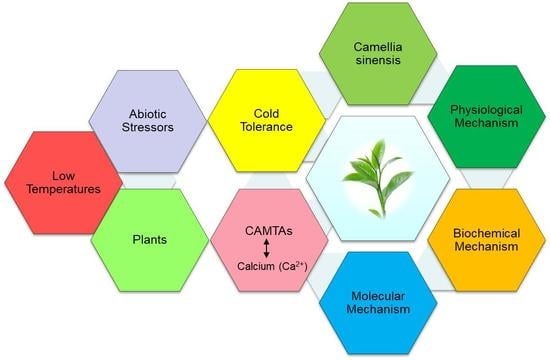
The Role of Calmodulin Binding Transcription Activator in Plants under Different Stressors: Physiological, Biochemical, Molecular Mechanisms of Camellia sinensis and Its Current Progress of CAMTAs
Abstract
1. Background and Importance of Camellia sinensis
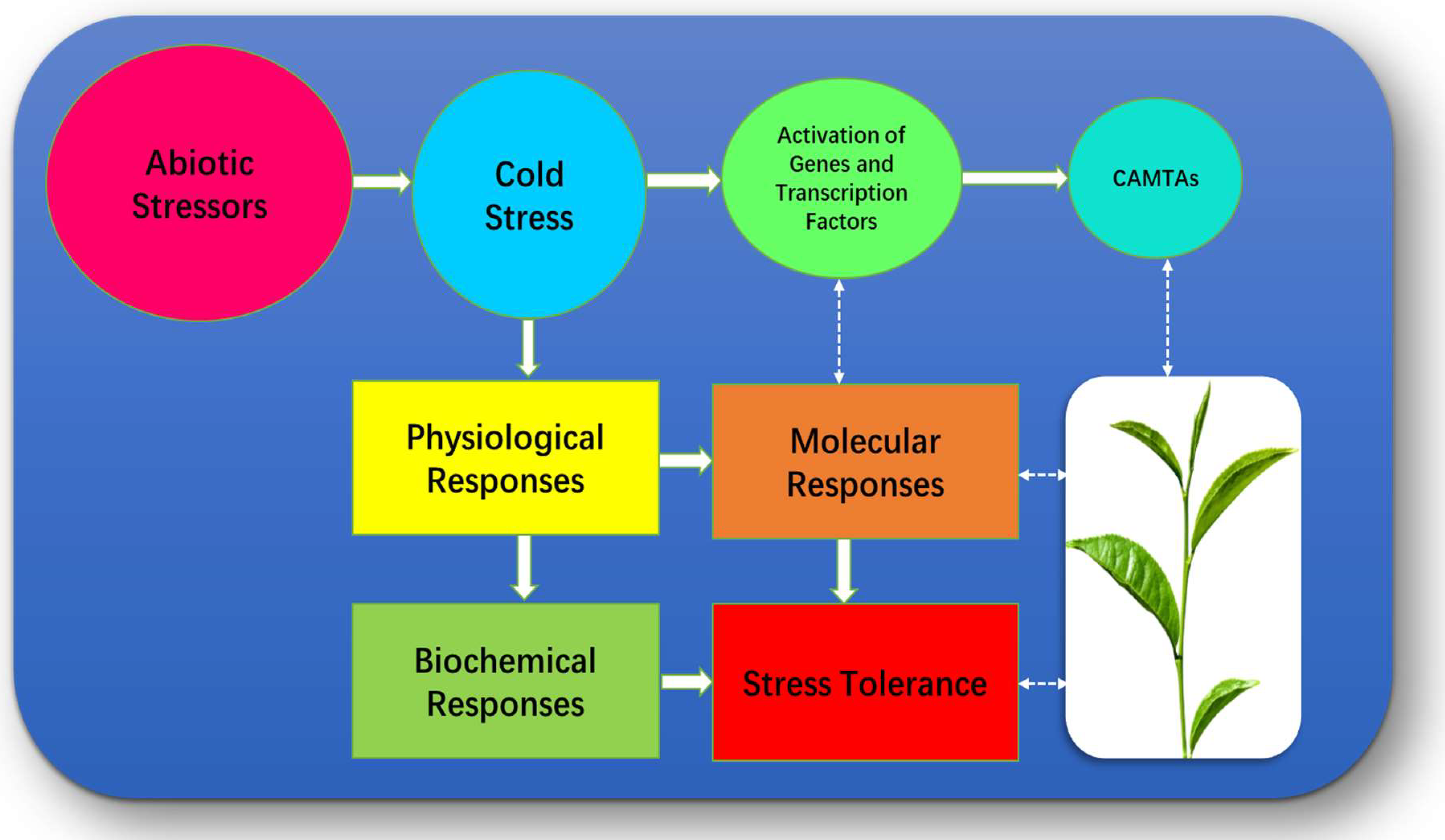
2. Camellia sinensis under Low Temperatures Stressors
3. Physiological Mechanisms Associated with Camellia sinensis
4. Biochemical Mechanisms Associated with Camellia sinensis
5. Molecular Mechanisms Associated with Camellia sinensis
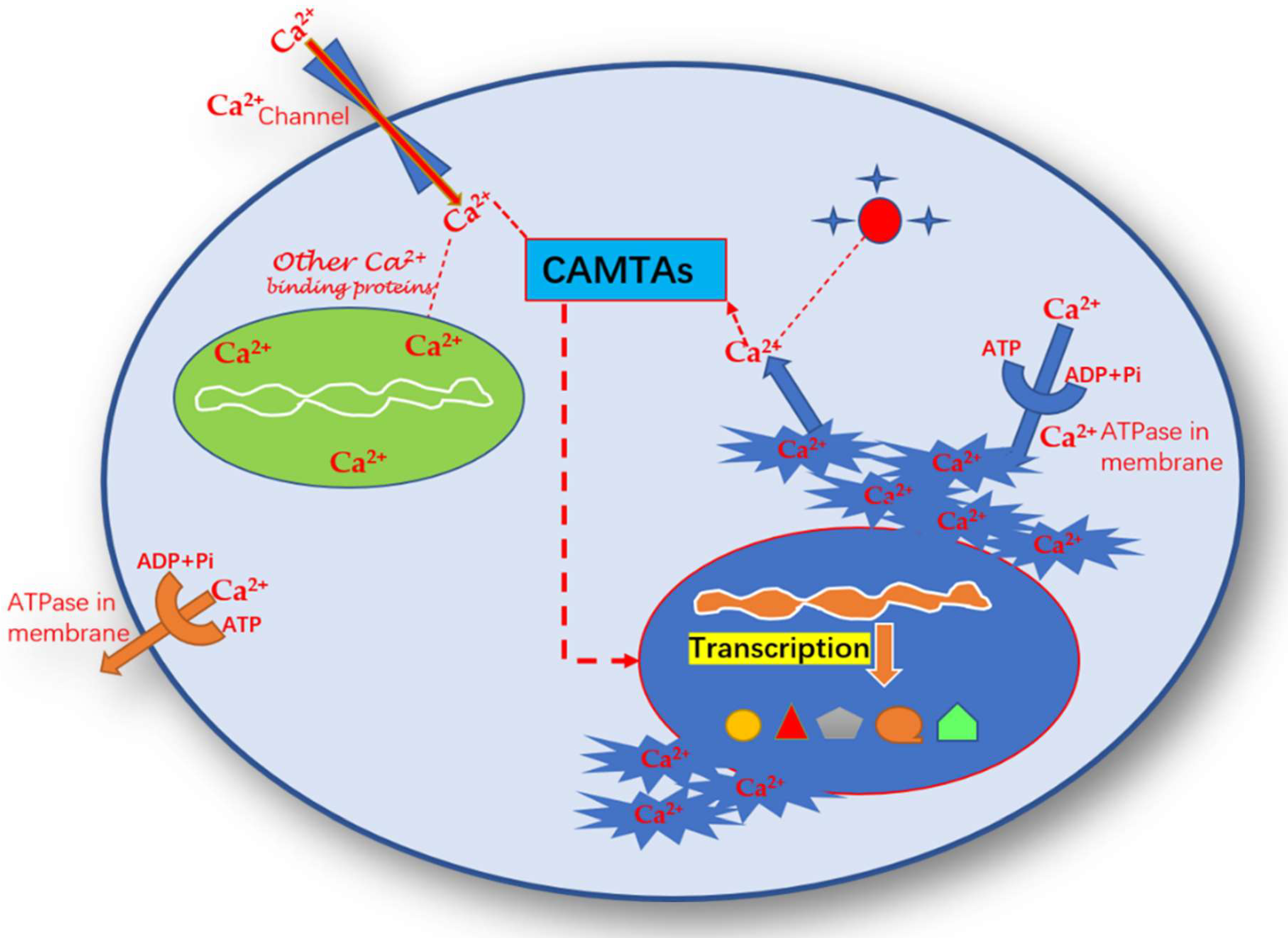
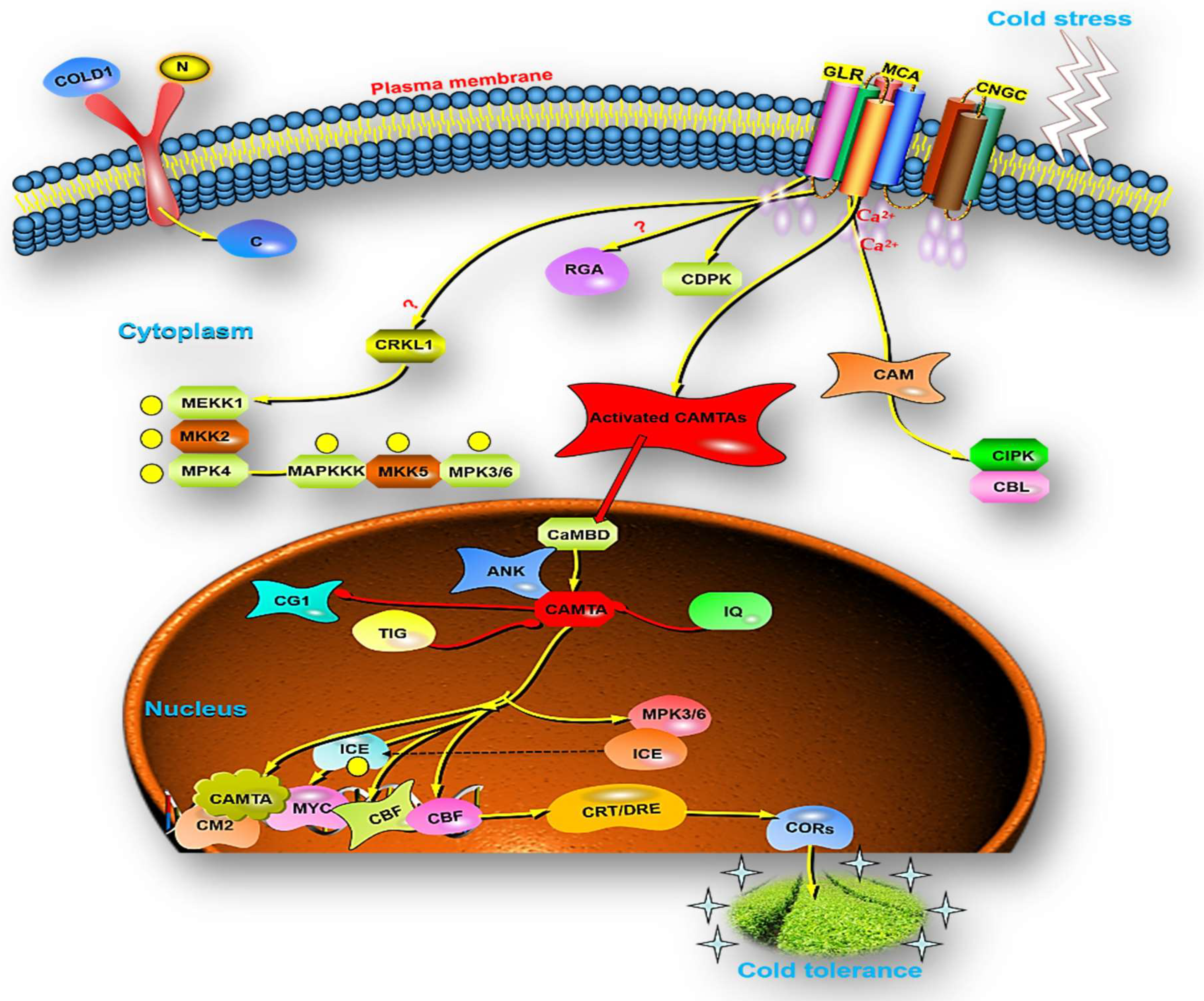
6. Role of Calmodulin Binding Transcription Activator in Plants
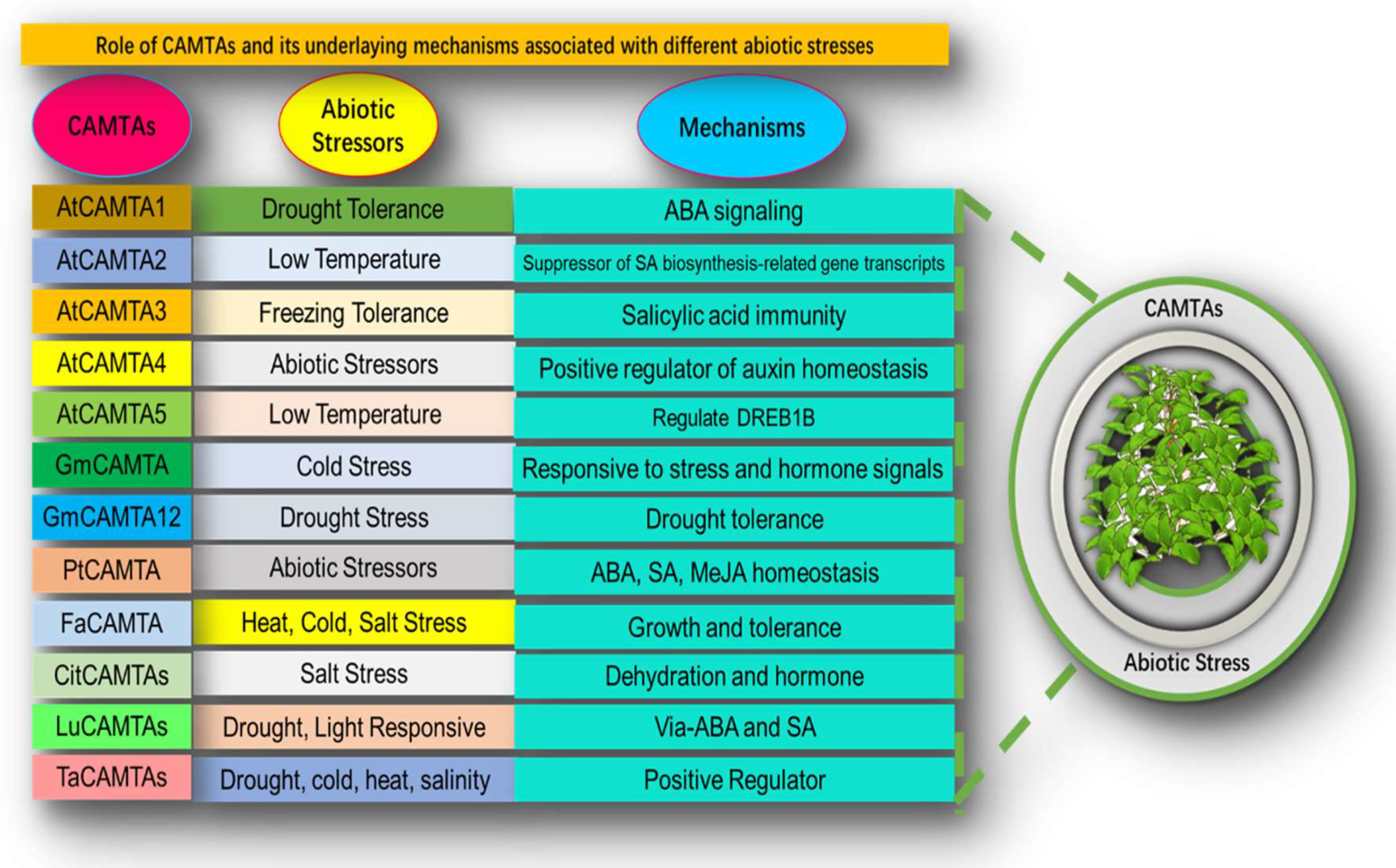
7. Role of Calmodulin Binding Transcription Activator under Different Stressors
8. Current Progress of CAMTAs in Camellia sinensis
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations Statistics Division (FAOSTAT). 2017. Available online: https://www.fao.org/statistics/en/ (accessed on 10 October 2022).
- Weisburger, J.H. Approaches for Chronic Disease Prevention Based on Current Understanding of Underlying Mechanisms. Am. J. Clin. Nutr. 2000, 71, 1710S–1714S. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Physiological and Defense Responses of Tea Plants to Elevated CO2: A Review. Front. Plant Sci. 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, C.; Sun, C.; Wu, L.; Wu, T. Disaster-Causing Hazard Division and Evaluation of Meteorological Disasters for Tea in Fujian Province. J. Nat. Disaster 2018, 27, 198–207. [Google Scholar]
- Yan, Y.; Jeong, S.; Park, C.-E.; Mueller, N.D.; Piao, S.; Park, H.; Joo, J.; Chen, X.; Wang, X.; Liu, J.; et al. Effects of Extreme Temperature on China’s Tea Production. Environ. Res. Lett. 2021, 16, 044040. [Google Scholar] [CrossRef]
- Finkler, A.; Ashery-Padan, R.; Fromm, H. CAMTAs: Calmodulin-Binding Transcription Activators from Plants to Human. FEBS Lett. 2007, 581, 3893–3898. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, J.; Xiang, Z.; Xi, P.; Zhao, D. Physiological Changes and Differential Gene Expression of Tea Plants (Camellia Sinensis (L.) Kuntze Var. Niaowangensis Q.H. Chen) Under Cold Stress. DNA Cell Biol. 2021, 40, 906–920. [Google Scholar] [CrossRef]
- Muoki, C.R.; Maritim, T.K.; Oluoch, W.A.; Kamunya, S.M.; Bore, J.K. Combating Climate Change in the Kenyan Tea Industry. Front. Plant Sci. 2020, 11, 339. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Lin, S.K.; Lin, J.; Liu, Q.L.; Ai, Y.F.; Ke, Y.Q.; Chen, C.; Zhang, Z.Y.; He, H. Time-Course of Photosynthesis and Non-Structural Carbon Compounds in the Leaves of Tea Plants (Camellia Sinensis L.) in Response to Deficit Irrigation. Agric. Water Manag. 2014, 144, 98–106. [Google Scholar] [CrossRef]
- Maritim, T.K.; Kamunya, S.M.; Mireji, P.; Mwendia, C.; Muoki, R.C.; Cheruiyot, E.K.; Wachira, F.N. Physiological and Biochemical Response of Tea [Camellia Sinensis (L.) O. Kuntze] to Water-Deficit Stress. J. Hortic. Sci. Biotechnol. 2015, 90, 395–400. [Google Scholar] [CrossRef]
- Zaman, S.; Hu, S.; Alam, M.A.; Du, H.; Che, S. The Accumulation of Fatty Acids in Different Organs of Purslane under Salt Stress. Sci. Hortic. 2019, 250, 236–242. [Google Scholar] [CrossRef]
- Zaman, S.; Shen, J.; Wang, S.; Wang, Y.; Ding, Z.; Song, D.; Wang, H.; Ding, S.; Pang, X.; Wang, M. Effects of Shading Nets on Reactive Oxygen Species Accumulation, Photosynthetic Changes, and Associated Physiochemical Attributes in Promoting Cold-Induced Damage in Camellia Sinensis (L.) Kuntze. Horticulturae 2022, 8, 637. [Google Scholar] [CrossRef]
- He, G.; Liu, X.; Cui, Z. Achieving global food security by focusing on nitrogen efficiency potentials and local production. Glob. Food Secur. 2021, 29, 100536. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Ding, Z.; Zhao, L. Global Transcriptional Analysis Reveals the Complex Relationship between Tea Quality, Leaf Senescence and the Responses to Cold-Drought Combined Stress in Camellia Sinensis. Front. Plant Sci. 2016, 7, 1858. [Google Scholar] [CrossRef]
- Silva, O.D.C.E. CG-1, a Parsley Light-Induced DNA-Binding Protein. Plant Mol. Biol. 1994, 25, 921–924. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. A Calmodulin-Binding/CGCG Box DNA-Binding Protein Family Involved in Multiple Signaling Pathways in Plants. J. Biol. Chem. 2002, 277, 45049–45058. [Google Scholar] [CrossRef]
- Li, B.; He, S.; Zheng, Y.; Wang, Y.; Lang, X.; Wang, H.; Fan, K.; Hu, J.; Ding, Z.; Qian, W. Genome-Wide Identification and Expression Analysis of the Calmodulin-Binding Transcription Activator (CAMTA) Family Genes in Tea Plant. BMC Genom. 2022, 23, 667. [Google Scholar] [CrossRef]
- Bouché, N.; Yellin, A.; Snedden, W.A.; Fromm, H. Plant-Specific Calmodulin-Binding Proteins. Annu. Rev. Plant Biol. 2005, 56, 435–466. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. An Early Ethylene Up-Regulated Gene Encoding a Calmodulin-Binding Protein Involved in Plant Senescence and Death. J. Biol. Chem. 2000, 275, 38467–38473. [Google Scholar] [CrossRef]
- Pant, P.; Iqbal, Z.; Pandey, B.K.; Sawant, S.V. Genome-Wide Comparative and Evolutionary Analysis of Calmodulin-Binding Transcription Activator (CAMTA) Family in Gossypium Species. Sci. Rep. 2018, 8, 5573. [Google Scholar] [CrossRef]
- Khan, I.; Abbas, T.; Anjum, K.; Abbas, S.Q.; Shagufta, B.I.; Ali Shah, S.A.; Akhter, N. Antimicrobial Potential of Aqueous Extract of Camellia Sinensis against Representative Microbes. Pak. J. Pharm. Sci. 2019, 32, 631–636. [Google Scholar] [PubMed]
- Wang, G.; Zeng, H.; Hu, X.; Zhu, Y.; Chen, Y.; Shen, C.; Wang, H.; Poovaiah, B.W.; Du, L. Identification and Expression Analyses of Calmodulin-Binding Transcription Activator Genes in Soybean. Plant Soil 2015, 386, 205–221. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, T.; Xu, L.; Pi, E.; Wang, S.; Wang, H.; Shen, C. Genome-Wide Identification of CAMTA Gene Family Members in Medicago Truncatula and Their Expression during Root Nodule Symbiosis and Hormone Treatments. Front. Plant Sci. 2015, 6, 459. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Han, J.; Wang, X.; Zhao, M.; Sun, X.; Wang, C.; Fang, J. Characterization of a Calmodulin-Binding Transcription Factor from Strawberry (Fragaria × Ananassa). Plant Genome 2015, 8, plantgenome2014-08. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Lu, C.; Sun, T.; Peng, T.; Han, X.; Qi, J.; Yan, S.; Tie, S. Identification and Expression Profiling Analysis of Calmodulin-Binding Transcription Activator Genes in Maize (Zea Mays L.) under Abiotic and Biotic Stresses. Front. Plant Sci. 2015, 6, 576. [Google Scholar] [CrossRef]
- Shangguan, L.; Wang, X.; Leng, X.; Liu, D.; Ren, G.; Tao, R.; Zhang, C.; Fang, J. Identification and Bioinformatic Analysis of Signal Responsive/Calmodulin-Binding Transcription Activators Gene Models in Vitis Vinifera. Mol. Biol. Rep. 2014, 41, 2937–2949. [Google Scholar] [CrossRef]
- Galon, Y.; Nave, R.; Boyce, J.M.; Nachmias, D.; Knight, M.R.; Fromm, H. Calmodulin-Binding Transcription Activator (CAMTA) 3 Mediates Biotic Defense Responses in Arabidopsis. FEBS Lett. 2008, 582, 943–948. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA Transcription Factors in Cold-Regulated Gene Expression and Freezing Tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef]
- Pandey, N.; Ranjan, A.; Pant, P.; Tripathi, R.K.; Ateek, F.; Pandey, H.P.; Patre, U.V.; Sawant, S.V. CAMTA 1 Regulates Drought Responses in Arabidopsis Thaliana. BMC Genom. 2013, 14, 216. [Google Scholar] [CrossRef]
- Ul Hassan, S.S.; Muhammad, I.; Abbas, S.Q.; Hassan, M.; Majid, M.; Jin, H.Z.; Bungau, S. Stress Driven Discovery of Natural Products from Actinobacteria with Anti-Oxidant and Cytotoxic Activities Including Docking and Admet Properties. Int. J. Mol. Sci. 2021, 22, 11432. [Google Scholar] [CrossRef]
- Hassan, S.S.u.; Jin, H.Z.; Abu-Izneid, T.; Rauf, A.; Ishaq, M.; Suleria, H.A. Stress-Driven Discovery in the Natural Products: A Gateway towards New Drugs. Biomed. Pharmacother. 2019, 109, 459–4677. [Google Scholar] [CrossRef]
- Shams, S.; Abbas, S.Q.; Muhammad, I.; Wu, J.; Yan, S.; Ali, F.; Majid, M.; Jin, H.; Bungau, S. Metals-Triggered Compound CDPDP Exhibits Anti-Arthritic Behavior by Downregulating the in Fl Ammatory Cytokines, and Modulating the Oxidative Storm in Mice Models with Extensive ADMET, Docking and Simulation Studies. Front. Pharmacol. 2022, 13, 1053744. [Google Scholar] [CrossRef]
- Hassan, S.S.; Shah, S.A.A.; Pan, C.; Fu, L.; Cao, X.; Shi, Y.; Wu, X.; Wang, K.; Wu, B. Production of an Antibiotic Enterocin from a Marine Actinobacteria Strain H1003 by Metal-Stress Technique with Enhanced Enrichment Using Response Surface Methodology. Pak. J. Pharm. Sci. 2017, 30, 313–324. [Google Scholar]
- Wang, X.-C.; Zhao, Q.-Y.; Ma, C.-L.; Zhang, Z.-H.; Cao, H.-L.; Kong, Y.-M.; Yue, C.; Hao, X.-Y.; Chen, L.; Ma, J.-Q.; et al. Global Transcriptome Profiles of Camellia Sinensis during Cold Acclimation. BMC Genom. 2013, 14, 415. [Google Scholar] [CrossRef]
- Yang, F.; Dong, F.; Hu, F.; Liu, Y.; Chai, J.; Zhao, H.; Lv, M.; Zhou, S. Genome-Wide Identification and Expression Analysis of the Calmodulin-Binding Transcription Activator (CAMTA) Gene Family in Wheat (Triticum Aestivum L.). BMC Genet. 2020, 21, 105. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium Signaling Network in Plants. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Ali, E.; Raza, M.A.; Cai, M.; Hussain, N.; Shahzad, A.N.; Hussain, M.; Ali, M.; Bukhari, S.A.H.; Sun, P. Calmodulin-Binding Transcription Activator (CAMTA) Genes Family: Genome-Wide Survey and Phylogenetic Analysis in Flax (Linum Usitatissimum). PLoS ONE 2020, 15, e0236454. [Google Scholar] [CrossRef]
- Kim, M.C.; Chung, W.S.; Yun, D.-J.; Cho, M.J. Calcium and Calmodulin-Mediated Regulation of Gene Expression in Plants. Mol. Plant 2009, 2, 13–21. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-Dependent Regulation of Photosynthesis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 993–1003. [Google Scholar] [CrossRef]
- Teige, M. Chloroplasts Use Calcium Signals to Call for Help under Heat Stress. Plant Cell Physiol. 2019, 60, 492–493. [Google Scholar] [CrossRef] [PubMed]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium Signaling and Salt Tolerance Are Diversely Entwined in Plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Chan, Z. Comparative Proteomic and Metabolomic Analyses Reveal Mechanisms of Improved Cold Stress Tolerance in Bermudagrass (Cynodon Dactylon (L.) Pers.) by Exogenous Calcium. J. Integr. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.-P. Calcium Signalling in Plant Biotic Interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant Signaling Networks Involving Ca2+ and Rboh/Nox-Mediated ROS Production under Salinity Stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef]
- Liu, Z.; Ying, H.; Chen, M.; Bai, J.; Xue, Y.; Yin, Y.; Batchelor, W.D.; Yang, Y.; Bai, Z.; Du, M.; et al. Optimization of China’s Maize and Soy Production Can Ensure Feed Sufficiency at Lower Nitrogen and Carbon Footprints. Nat. Food 2021, 2, 426–433. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Buatois, L.A.; Mángano, M.G. Potential and Problems in Evaluating Secular Changes in the Diversity of Animal-Substrate Interactions at Ichnospecies Rank. Terra Nova 2022, 34, 433–440. [Google Scholar] [CrossRef]
- Abbasi, F.; Onodera, H.; Toki, S.; Tanaka, H.; Komatsu, S. OsCDPK13, a Calcium-Dependent Protein Kinase Gene from Rice, Is Induced by Cold and Gibberellin in Rice Leaf Sheath. Plant Mol. Biol. 2004, 55, 541–552. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Chandar, S.S.; Rao, K.V.B. Protease Inhibitors from Marine Actinobacteria as a Potential Source for Antimalarial Compound. PLoS ONE 2014, 9, e90972. [Google Scholar] [CrossRef]
- Galon, Y.; Snir, O.; Fromm, H. How Calmodulin Binding Transcription Activators (CAMTAs) Mediate Auxin Responses. Plant Signal. Behav. 2010, 5, 1311–1314. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA Transcription Factors and Salicylic Acid in Configuring the Low-Temperature Transcriptome and Freezing Tolerance of Arabidopsis. Plant J. 2013, 75, 364–376. [Google Scholar] [CrossRef]
- Kidokoro, S.; Yoneda, K.; Takasaki, H.; Takahashi, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Different Cold-Signaling Pathways Function in the Responses to Rapid and Gradual Decreases in Temperature. Plant Cell 2017, 29, 760–774. [Google Scholar] [CrossRef]
- Rachmi, C.N.; Agho, K.E.; Li, M.; Baur, L.A. Stunting, Underweight and Overweight in Children Aged 2.0–4.9 Years in Indonesia: Prevalence Trends and Associated Risk Factors. PLoS ONE 2016, 11, e0154756. [Google Scholar] [CrossRef]
- Weckwerth, P.; Ehlert, B.; Romeis, T. ZmCPK1, a Calcium-Independent Kinase Member of the Zea Mays CDPK Gene Family, Functions as a Negative Regulator in Cold Stress Signalling. Plant Cell Environ. 2015, 38, 544–558. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Aleynova, O.A.; Manyakhin, A.Y.; Kiselev, K.V. The Role of Calcium-Dependent Protein Kinase Genes CPK16, CPK25, CPK30, and CPK32 in Stilbene Biosynthesis and the Stress Resistance of Grapevine Vitis Amurensis Rupr. Appl. Biochem. Microbiol. 2018, 54, 410–417. [Google Scholar] [CrossRef]
- Li, M.; Hu, W.; Ren, L.; Jia, C.; Liu, J.; Miao, H.; Guo, A.; Xu, B.; Jin, Z. Identification, Expression, and Interaction Network Analyses of the CDPK Gene Family Reveal Their Involvement in the Development, Ripening, and Abiotic Stress Response in Banana. Biochem. Genet. 2020, 58, 40–62. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Zhu, Y.; Zhang, L.; Chen, T.; Zhou, F.; Chen, H.; Lin, Y. The Calcium-Dependent Kinase OsCPK24 Functions in Cold Stress Responses in Rice. J. Integr. Plant Biol. 2018, 60, 173–188. [Google Scholar] [CrossRef]
- Mo, C.; Wan, S.; Xia, Y.; Ren, N.; Zhou, Y.; Jiang, X. Expression Patterns and Identified Protein-Protein Interactions Suggest That Cassava CBL-CIPK Signal Networks Function in Responses to Abiotic Stresses. Front. Plant Sci. 2018, 9, 269. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Kiselev, K.V.; Ogneva, Z.V.; Dubrovina, A.S. The Grapevine Calmodulin-like Protein Gene CML21 Is Regulated by Alternative Splicing and Involved in Abiotic Stress Response. Int. J. Mol. Sci. 2020, 21, 7939. [Google Scholar] [CrossRef]
- Tang, M.; Xu, C.; Cao, H.; Shi, Y.; Chen, J.; Chai, Y.; Li, Z. Tomato Calmodulin-like Protein SlCML37 Is a Calcium (Ca2+) Sensor That Interacts with Proteasome Maturation Factor SlUMP1 and Plays a Role in Tomato Fruit Chilling Stress Tolerance. J. Plant Physiol. 2021, 258–259, 153373. [Google Scholar] [CrossRef]
- Xu, L.; Zahid, K.R.; He, L.; Zhang, W.; He, X.; Zhang, X.; Yang, X.; Zhu, L. GhCAX3 Gene, a Novel Ca2+/H+ Exchanger from Cotton, Confers Regulation of Cold Response and ABA Induced Signal Transduction. PLoS ONE 2013, 8, e66303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ding, Y.; Shi, Y.; Ma, L.; Wang, Y.; Song, C.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The Calcium Transporter ANNEXIN1 Mediates Cold-Induced Calcium Signaling and Freezing Tolerance in Plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 Mediate Cold Acclimation-Induced Chilling Tolerance by Regulating Apoplastic H2O2 Production and Redox Homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, L.; Wei, J.; Chen, Q.; Yang, Y.; Hu, X.; Kong, X. The Glutamate Receptors AtGLR1.2 and AtGLR1.3 Increase Cold Tolerance by Regulating Jasmonate Signaling in Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2018, 506, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Yao, L.; Ding, C.; Lei, L.; Hao, X.; Li, N.; Zeng, J.; Yang, Y.; Wang, X. Characterization of CBL–CIPK Signaling Complexes and Their Involvement in Cold Response in Tea Plant. Plant Physiol. Biochem. 2020, 154, 195–203. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.; Zhu, S.; Wan, H.; Yang, Y.; et al. Transcriptional Activation and Phosphorylation of OsCNGC9 Confer Enhanced Chilling Tolerance in Rice. Mol. Plant 2021, 14, 315–329. [Google Scholar] [CrossRef]
- Catalá, R.; Santos, E.; Alonso, J.M.; Ecker, J.R.; Martínez-Zapater, J.M.; Salinas, J. Mutations in the Ca2+/H+ Transporter CAX1 Increase CBF/DREB1 Expression and the Cold-Acclimation Response in Arabidopsis. Plant Cell 2003, 15, 2940–2951. [Google Scholar] [CrossRef]
- Su, W.; Zhang, C.; Wang, D.; Ren, Y.; Sun, T.; Feng, J.; Su, Y.; Xu, L.; Shi, M.; Que, Y. The CaCA Superfamily Genes in Saccharum: Comparative Analysis and Their Functional Implications in Response to Biotic and Abiotic Stress. BMC Genom. 2021, 22, 549. [Google Scholar] [CrossRef]
- Weiland, M.; Mancuso, S.; Baluska, F.; Weiland, M.; Mancuso, S.; Baluska, F. Signalling via Glutamate and GLRs in Arabidopsis Thaliana. Funct. Plant Biol. 2015, 43, 1–25. [Google Scholar] [CrossRef]
- Shkolnik, D.; Finkler, A.; Pasmanik-Chor, M.; Fromm, H. Calmodulin-Binding Transcription Activator 6: A Key Regulator of Na+ Homeostasis during Germination. Plant Physiol. 2019, 180, 1101–1118. [Google Scholar] [CrossRef]
- Wang, C.; Shang, J.-X.; Chen, Q.-X.; Oses-Prieto, J.A.; Bai, M.-Y.; Yang, Y.; Yuan, M.; Zhang, Y.-L.; Mu, C.-C.; Deng, Z.; et al. Identification of BZR1-Interacting Proteins as Potential Components of the Brassinosteroid Signaling Pathway in Arabidopsis Through Tandem Affinity Purification. Mol. Cell. Proteom. 2013, 12, 3653–3665. [Google Scholar] [CrossRef]
- Kim, Y.S.; An, C.; Park, S.; Gilmour, S.J.; Wang, L.; Renna, L.; Brandizzi, F.; Grumet, R.; Thomashow, M.F. CAMTA-Mediated Regulation of Salicylic Acid Immunity Pathway Genes in Arabidopsis Exposed to Low Temperature and Pathogen Infection. Plant Cell 2017, 29, 2465–2477. [Google Scholar] [CrossRef]
- Beck, M.; Zhou, J.; Faulkner, C.; MacLean, D.; Robatzek, S. Spatio-Temporal Cellular Dynamics of the Arabidopsis Flagellin Receptor Reveal Activation Status-Dependent Endosomal Sorting. Plant Cell 2012, 24, 4205–4219. [Google Scholar] [CrossRef]
- Kakar, K.U.; Nawaz, Z.; Cui, Z.; Cao, P.; Jin, J.; Shu, Q.; Ren, X. Evolutionary and Expression Analysis of CAMTA Gene Family in Nicotiana Tabacum Yielded Insights into Their Origin, Expansion and Stress Responses. Sci. Rep. 2018, 8, 10322. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, F.; Zhou, H.; Liu, N.; Niu, X.; Yan, C.; Zhang, L.; Han, S.; Hou, C.; Wang, D. TaCAMTA4, a Calmodulin-Interacting Protein, Involved in Defense Response of Wheat to Puccinia Triticina. Sci. Rep. 2019, 9, 641. [Google Scholar] [CrossRef]
- Rahman, H.; Xu, Y.-P.; Zhang, X.-R.; Cai, X.-Z. Brassica Napus Genome Possesses Extraordinary High Number of CAMTA Genes and CAMTA3 Contributes to PAMP Triggered Immunity and Resistance to Sclerotinia Sclerotiorum. Front. Plant Sci. 2016, 7, 581. [Google Scholar] [CrossRef]
- Noman, M.; Jameel, A.; Qiang, W.-D.; Ahmad, N.; Liu, W.-C.; Wang, F.-W.; Li, H.-Y. Overexpression of GmCAMTA12 Enhanced Drought Tolerance in Arabidopsis and Soybean. Int. J. Mol. Sci. 2019, 20, 4849. [Google Scholar] [CrossRef]
- Sun, T.; Huang, J.; Xu, Y.; Verma, V.; Jing, B.; Sun, Y.; Ruiz Orduna, A.; Tian, H.; Huang, X.; Xia, S.; et al. Redundant CAMTA Transcription Factors Negatively Regulate the Biosynthesis of Salicylic Acid and N-Hydroxypipecolic Acid by Modulating the Expression of SARD1 and CBP60g. Mol. Plant 2020, 13, 144–156. [Google Scholar] [CrossRef]
- Laluk, K.; Prasad, K.V.S.K.; Savchenko, T.; Celesnik, H.; Dehesh, K.; Levy, M.; Mitchell-Olds, T.; Reddy, A.S.N. The Calmodulin-Binding Transcription Factor SIGNAL RESPONSIVE1 Is a Novel Regulator of Glucosinolate Metabolism and Herbivory Tolerance in Arabidopsis. Plant Cell Physiol. 2012, 53, 2008–2015. [Google Scholar] [CrossRef]
- Tokizawa, M.; Kobayashi, Y.; Saito, T.; Kobayashi, M.; Iuchi, S.; Nomoto, M.; Tada, Y.; Yamamoto, Y.Y.; Koyama, H. Sensitive to proton rhizotoxicity1, calmodulin binding transcription activator2, and Other Transcription Factors Are Involved in Aluminum-Activated Malate Transporter1 Expression. Plant Physiol. 2015, 167, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhao, M.; Xing, F.; Mao, G.; Wang, Y.; Dai, Y.; Niu, M.; Yuan, H. Identification and Expression Analysis of CAMTA Genes in Tea Plant Reveal Their Complex Regulatory Role in Stress Responses. Front. Plant Sci. 2022, 13, 910768. [Google Scholar] [CrossRef] [PubMed]

| Gene | Ca2+ Component | Species | References |
|---|---|---|---|
| CAMTA5 | Transcription factor | A. thaliana | [53] |
| MdCPK1a | Ca2+sensor | M. domestica | [54] |
| ZmCPK1 | Ca2+sensor | Z. mays | [55] |
| VaCPK30 | Ca2+sensor | V. amurensis | [56] |
| MaCDPK7 | Ca2+sensor | M. acuminata | [57] |
| OsCPK24 | Ca2+sensor | O. sativa | [58] |
| MeCIPK7 | Ca2+sensor | M. esculenta | [59] |
| MsCML46 | Ca2+sensor | M. sativa | [32] |
| CML21v1, | Ca2+sensor | V. amurensis | [60] |
| SlCML37 | Ca2+sensor | S. lycopersicum | [61] |
| GhCAX3 | Ca2+ channel | G. hirsutum | [62] |
| ANNEXIN1 | Ca2+ channel | A. thaliana | [63] |
| GLR3.5 | Ca2+ channel | L. lycopersicum | [64] |
| AtGLR1.3 | Ca2+ channel | A. thaliana | [65] |
| AtCNGC4 | Ca2+ channel | A. thaliana | [66] |
| ZjCNGC2 | Ca2+ channel | Z. jujuba | [67] |
| CNGC9 | Ca2+ channel | O. sativa | [68] |
| AtCAX1 | Ca2+ channel | A. thaliana | [69] |
| Ca2C/cation antiporter | Ca2+ channel | Saccharum spp. | [70] |
| AtGLR3.4 | Ca2+ channel | A. thaliana | [71] |
| CAMTA3 | Transcription factor | A. thaliana | [29] |
| Gene Names | Role | Species | Reference |
|---|---|---|---|
| CAMTA | Biotic/abiotic stress | P. trichocarpa | [10] |
| TaCAMTAs | Drought, cold, heat, and salinity | T. aestivum | [36] |
| FaCAMTA | Heat, cold, and salt stress | F. ananassa | [13] |
| ZmCAMTA | Biotic/abiotic stress tolerance | Z. mays | [14] |
| VvCAMTA1 | Ca2+ signal transduction | V. vinifera | [15] |
| GmCAMTA | Responsive to stress and hormone signals | G. max | [11] |
| LuCAMTAs | ABA, SA, drought, low temperature and light responsive | L. usitatissimum | [39] |
| NtabCAMTAs | Drought, cold, cadmium, and black shank stress | N. tabacum | [76] |
| TaCAMTA4 | Negative regulator of defense response against P. triticina | T. aestivum | [77] |
| BnCAMTA | Stress-inducible and phytohormonal regulation | B. napus | [78] |
| AtCAMTA6 | Na+ homeostasis in seed germination | A. thaliana | [72] |
| AtCAMTA5 | BZR1-associated protein; BR signaling | N. benthamiana | [35] |
| AtCAMTA4 | Positive regulator of auxin homeostasis | A. thaliana | [51] |
| GmCAMTA12 | Drought tolerance | [79] | |
| AtCAMTA1 | Drought tolerance via ABA signaling | A. thaliana | [30] |
| AtCAMTA2 | Suppressor of SA biosynthesis-related gene transcripts | A. thaliana | [80] |
| AtCAMTA3 | Plant defenses against insect herbivory via SA-JA crosstalk | A. thaliana | [81] |
| AtCAMTA5 | BZR1-associated protein; BR signaling | N. benthamiana | [73] |
| CAMTA1 | Cold acclimatization | A. thaliana | [29] |
| CAMTA2 | Activator of AtALMT1 (metal toxicity) | A. thaliana | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, S.; Hassan, S.S.u.; Ding, Z. The Role of Calmodulin Binding Transcription Activator in Plants under Different Stressors: Physiological, Biochemical, Molecular Mechanisms of Camellia sinensis and Its Current Progress of CAMTAs. Bioengineering 2022, 9, 759. https://doi.org/10.3390/bioengineering9120759
Zaman S, Hassan SSu, Ding Z. The Role of Calmodulin Binding Transcription Activator in Plants under Different Stressors: Physiological, Biochemical, Molecular Mechanisms of Camellia sinensis and Its Current Progress of CAMTAs. Bioengineering. 2022; 9(12):759. https://doi.org/10.3390/bioengineering9120759
Chicago/Turabian StyleZaman, Shah, Syed Shams ul Hassan, and Zhaotang Ding. 2022. "The Role of Calmodulin Binding Transcription Activator in Plants under Different Stressors: Physiological, Biochemical, Molecular Mechanisms of Camellia sinensis and Its Current Progress of CAMTAs" Bioengineering 9, no. 12: 759. https://doi.org/10.3390/bioengineering9120759
APA StyleZaman, S., Hassan, S. S. u., & Ding, Z. (2022). The Role of Calmodulin Binding Transcription Activator in Plants under Different Stressors: Physiological, Biochemical, Molecular Mechanisms of Camellia sinensis and Its Current Progress of CAMTAs. Bioengineering, 9(12), 759. https://doi.org/10.3390/bioengineering9120759