumonic Plague in Human Precision-Cut Lung Slices Highlights a Role for the Plasminogen Activator Protease in Facilitating Type 3 Secretion. Infect. Immun. 2019, 87, e00175-19. [Google Scholar] [CrossRef]
- Hesse, C.; Sewald, K.; Jonigk, D.; Warnecke, G.; Fieguth, H.-G.; de Maat, S.; Maas, C.; Bonella, F.; Preissner, K.T.; Weiss, B.; et al. Coagulation factor XII regulates inflammatory responses in human lungs. Thromb. Haemost. 2017, 117, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Danov, O.; Lasswitz, L.; Obernolte, H.; Hesse, C.; Braun, A.; Wronski, S.; Sewald, K. Rupintrivir reduces RV-induced TH-2 cytokine IL-4 in precision-cut lung slices (PCLS) of HDM-sensitized mice ex vivo. Respir. Res. 2019, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Booth, J.L.; Duggan, E.S.; Patel, K.B.; Coggeshall, K.M.; Metcalf, J.P. Human lung innate immune cytokine response to adenovirus type 7. J. Gen. Virol. 2010, 91, 1155–1163. [Google Scholar] [CrossRef]
- Limkar, A.; Percopo, C.; Redes, J.; Druey, K.; Rosenberg, H. Persistent Airway Hyperresponsiveness Following Recovery from Infection with Pneumonia Virus of Mice. Viruses 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Danov, O.; Delgado, S.M.J.; Obernolte, H.; Seehase, S.; Dehmel, S.; Braubach, P.; Fieguth, H.-G.; Matschiner, G.; Fitzgerald, M.; Jonigk, D.; et al. Human lung tissue provides highly relevant data about efficacy of new anti-asthmatic drugs. PLoS ONE 2018, 13, e0207767. [Google Scholar] [CrossRef]
- Corrigendum. Am. J. Physiol Lung Cell. Mol. Physiol 2020, 318, L844. [CrossRef] [PubMed]
- Bui, C.B.; Kolodziej, M.; Lamanna, E.; Elgass, K.; Sehgal, A.; Rudloff, I.; Schwenke, D.O.; Tsuchimochi, H.; Kroon, M.A.G.M.; Cho, S.X.; et al. Interleukin-1 Receptor Antagonist Protects Newborn Mice Against Pulmonary Hypertension. Front. Immunol. 2019, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Bläsche, R.; Kasper, M.; Barth, K. A Co-Culture System with an Organotypic Lung Slice and an Immortal Alveolar Macrophage Cell Line to Quantify Silica-Induced Inflammation. PLoS ONE 2015, 10, e0117056. [Google Scholar] [CrossRef] [PubMed]
- Rittchen, S.; Jandl, K.; Lanz, I.; Reiter, B.; Ferreirós, N.; Kratz, D.; Lindenmann, J.; Brcic, L.; Bärnthaler, T.; Atallah, R.; et al. Monocytes and Macrophages Serve as Potent Prostaglandin D2 Sources during Acute, Non-Allergic Pulmonary Inflammation. Int. J. Mol. Sci. 2021, 22, 11697. [Google Scholar] [CrossRef]
- Dragan, A.L.; Voth, D.E. Take my breath away: Studying pathogen invasion of the human lung using primary tissue models. Pathog. Dis. 2021, 79, ftab016. [Google Scholar] [CrossRef] [PubMed]
- Molina-Torres, C.A.; Flores-Castillo, O.N.; Carranza-Torres, I.E.; Guzman-Delgado, N.E.; Viveros-Valdez, E.; Vera-Cabrera, L.; Ocampo-Candiani, J.; Verde-Star, J.; Castro-Garza, J.; Carranza-Rosales, P. Ex vivo infection of murine precision-cut lung tissue slices with Mycobacterium abscessus: A model to study antimycobacterial agents. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 52. [Google Scholar] [CrossRef]
- Wu, M.-L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.D.; Katoch, V.M. Animal models of tuberculosis. Tuberculosis 2005, 85, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Mongey, R.; Wang, P.; Rothery, S.; Gaboriau, D.C.; Hind, M.; Griffiths, M.; Dean, C.H. The acid injury and repair (AIR) model: A novel ex-vivo tool to understand lung repair. Biomaterials 2021, 267, 120480. [Google Scholar] [CrossRef]
- Meduri, G.U.; Annane, D.; Chrousos, G.P.; Marik, P.E.; Sinclair, S.E. Activation and Regulation of Systemic Inflammation in ARDS: Rationale for prolonged glucocorticoid therapy. Chest 2009, 136, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Jandl, K.; Stacher, E.; Bálint, Z.; Sturm, E.M.; Maric, J.; Peinhaupt, M.; Luschnig, P.; Aringer, I.; Fauland, A.; Konya, V.; et al. Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J. Allergy Clin. Immunol. 2016, 137, 833–843. [Google Scholar] [CrossRef]
- Rausch, S.M.K.; Haberthür, D.; Stampanoni, M.; Schittny, J.C.; Wall, W.A. Local Strain Distribution in Real Three-Dimensional Alveolar Geometries. Ann. Biomed. Eng. 2011, 39, 2835–2843. [Google Scholar] [CrossRef]
- Shi, Z.; Ye, W.; Zhang, J.; Zhang, F.; Yu, D.; Yu, H.; Chen, B.; Zhou, M.; Sun, H. LipoxinA4 attenuates acute pancreatitis-associated acute lung injury by regulating AQP-5 and MMP-9 expression, anti-apoptosis and PKC/SSeCKS-mediated F-actin activation. Mol. Immunol. 2018, 103, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef]
- McVey, M.J.; Kapur, R.; Cserti-Gazdewich, C.; Semple, J.W.; Karkouti, K.; Kuebler, W.M. Transfusion-related Acute Lung Injury in the Perioperative Patient. Anesthesiology 2019, 131, 693–715. [Google Scholar] [CrossRef]
- Hodinka, R.L. Respiratory RNA Viruses. Microbiol. Spectr. 2016, 4, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Clementi, N.; Ghosh, S.; De Santis, M.; Castelli, M.; Criscuolo, E.; Zanoni, I.; Clementi, M.; Mancini, N. Viral Respiratory Pathogens and Lung Injury. Clin. Microbiol. Rev. 2021, 34, e00103-20. [Google Scholar] [CrossRef] [PubMed]
- Goris, K.; Uhlenbruck, S.; Schwegmann-Wessels, C.; Köhl, W.; Niedorf, F.; Stern, M.; Hewicker-Trautwein, M.; Bals, R.; Taylor, G.; Braun, A.; et al. Differential Sensitivity of Differentiated Epithelial Cells to Respiratory Viruses Reveals Different Viral Strategies of Host Infection. J. Virol. 2009, 83, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Reamon-Buettner, S.M.; Niehof, M.; Hirth, N.; Danov, O.; Obernolte, H.; Braun, A.; Warnecke, J.; Sewald, K.; Wronski, S. Transcriptomic Analysis Reveals Priming of The Host Antiviral Interferon Signaling Pathway by Bronchobini® Resulting in Balanced Immune Response to Rhinovirus Infection in Mouse Lung Tissue Slices. Int. J. Mol. Sci. 2019, 20, 2242. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R.M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 2020, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, N.; Wayne, G.; Birchall, M.; Song, W. Recent advances in human respiratory epithelium models for drug discovery. Biotechnol. Adv. 2022, 54, 107832. [Google Scholar] [CrossRef]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef]
- Kling, M.A. A review of respiratory system anatomy, physiology, and disease in the mouse, rat, hamster, and gerbil. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 287–337. [Google Scholar] [CrossRef]
- Wright, J.L.; Cosio, M.; Churg, A. Animal models of chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L1–L15. [Google Scholar] [CrossRef]
- Rackley, C.R.; Stripp, B.R. Building and maintaining the epithelium of the lung. J. Clin. Investig. 2012, 122, 2724–2730. [Google Scholar] [CrossRef]
- Plopper, C.G.; Hill, L.H.; Mariassy, A.T. Ultrastructure of the Nonciliated Bronchiolar Epithelial (Clara) Cell of Mammalian Lung. III. A Study of Man with Comparison of 15 Mammalian Species. Exp. Lung Res. 1980, 1, 171–180. [Google Scholar] [CrossRef]
- Fricker, M.; Deane, A.; Hansbro, P.M. Animal models of chronic obstructive pulmonary disease. Expert Opin. Drug Discov. 2014, 9, 629–645. [Google Scholar] [CrossRef]
- Jones, B.; Donovan, C.; Liu, G.; Gomez, H.M.; Chimankar, V.; Harrison, C.L.; Wiegman, C.H.; Adcock, I.M.; Knight, D.A.; Hirota, J.A.; et al. Animal models of COPD: What do they tell us? Respirology 2017, 22, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, E.M.; Culha, S.; Menzen, M.H.; Bidan, C.M.; Gosens, R. Elastase-Induced Parenchymal Disruption and Airway Hyper Responsiveness in Mouse Precision Cut Lung Slices: Toward an Ex vivo COPD Model. Front. Physiol. 2016, 7, 657. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, H.; Bidan, C.M.; Brook, B.S.; Zuidhof, A.B.; Elzinga, C.R.; Smit, M.; Oldenburger, A.; Gosens, R.; Timens, W.; Meurs, H. Small airway hyperresponsiveness in COPD: Relationship between structure and function in lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L537–L546. [Google Scholar] [CrossRef]
- Temann, A.; Golovina, T.; Neuhaus, V.; Thompson, C.; Chichester, J.A.; Braun, A.; Yusibov, V. Evaluation of inflammatory and immune responses in long-term cultured human precision-cut lung slices. Hum. Vaccines Immunother. 2017, 13, 351–358. [Google Scholar] [CrossRef]
- Cardet, J.C.; Bulkhi, A.A.; Lockey, R.F. Nonrespiratory Comorbidities in Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 3887–3897. [Google Scholar] [CrossRef]
- Bedard, A.; Li, Z.; Ait-Hadad, W.; Camargo, C.A., Jr.; Leynaert, B.; Pison, C.; Dumas, O.; Varraso, R. The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. Int J. Environ. Res. Public Health 2021, 18, 3013. [Google Scholar] [CrossRef]
- Lauenstein, L.; Switalla, S.; Prenzler, F.; Seehase, S.; Pfennig, O.; Forster, C.; Fieguth, H.; Braun, A.; Sewald, K. Assessment of immunotoxicity induced by chemicals in human precision-cut lung slices (PCLS). Toxicol. In Vitro 2014, 28, 588–599. [Google Scholar] [CrossRef]
- Li, G.; Cohen, J.A.; Martines, C.; Ram-Mohan, S.; Brain, J.D.; Krishnan, R.; Ai, X.; Bai, Y. Preserving Airway Smooth Muscle Contraction in Precision-Cut Lung Slices. Sci. Rep. 2020, 10, 6480. [Google Scholar] [CrossRef]
- Donovan, C.; Bailey, S.R.; Tran, J.; Haitsma, G.; Ibrahim, Z.A.; Foster, S.; Tang, M.L.K.; Royce, S.G.; Bourke, J.E. Rosiglitazone elicits in vitro relaxation in airways and precision cut lung slices from a mouse model of chronic allergic airways disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1219–L1228. [Google Scholar] [CrossRef]
- Parikh, V.; Scala, J.; Patel, R.; Corbi, C.; Lo, D.; Bochkov, Y.A.; Kennedy, J.L.; Kurten, R.C.; Liggett, S.B.; Gern, J.E.; et al. Rhinovirus C15 Induces Airway Hyperresponsiveness via Calcium Mobilization in Airway Smooth Muscle. Am. J. Respir. Cell Mol. Biol. 2020, 62, 310–318. [Google Scholar] [CrossRef]
- Ram-Mohan, S.; Bai, Y.; Schaible, N.; Ehrlicher, A.J.; Cook, D.P.; Suki, B.; Stoltz, D.A.; Solway, J.; Ai, X.; Krishnan, R. Tissue traction microscopy to quantify muscle contraction within precision-cut lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L323–L330. [Google Scholar] [CrossRef]
- Ågren, L.; Elfsmark, L.; Akfur, C.; Jonasson, S. High concentrations of ammonia induced cytotoxicity and bronchoconstriction in a precision-cut lung slices rat model. Toxicol. Lett. 2021, 349, 51–60. [Google Scholar] [CrossRef]
- Pybus, H.J.; Tatler, A.L.; Edgar, L.T.; O’Dea, R.D.; Brook, B.S. Reduced biomechanical models for precision-cut lung-slice stretching experiments. J. Math. Biol. 2021, 82, 35. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Bai, Y.; Ai, X. Utilizing the Precision-Cut Lung Slice to Study the Contractile Regulation of Airway and Intrapulmonary Arterial Smooth Muscle. J. Vis. Exp. 2022, 183, e63932. [Google Scholar] [CrossRef]
- Wright, J.L.; Zhou, S.; Preobrazhenska, O.; Marshall, C.; Sin, D.D.; Laher, I.; Golbidi, S.; Churg, A.M. Statin Reverses Smoke-induced Pulmonary Hypertension and Prevents Emphysema but Not Airway Remodeling. Am. J. Respir. Crit. Care Med. 2011, 183, 50–58. [Google Scholar] [CrossRef]
- Suleiman, S.; Klassen, S.; Katz, I.; Balakirski, G.; Krabbe, J.; Von Stillfried, S.; Kintsler, S.; Braunschweig, T.; Babendreyer, A.; Spillner, J.; et al. Argon reduces the pulmonary vascular tone in rats and humans by GABA-receptor activation. Sci. Rep. 2019, 9, 1902. [Google Scholar] [CrossRef]
- Hoy, R.F.; Chambers, D.C. Silica-related diseases in the modern world. Allergy 2020, 75, 2805–2817. [Google Scholar] [CrossRef]
- Du, S.; Li, C.; Lu, Y.; Lei, X.; Zhang, Y.; Li, S.; Liu, F.; Chen, Y.; Weng, D.; Chen, J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics 2019, 9, 1878–1892. [Google Scholar] [CrossRef]

| Types of Respiratory Inflammatory Diseases | Method of Preparing the Model | Human/Mouse Lung Slices | Inflammatory Response Evaluation Index | References | |
|---|---|---|---|---|---|
| Bacterialinfection inflammation | Pseudomonas aeruginosa | PCLSs were treated with 5 μg/mL ultrapure Pseudomonas aeruginosa-derived flagellin or 0.1 μg/mL ultrapure LPS derived from Salmonella R595 | Mouse | Both the chemokines KC and MIP-2 are involved in the early innate immune response by recruiting neutrophils. Dendritic cells release IL1β, IL12, TNF-α, IL-23, IL-6, and IL-8 | [47] |
| Mycobacterium abscess | 250 μL DMEM/F12 complete medium was added to PCLSs in a 24-well microplate and inoculated with 1.5 × 10 CFU/plate of M. abscess virulent strain L948 (ATCC 19977) | Mouse | Multinucleated cells, epithelioid cells, foamy macrophages, multinucleated giant cells, and early granulomas | [48] | |
| Staphylococcus aureus | Staphylococcus aureus cultured in trypsin soybean broth (TSB) lacking sodium pyruvate was incubated with hPCLSs | Human | Large amounts of IL-6 and TNF-α and IL-8 are secreted by human alveolar macrophages | [49] | |
| Yersinia pestis | 105 or 107 CO92 wild-type strains or CO92 Yersinia pestis strains were infected with hPCLSs in 48-well plates | Human | Alveolar macrophages are the main host cell targets in the early post-infection period, and hPCLSs infection leads to increased levels of TNF-α, IL-6 and IL-8 | [50] | |
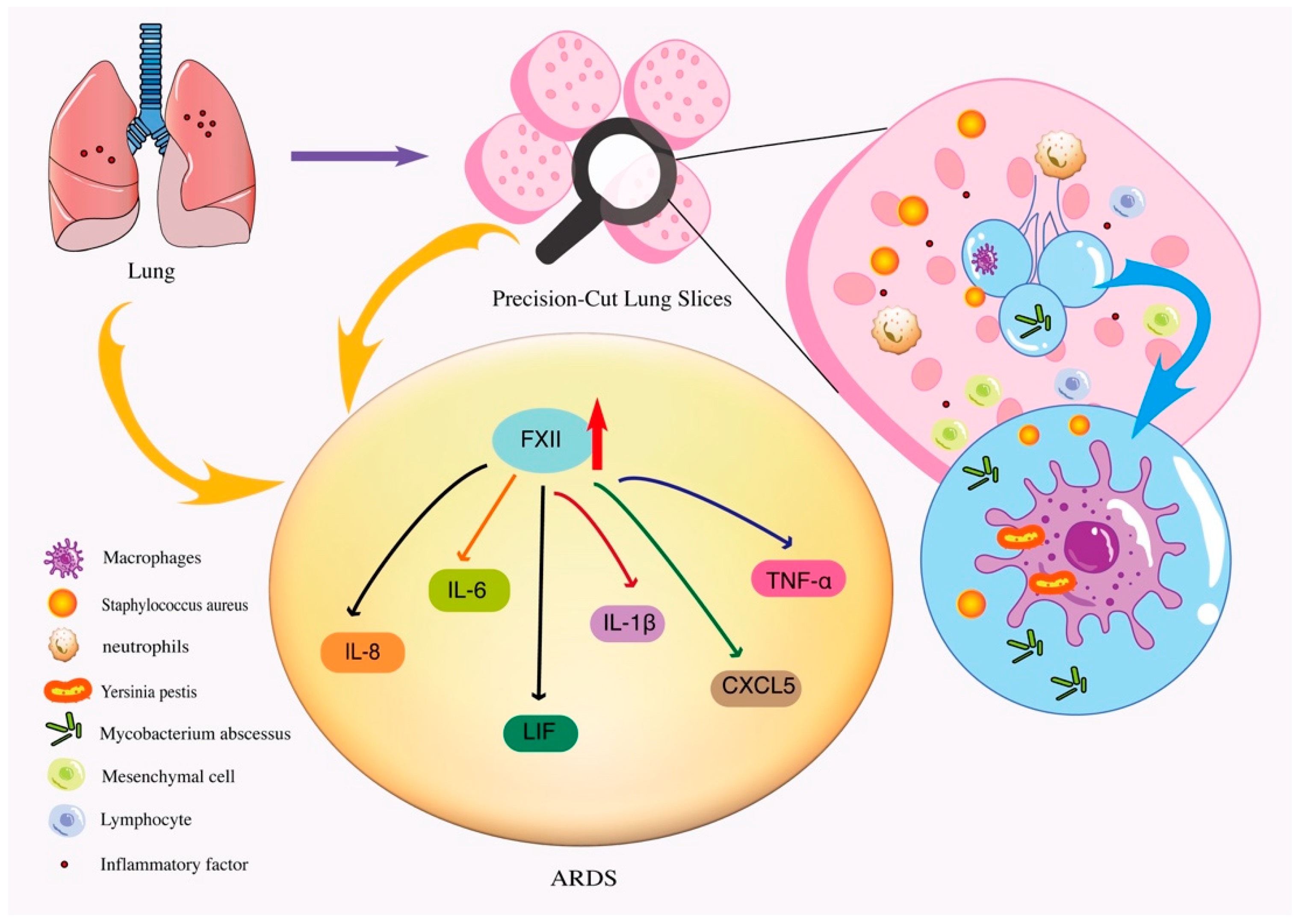
| ARDS | PCLSs were exposed to FXII or FXIIa, and the expression of inflammatory mediators was assessed using cytokine/chemokine PCR arrays. | Human | Recruitment of neutrophils and macrophages to the injury/infection site initiates the production of inflammatory mediators such as TNF-α, IL-1β, IL-6 and IL-8 | [51] | |
| Respiratory viral infection | Rhinovirus (RV) | Infect PCLSs with 250 µL of 2 × 105 IU/mL RV, UV-inactivated RV at 33 °C, the optimal replication temperature for RV | Mouse | Increased levels of IFN-α, IFN-β, IFN-γ, MCP-1, TNF-α, IP-10, IL-6, IL-10, and IL-17A were observed on PCLSs | [52] |
| Adenovirus (Ad) | Lung slices in separate wells were exposed to 1 × 109 pfu/well Ad7 and incubated for 8 h at 37 °C, 5% CO2 | Human | Neutrophil chemokine IL-8 is mainly derived from epithelial cells, while monocyte and lymphocyte chemokine IP-10 is derived from macrophages and epithelial cells | [53] | |
| Pneumonia virus (PVM) | Intranasal inoculation of 50 µL of mouse pneumonia virus (PVM) strain J3666 and the lungs were harvested to make PCLSs | Mouse | neutrophils recruited to the lung and airway were detected in PCLSs and inflammatory cytokines IL-6, CCL2, and CXCL10were detected in BALF | [54] | |
| Chronic Obstructive Pulmonary Disease (COPD) | Lung slices of mice induced with exogenous elastase to make a COPD model | Mouse | Elastase leads to increased secretion of IL-6, MCP-1, and KC on PCLSs | [20] | |
| Asthma | PCLSs is obtained from the lung lobes of patients undergoing lobectomy for cancer. | Human | IL-13 induced release of proinflammatory cytokines eotaxin-3 and TARC in hPCLS. | [55] | |
| Idiopathic fibrosis (IPF) | A combination of pro-fibrotic growth factors and signaling molecules was used to construct an early fibrosis model in PCLSs from patients without ILD/IPF. | Human | Pro-inflammatory factor IL1B is upregulated on PCLSs | [56] | |
| pulmonary arterial hypertension (PAH) | C57BL/6J mice were intraperitoneally injected with 150 μg/kg lipopolysaccharide (LPS) on the 14th day of gestation, and lung tissue was taken for biopsy on the 28th day | Mouse | The interleukin-1 receptor antagonist down-regulates the pro-inflammatory factor IL-1β | [57] | |
| Silicosis | A co-culture system of alveolar epithelial cells and macrophages was prepared in PCLSs | Mouse | Macrophages secrete IL-6, TNF-α, and IL-1β | [58] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, P.; Wang, Y.; Liu, Y.; Yang, H.; Zhou, G.; Wu, X.; Wen, Q. Application of Precision-Cut Lung Slices as an In Vitro Model for Research of Inflammatory Respiratory Diseases. Bioengineering 2022, 9, 767. https://doi.org/10.3390/bioengineering9120767
Liu Y, Wu P, Wang Y, Liu Y, Yang H, Zhou G, Wu X, Wen Q. Application of Precision-Cut Lung Slices as an In Vitro Model for Research of Inflammatory Respiratory Diseases. Bioengineering. 2022; 9(12):767. https://doi.org/10.3390/bioengineering9120767
Chicago/Turabian StyleLiu, Yan, Ping Wu, Yin Wang, Yansong Liu, Hongfang Yang, Guohua Zhou, Xiaoqi Wu, and Qingping Wen. 2022. "Application of Precision-Cut Lung Slices as an In Vitro Model for Research of Inflammatory Respiratory Diseases" Bioengineering 9, no. 12: 767. https://doi.org/10.3390/bioengineering9120767
APA StyleLiu, Y., Wu, P., Wang, Y., Liu, Y., Yang, H., Zhou, G., Wu, X., & Wen, Q. (2022). Application of Precision-Cut Lung Slices as an In Vitro Model for Research of Inflammatory Respiratory Diseases. Bioengineering, 9(12), 767. https://doi.org/10.3390/bioengineering9120767








