Effects of Electrical Pulse Width and Output Irradiance on Intense Pulse Light Inactivation
Abstract
1. Introduction
2. Materials and Methods
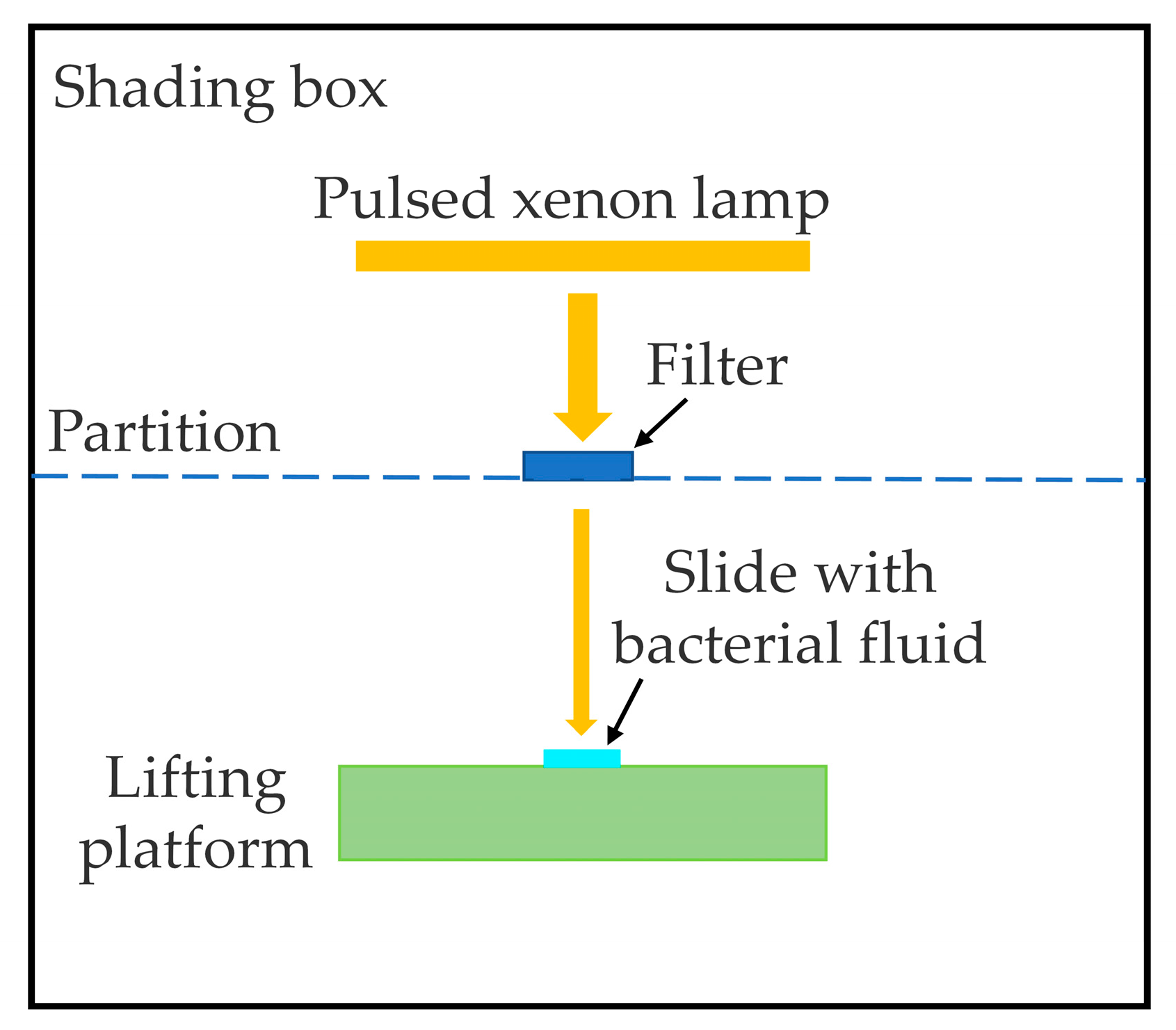
2.1. Experimental Setup
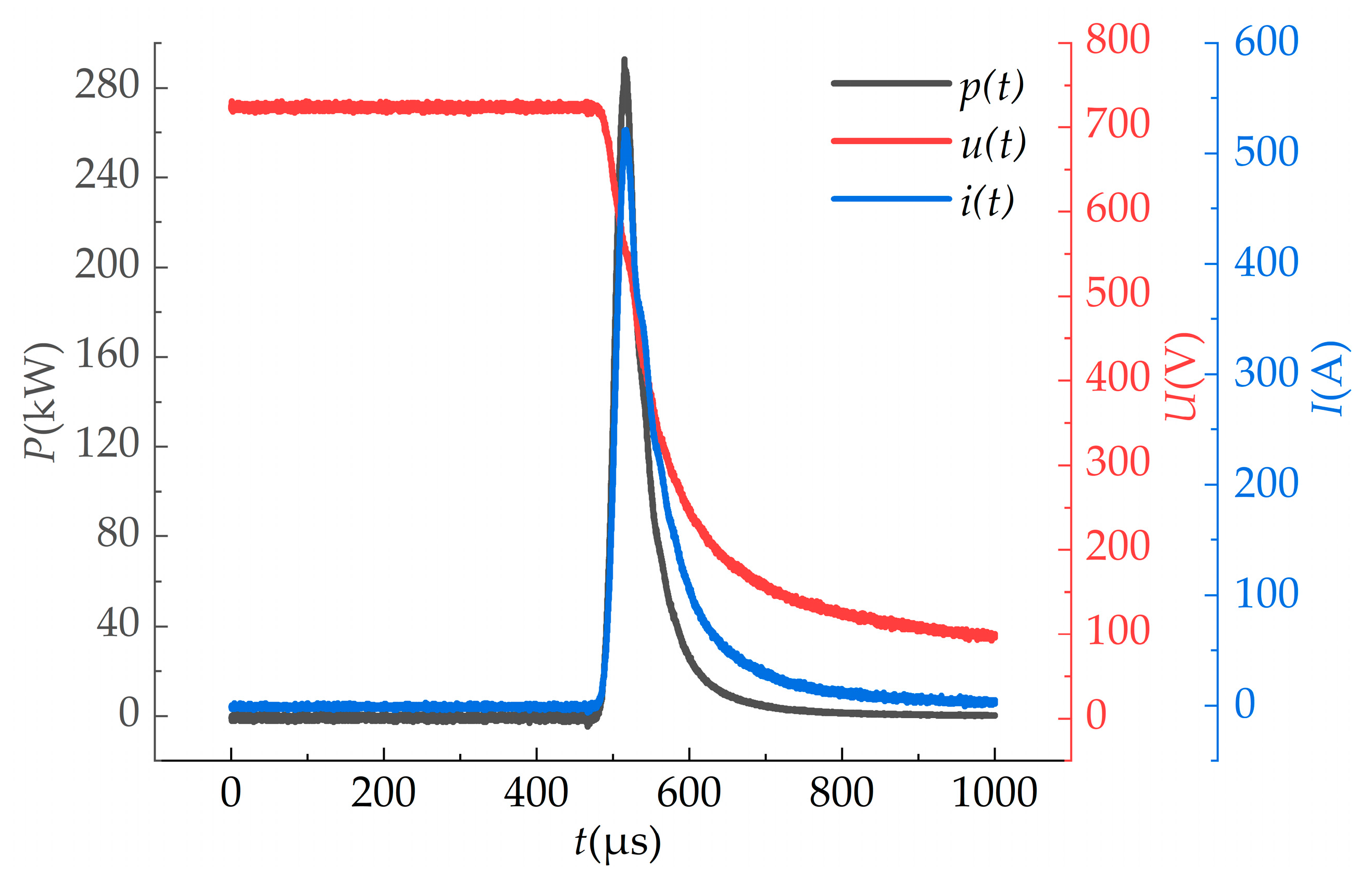
2.2. Calculation of Photoelectric Parameters
2.3. Inactivation Experimental Setup
2.4. Inactivation Experiment Process
3. Results and Discussion
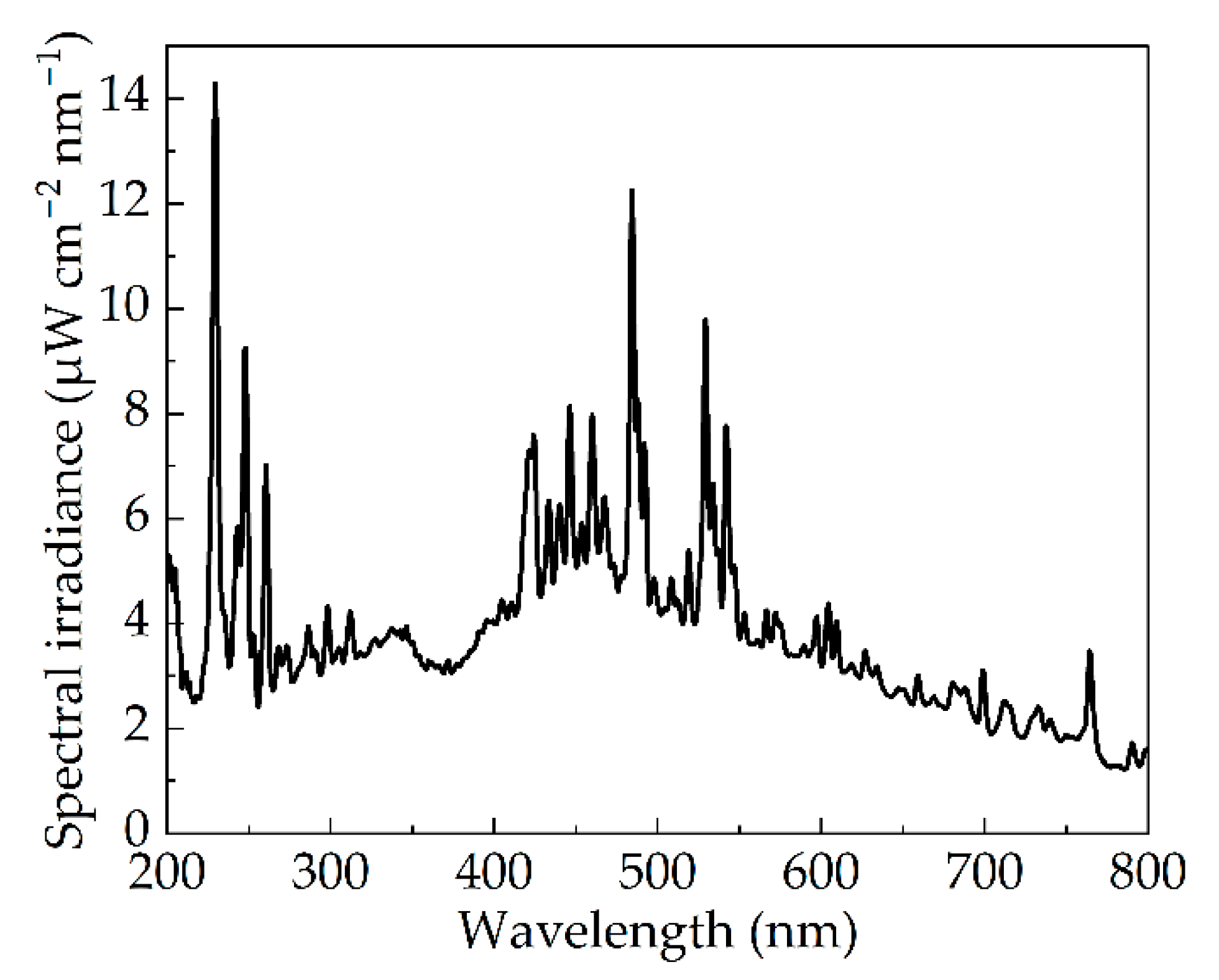
3.1. Photoelectric Parameters of Pulsed Xenon Lamp
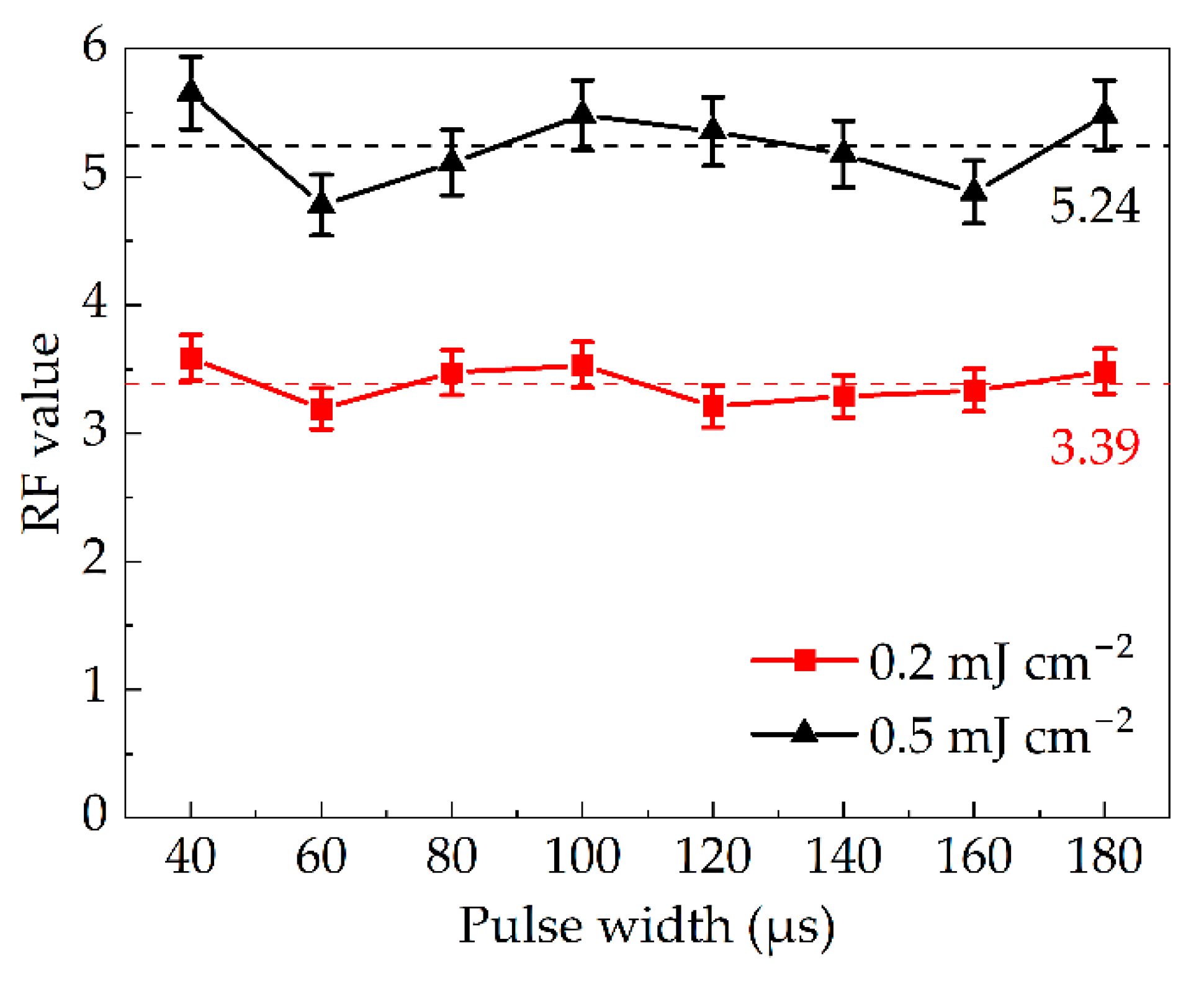
3.2. Effect of Pulse Width on Inactivation Efficiency
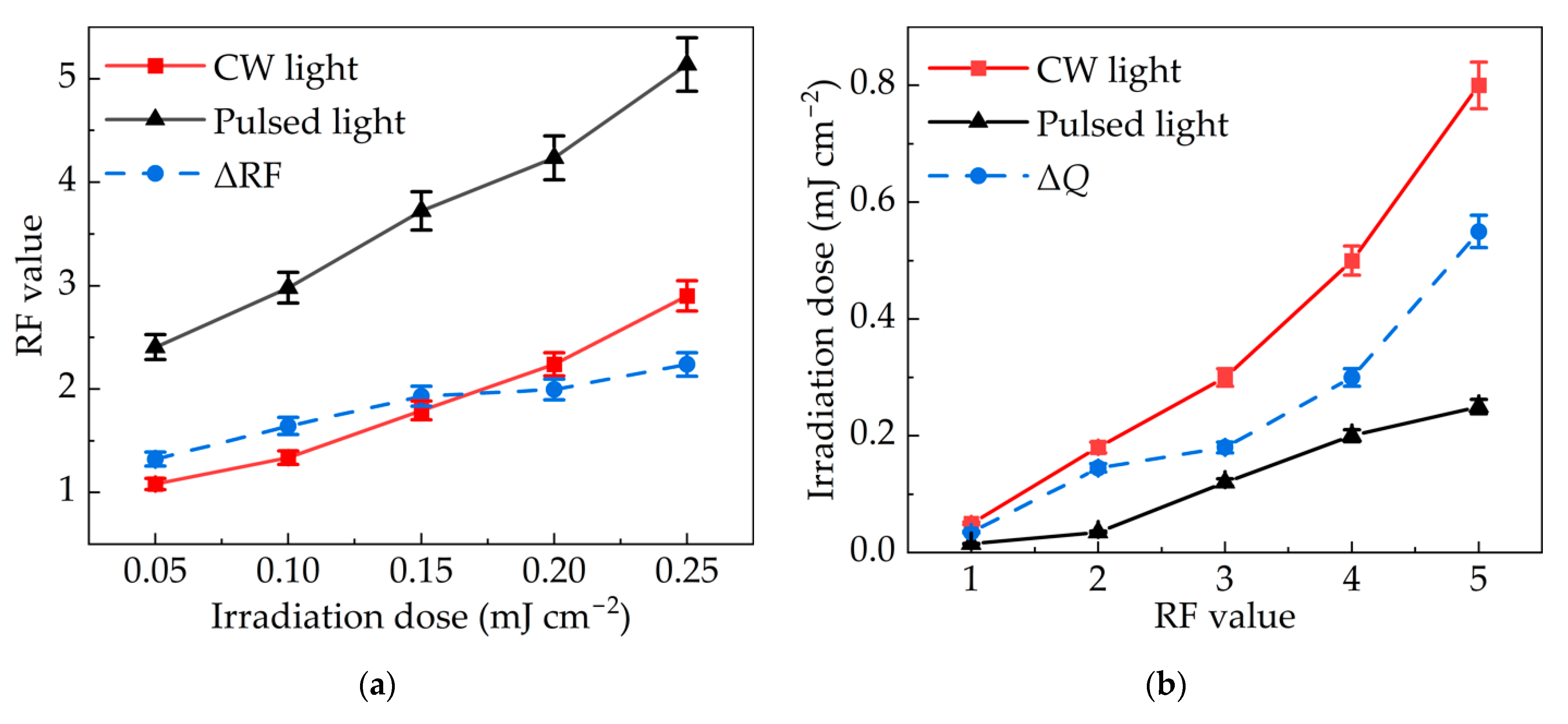
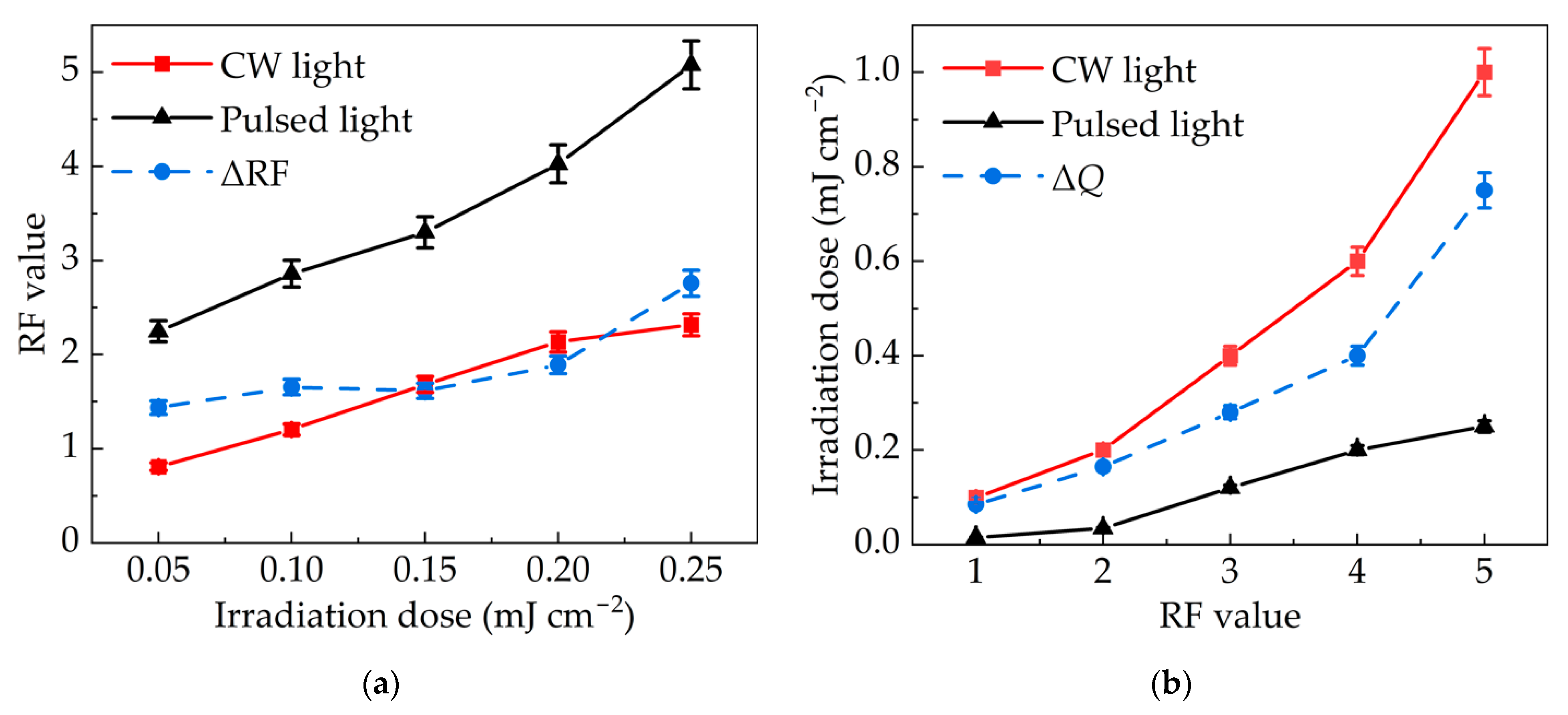
3.3. Comparison of Inactivation Effects between Pulsed Light and Continuous Light
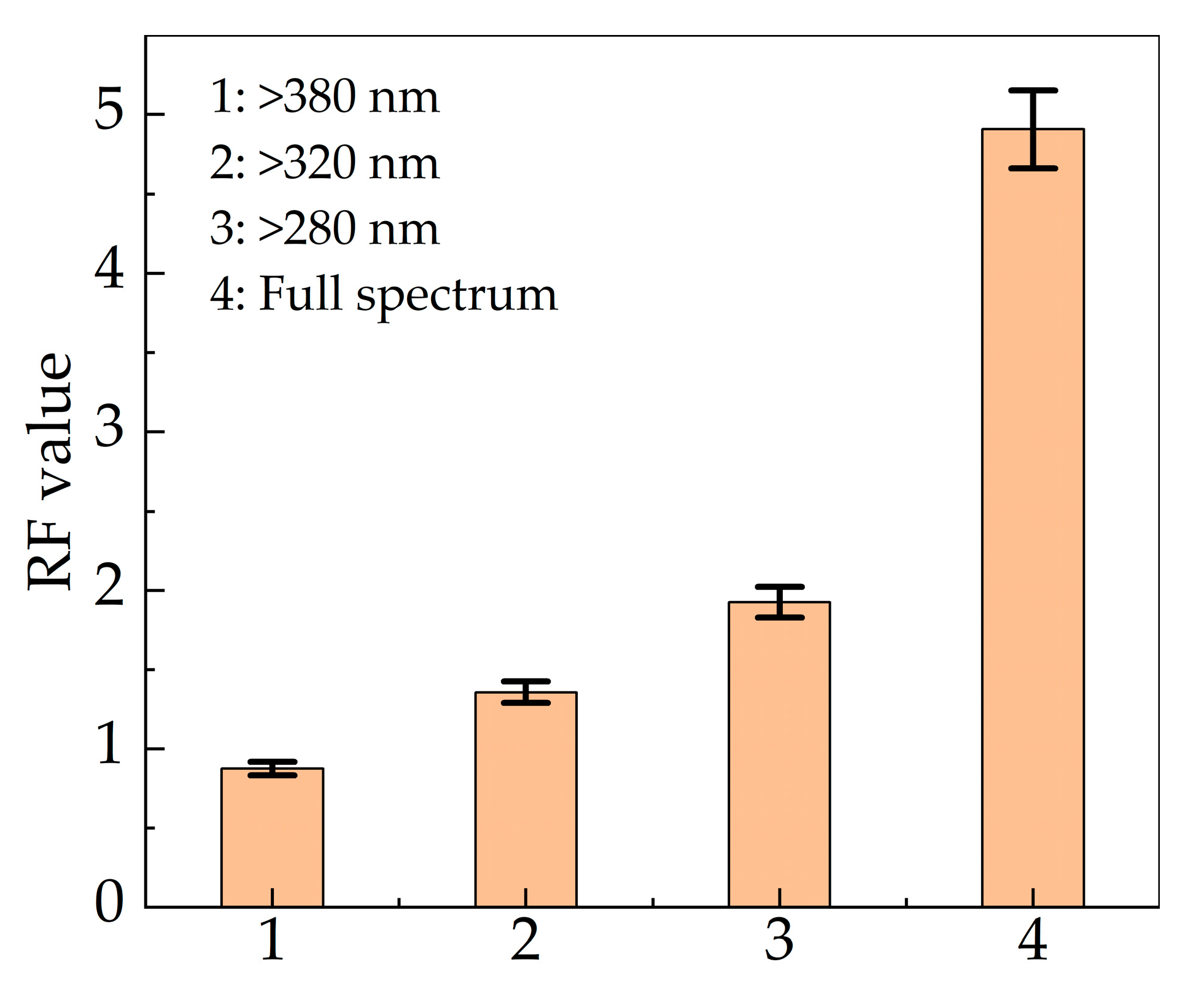
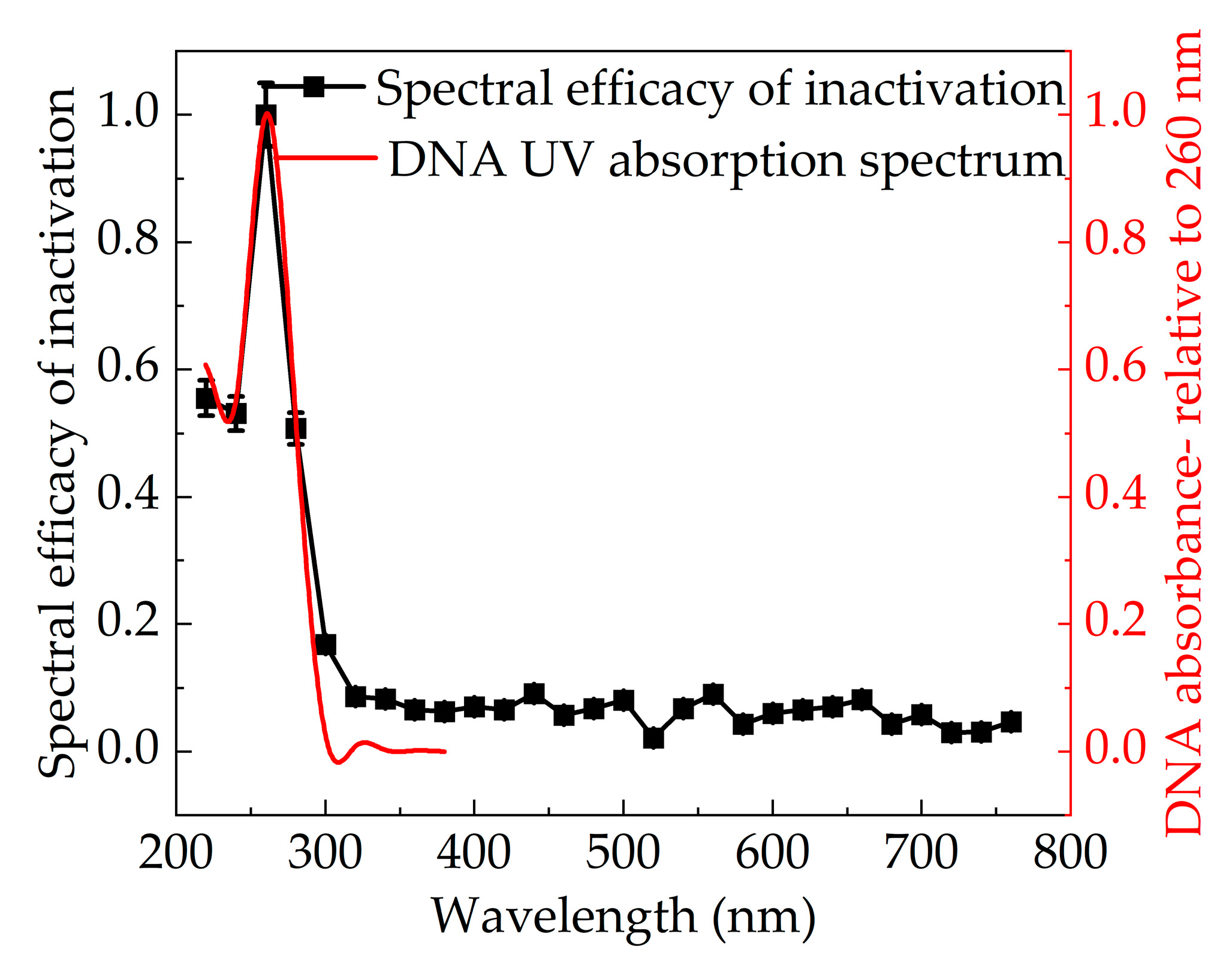
3.4. Effect of Wavelength on Inactivation Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Symbols/Acronyms
| Symbol/Acronym | Interpretation |
| IPL | Intense Pulse Light |
| RF | Reduction Factor |
| UV | Ultraviolet |
| CW | Continuous-wave |
| u(t) | Transient Voltage |
| i(t) | Transient Current |
| p(t) | Transient Electric Power |
| Pelec | Effective Power |
| T | Pulse Period |
| Psp | Single-pulsed Electric Power |
| f | Frequency |
| FWHM | Full Width at Half Maxima |
| Pr1 | Radiant Flux (Radiant Power) |
| E1 | Irradiance |
| L | Length of the Light Source |
| D | Distance |
| α | Half Apex Angle |
| η | Radiant Efficiency |
| Q | Irradiation Dose |
| τ | Optical Pulse Width |
| N | Flash Times |
| Q1 | Single-pulsed Irradiation Dose |
| N0 | Number of the Control Group |
| N1 | Number of the Experimental Group |
| μ | Mean of Sample |
| σ | Standard Deviation of Sample |
| Pmax | Peak Power |
| CV | Coefficient of Variation |
| ΔRF | Difference of Reduction Factor |
| ΔQ | Difference of Dose |
| E | Average Irradiance Per Second |
| LWP | Long-Wave Pass |
References
- Chen, D.; Wiertzema, J.R.; Peng, P.; Cheng, Y.; Wang, Y.; Liu, J.; Ma, Y.; Mosher, W.; Kang, M.; Min, M.; et al. Catalytic intense pulse light inactivation of Cronobacter sakazakii and other pathogens in non-fat dry milk and wheat flour. Food Chem. 2020, 332, 127420. [Google Scholar] [CrossRef]
- Júnior, L.M.; Cristianini, M.; Anjos, C.A.R. Packaging aspects for processing and quality of foods treated by pulsed light. J. Food Process. Pres. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Feng, D.; Shi, J.; Sun, D. Inactivation of microalgae in ballast water with pulse intense light treatment. Mar. Pollut. Bull. 2015, 90, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Park, J.Y.; Chung, M.S.; Cheigh, C.I. Microbial inactivation in fresh and minimally processed foods by intense pulsed light (IPL) treatment. Food Sci. Biotechnol. 2021, 30, 939–948. [Google Scholar] [CrossRef]
- Kang, M.W.; Chen, D.; Ruan, R.; Vickers, Z.M. The effect of intense pulsed light on the sensory properties of nonfat dry milk. J. Food Sci. 2021, 86, 4119–4133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, B.; Ma, F.; Zheng, H. The design and theoretical analysis of major components of pulse light sterilization equipment. Procedia. Eng. 2012, 37, 260–267. [Google Scholar] [CrossRef][Green Version]
- Ramos, C.C.R.; Roque, J.L.A.; Sarmiento, D.B.; Suarez, L.E.G.; Sunio, J.T.P.; Tabungar, K.I.B.; Tengco, G.S.C.; Rio, P.C.; Hilario, A.L. Use of ultraviolet-C in environmental sterilization in hospitals: A systematic review on efficacy and safety. Int. J. Health Sci. 2020, 14, 52–65. [Google Scholar]
- Jang, H.; Nguyen, M.C.; Noh, S.; Cho, W.K.; Sohn, Y.; Yee, K.; Jung, H.; Kim, J. UV laser sterilization of Bacillus atrophaeus spores on ceramic tiles. Ceram. Int. 2022, 48, 1446–1450. [Google Scholar] [CrossRef]
- Kwok, K.C.; Shiu, Y.W.; Yeung, C.H.; Niranjan, K. Effect of thermal processing on available lysine, thiamine and riboflavin content in soymilk. J. Sci. Food Agric. 1998, 77, 473–478. [Google Scholar] [CrossRef]
- Franco-Vega, A.; Reyes-Jurado, F.; González-Albarrán, D.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Developments and Advances of High Intensity Pulsed Light and its Combination with Other Treatments for Microbial Inactivation in Food Products. Food Eng. Rev. 2021, 13, 741–768. [Google Scholar] [CrossRef]
- MacGregor, S.J.; Rowan, N.J.; Mcllvaney, L.; Anderson, J.G.; Fouracre, R.A.; Farish, O. Light inactivation of food-related pathogenic bacteria using a pulsed power source. Lett. Appl. Microbiol. 1998, 27, 67–70. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Demirci, A.; Irudayaraj, J. Inactivation of Staphylococcus aureus by pulsed UV light sterilization. J. Food Protect. 2004, 67, 1027–1030. [Google Scholar] [CrossRef]
- Yi, J.Y.; Lee, N.H.; Chung, M.S. Inactivation of bacteria and murine norovirus in untreated groundwater using a pilot-scale continuous-flow intense pulsed light (IPL) system. LWT-Food Sci. Technol. 2016, 66, 108–113. [Google Scholar] [CrossRef]
- Jo, H.L.; Hwang, H.J.; Chung, M.S. Inactivation of Bacillus subtilis spores at various germination and outgrowth stages using intense pulsed light. Food Microbiol. 2019, 82, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Irudayaraj, J.; Demirci, A.; Geiser, D. Pulsed UV-light treatment of corn meal for inactivation of Aspergillus niger spores. Int. J. Food Sci. Technol. 2003, 38, 883–888. [Google Scholar] [CrossRef]
- Luo, W.; Chen, A.; Chen, M.; Dong, W.; Hou, X. Comparison of sterilization efficiency of pulsed and continuous UV light using tunable frequency UV system. Innov. Food Sci. Emerg. 2014, 26, 220–225. [Google Scholar] [CrossRef]
- Gillespie, J.B.; Maclean, M.; Given, M.J.; Wilson, M.P.; Judd, M.D.; Timoshkin, I.V.; MacGregor, S.J. Efficacy of Pulsed 405-nm Light-Emitting Diodes for Antimicrobial Photodynamic Inactivation: Effects of Intensity, Frequency, and Duty Cycle. Photomed. Laser Surg. 2017, 35, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Cheigh, C.I.; Jeong, E.A.; Shin, J.K.; Chung, M.S. Nonthermal sterilization of Listeria monocytogenes in infant foods by intense pulsed-light treatment. J. Food Eng. 2010, 97, 504–509. [Google Scholar] [CrossRef]
- Hierro, E.; Hospital, X.F.; Fernández-León, M.F.; Caballero, N.; Cerdán, B.; Fernández, M. Impact of voltage and pulse delivery mode on the efficacy of pulsed light for the inactivation of Listeria. Innov. Food Sci. Emerg. 2022, 77, 102973. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Hong, C.; Telebielaigen, S.; Nsor-Atindana, J.; Duan, Y.; Zhong, F. Optimization of spiral continuous flow-through pulse light sterilization for Escherichia coli in red grape juice by response surface methodology. Food Control. 2019, 105, 8–12. [Google Scholar] [CrossRef]
- Prasad, A.; Gänzle, M.; Roopesh, M.S. Inactivation of Escherichia Coli and Salmonella Using 365 and 395 nm High Intensity Pulsed Light Emitting Diodes. Foods 2019, 8, 679. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Devlieghere, F.; Bonduelle, V.; Debevere, J. Factors affecting the inactivation of micro-organisms by intense light pulses. J. Appl. Microbiol. 2005, 99, 460–470. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Ouyang, B.; Demirci, A. Utilization of pulsed UV light for inactivation of Salmonella Enteritidis on shelled walnuts. LWT-Food Sci. Technol. 2020, 134, 110023. [Google Scholar] [CrossRef]
- Mengarda Buosi, D.T.; de Moraes, J.O.; Cheng, Y.; Cheng, R.A.; Moraru, C.I.; Carciofi, B.A.M. Effective pulsed light treatments for inactivating Salmonella enterica serotypes. Food Control 2022, 135, 108776. [Google Scholar] [CrossRef]
- Wang, T.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G.A. Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Res. 2005, 39, 2921–2925. [Google Scholar] [CrossRef] [PubMed]
- Alzueta, O.R.; Cuquerella, M.C.; Miranda, M.A. Transient UV-vis absorption spectroscopic characterisation of 2′-methoxyacetophenone as a DNA photosensitizer. Spectrochim. Acta A 2019, 218, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Linden, K.G.; Shin, G.; Sobsey, M.D. Comparative effectiveness of UV wavelengths for the inactivation of Cryptosporidium parvum oocysts in water. Water Sci. Technol. 2001, 43, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 285, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Daynouri, F.; Robinson, J. Evaluation of the Keitz Method for Quantifying the UV Output of Medium Pressure Mercury Lamps. Ozone Sci. Eng. 2012, 34, 310–314. [Google Scholar] [CrossRef]
- Daeschlein, G.; Woedtke, T.V.; Kindel, E.; Brandenburg, R.; Weltmann, K.D.; Jünger, M. Antibacterial Activity of an Atmospheric Pressure Plasma Jet Against Relevant Wound Pathogens in vitro on a Simulated Wound Environment. Plasma Process. Polym. 2010, 7, 224–230. [Google Scholar] [CrossRef]
- Kolobov, V.I.; Arslanbekov, R.R. Towards adaptive kinetic-fluid simulations of weakly ionized plasmas. J. Comput. Phys. 2012, 231, 839–869. [Google Scholar] [CrossRef]
- Cassar, J.R.; Mills, E.W.; Demirci, A. Characterization of pulsed light for microbial inactivation. J. Food Eng. 2022, 334, 111152. [Google Scholar] [CrossRef]
- Fiske, D.W.; Baer, D.M. The coefficient of variation: An evaluation. Educ. Psychol. Meas. 1955, 15, 396–403. [Google Scholar] [CrossRef]


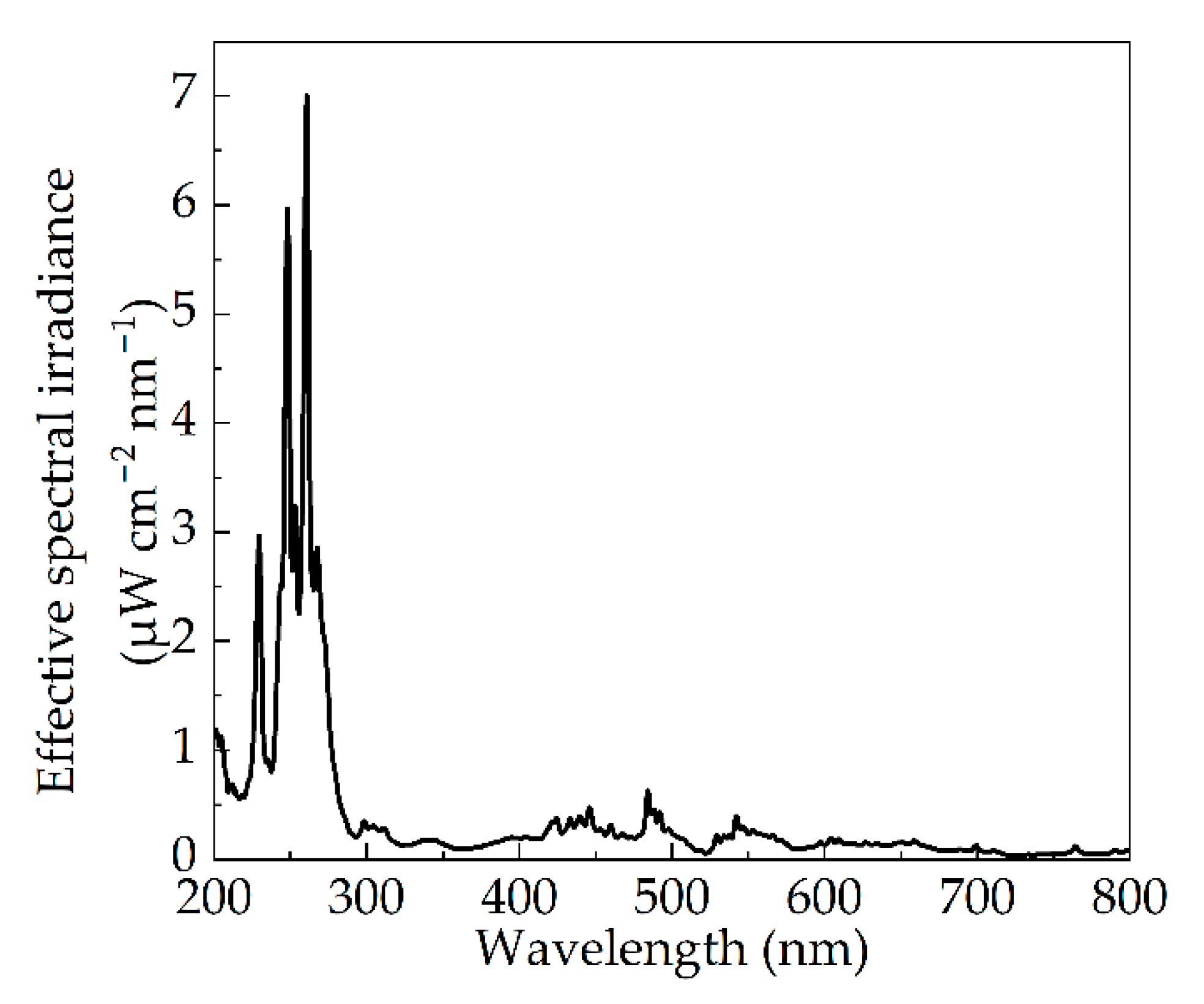
| Pulse Width (μs) | Psp (W) | Pmax (kW) | E1 (mW cm−2) | Pr1 (W) | η (%) | FWHM (μs) |
|---|---|---|---|---|---|---|
| 40 | 10.5 | 292.8 | 4.3 | 5.5 | 52.4 | 40.0 |
| 60 | 13.0 | 297.6 | 5.1 | 6.6 | 50.8 | 60.0 |
| 80 | 24.9 | 316.8 | 9.9 | 12.6 | 50.6 | 80.0 |
| 100 | 28.9 | 316.8 | 11.4 | 14.6 | 50.5 | 100.0 |
| 120 | 32.4 | 316.8 | 13.1 | 16.8 | 51.9 | 120.0 |
| Band | Percentage in This Work | Percentage in Ref. [32] |
|---|---|---|
| UVC | 15.1% | 7.23% |
| UVB | 5.41% | 5.37% |
| UVA | 9.99% | 16.4% |
| UV | 30.5% | 29.0% |
| Visible | 69.5% | 71.0% |
| Wavelength (nm) | RF Value |
|---|---|
| 400 | 0.39 ± 0.009 |
| 440 | 0.32 ± 0.012 |
| 480 | 0.25 ± 0.013 |
| 540 | 0.30 ± 0.013 |
| 580 | 0.28 ± 0.021 |
| 600 | 0.43 ± 0.018 |
| 680 | 0.34 ± 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Shen, D.; Yuan, Q.; Wu, Z.; Wang, J.; Li, F.; Han, Q.; Zhang, S. Effects of Electrical Pulse Width and Output Irradiance on Intense Pulse Light Inactivation. Bioengineering 2022, 9, 730. https://doi.org/10.3390/bioengineering9120730
Xie S, Shen D, Yuan Q, Wu Z, Wang J, Li F, Han Q, Zhang S. Effects of Electrical Pulse Width and Output Irradiance on Intense Pulse Light Inactivation. Bioengineering. 2022; 9(12):730. https://doi.org/10.3390/bioengineering9120730
Chicago/Turabian StyleXie, Shuge, Diya Shen, Qing Yuan, Zairui Wu, Junyuan Wang, Fusheng Li, Qiuyi Han, and Shanduan Zhang. 2022. "Effects of Electrical Pulse Width and Output Irradiance on Intense Pulse Light Inactivation" Bioengineering 9, no. 12: 730. https://doi.org/10.3390/bioengineering9120730
APA StyleXie, S., Shen, D., Yuan, Q., Wu, Z., Wang, J., Li, F., Han, Q., & Zhang, S. (2022). Effects of Electrical Pulse Width and Output Irradiance on Intense Pulse Light Inactivation. Bioengineering, 9(12), 730. https://doi.org/10.3390/bioengineering9120730








