A Rapid and Sensitive Aptamer-Based Biosensor for Amnesic Shellfish Toxin Domoic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
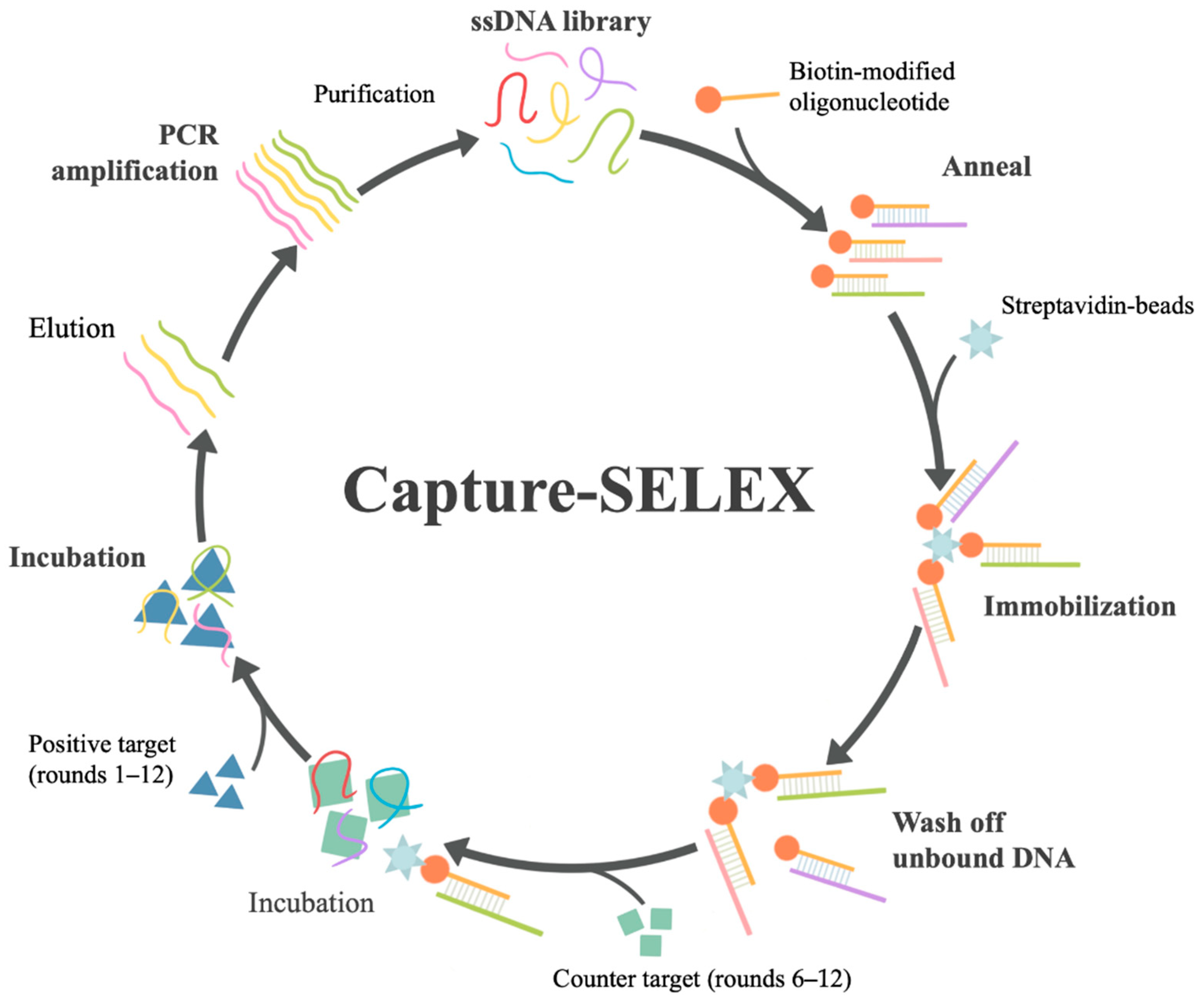
2.3. Aptamer Selection by Capture-SELEX
2.3.1. The ssDNA Library and Primers
2.3.2. Hybridization and Immobilization of the ssDNA Library
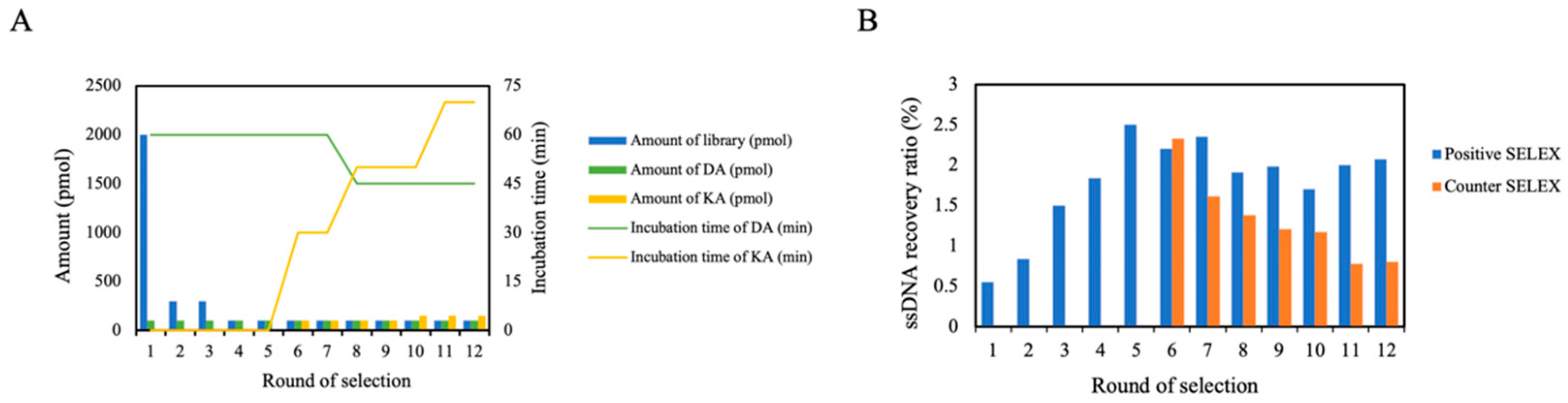
2.3.3. Screening Procedure
2.4. Sequencing of ssDNA
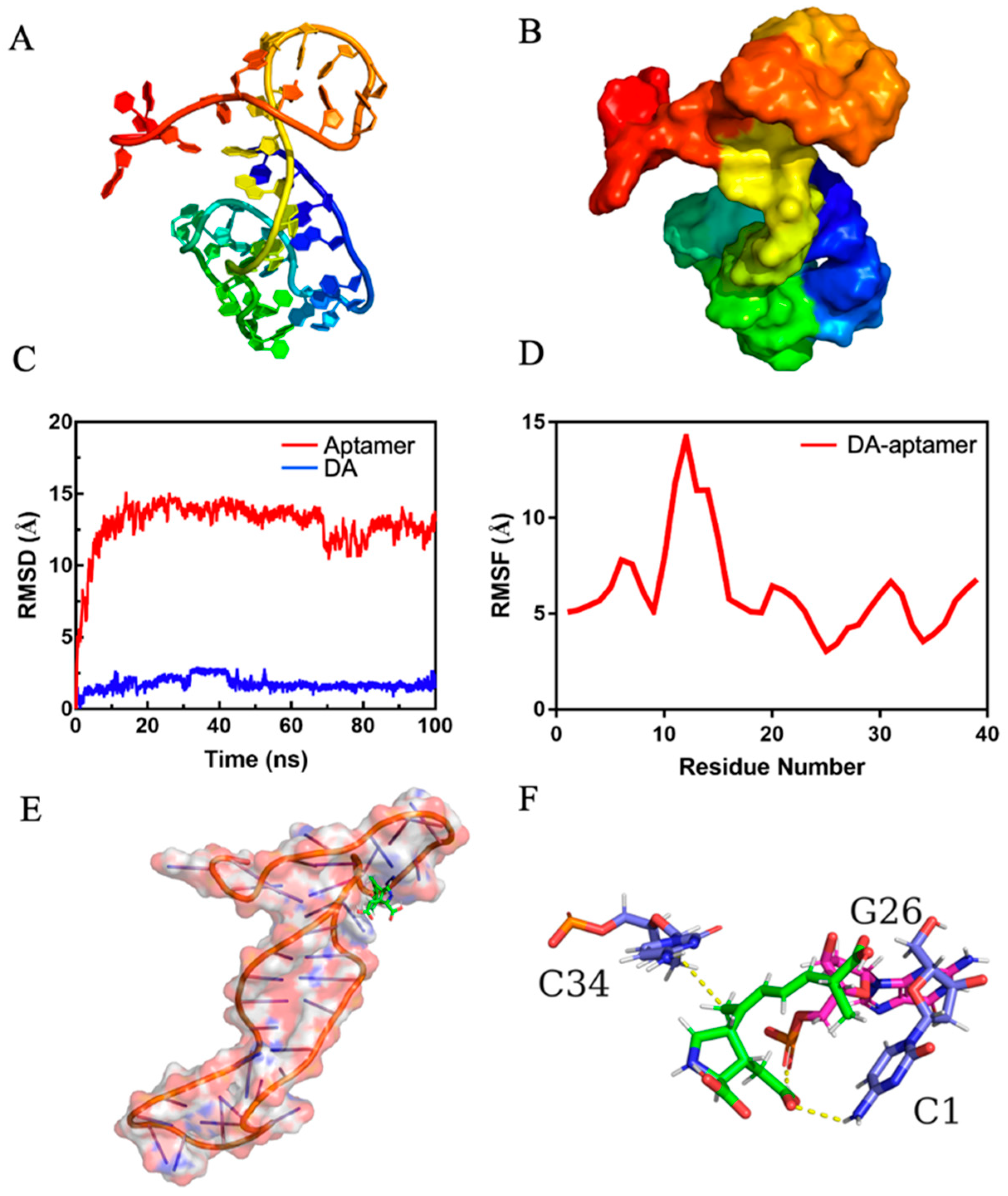
2.5. Interaction Mechanism of Aptamer and DA
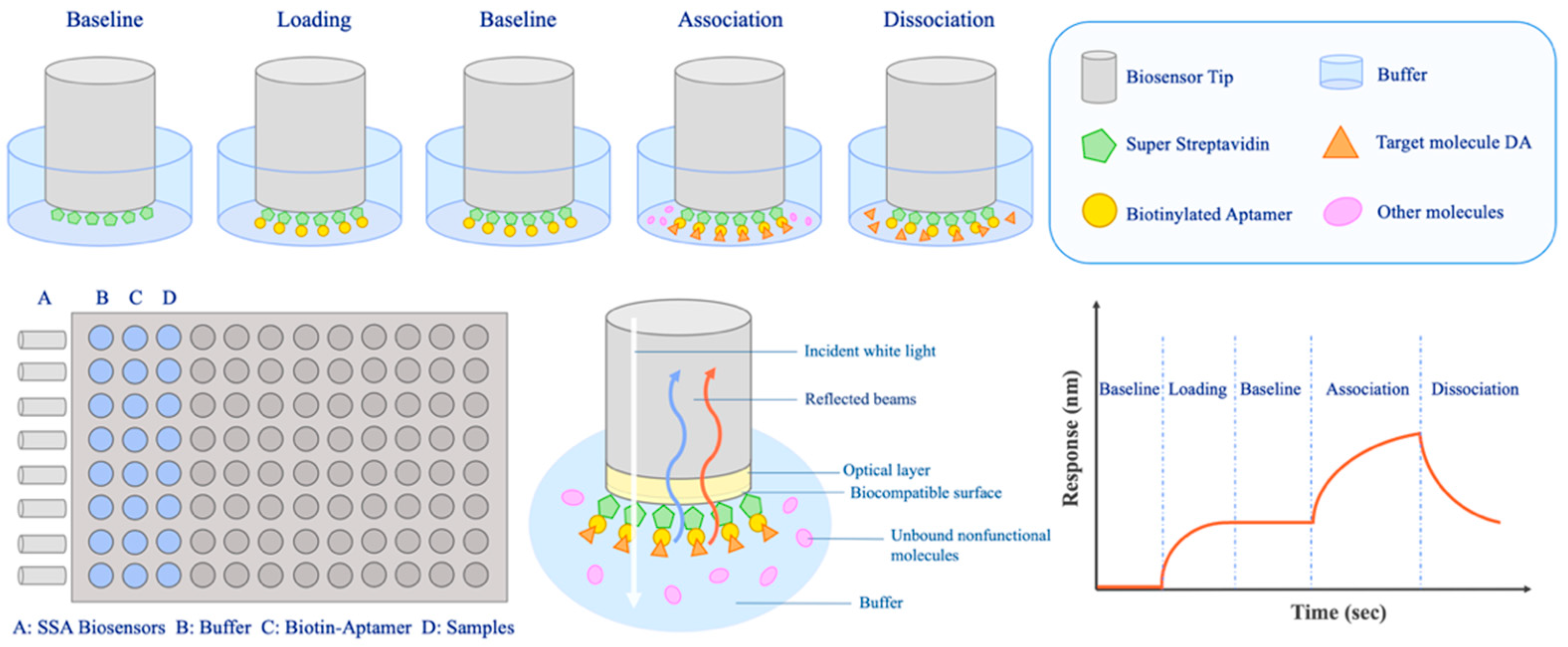
2.6. Biolayer Interferometry (BLI) Assay
2.6.1. Preparation of Sensors and Aptamers
2.6.2. Detection Procedure of BLI
2.7. Property Assessment of Aptasensor
2.8. Treatment of Real Samples
2.8.1. Seawater Samples
2.8.2. Shellfish Samples
3. Results and Discussion
3.1. Selection of DA Aptamer by Capture-SELEX In Vitro
3.2. Acquisition and Affinity Identification of Candidate Aptamers
3.3. Truncation of Aptamer C1
3.4. Identification of Affinity and Specificity of Aptamer C1-d
3.5. Molecular Docking and Molecular Dynamics Simulations
3.6. BLI-Based Aptasensor for DA Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quilliam, M.A.; Wright, J.C. The Amnesic Shellfish Poisoning Mystery. Anal. Chem. 1989, 61, 1053A–1060A. [Google Scholar] [CrossRef]
- Perl, T.M.; Bédard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.D.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 1990, 322, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.N.; Teng, S.T.; Lim, H.C.; Kotaki, Y.; Bates, S.S.; Leaw, C.P.; Lim, P.T. Diatom Nitzschia navis-varingica (Bacillariophyceae) and its domoic acid production from the mangrove environments of Malaysia. Harmful Algae 2016, 60, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Livramento, F.; Rangel, I.M. Amnesic shellfish poisoning (ASP) toxins in plankton and molluscs from Luanda Bay, Angola. Toxicon 2010, 55, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Lail, E.M.; Skrabal, S.A.; Kieber, R.J.; Bouillon, R.-C.; Wright, J.L.C. The role of particles on the biogeochemical cycling of domoic acid and its isomers in natural water matrices. Harmful Algae 2007, 6, 651–657. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Sim, P.G.; McCulloch, A.W.; McInnes, A.G. High-Performance Liquid Chromatography of Domoic Acid, a Marine Neurotoxin, with Application to Shellfish and Plankton. Int. J. Environ. Anal. Chem. 2006, 36, 139–154. [Google Scholar] [CrossRef]
- Zhao, J.; Thibault, P.; Quilliam, M.A. Analysis of domoic acid and isomers in seafood by capillary electrophoresis. Electrophoresis 1997, 18, 268–276. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Faustman, E.M. Domoic acid as a developmental neurotoxin. Neurotoxicology 2010, 31, 409–423. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Noren, D.P.; Schultz, I.R.; Bogard, S.M.; Wilson, J.; Eberhart, B.T. Uptake, tissue distribution and excretion of domoic acid after oral exposure in coho salmon (Oncorhynchus kisutch). Aquat. Toxicol. 2007, 81, 266–274. [Google Scholar] [CrossRef]
- Berman, F.W.; Murray, T.F. Domoic Acid Neurotoxicity in Cultured Cerebellar Granule Neurons Is Mediated Predominantly by NMDA Receptors that Are Activated as a Consequence of Excitatory Amino Acid Release. J. Neurochem. 1997, 69, 693–703. [Google Scholar] [CrossRef]
- Larm, J.A.; Bert, P.M.; Cheung, N.S. Neurotoxin Domoic Acid Produces Cytotoxicity VIa Kainate- and AMPA-Sensitive Receptors in Cultured Cortical Neurones. Neurochem. Int. 1997, 31, 677–682. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.P.; Domenico, A.D.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Marine biotoxins in shellfish—Domoic acid. EFSA J. 2009, 1181, 1–61. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid Extraction and Cleanup for Liquid Chromatographic Determination of Domoic Acid in Unsalted Seafood. J. AOAC Int. 1995, 78, 543–554. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Charbonneau, C.F.; Ménard, C.; Quilliam, M.A.; Sim, P.G. Liquid chromatographic determination of domoic acid in shellfish products using the paralytic shellfish poison extraction procedure of the association of official analytical chemists. J. Chromatogr. 1989, 462, 349–356. [Google Scholar] [CrossRef]
- Lopez-Rivera, A.; Suarez-Isla, B.A.; Eilers, P.P.; Beaudry, C.G.; Hall, S.; Fernandez Amandi, M.; Furey, A.; James, K.J. Improved high-performance liquid chromatographic method for the determination of domoic acid and analogues in shellfish: Effect of pH. Anal. Bioanal. Chem. 2005, 381, 1540–1545. [Google Scholar] [CrossRef]
- Saeed, A.; Ling, S.; Yuan, J.; Wang, S. The Preparation and Identification of a Monoclonal Antibody against Domoic Acid and Establishment of Detection by Indirect Competitive ELISA. Toxins 2017, 9, 250. [Google Scholar] [CrossRef]
- Kleivdal, H.; Kristiansen, S.-I.; Nilsen, M.V.; Briggs, L. Single-Laboratory Validation of the Biosense Direct Competitive Enzyme-Linked Immunosorbent Assay (ELISA) for Determination of Domoic Acid Toxins in Shellfish. J. AOAC Int. 2007, 94, 1000–1010. [Google Scholar] [CrossRef]
- Yu, F.; Wu, T.; Chi, T.; Su, M. Development of a Sensitive Enzyme-Linked Immunosorbent Assay for the Determination of Domoic Acid in Shellfish. J. Agric. Food Chem. 2004, 52, 5334–5339. [Google Scholar] [CrossRef]
- Maucher, J.M.; Ramsdell, J.S. Ultrasensitive detection of domoic acid in mouse blood by competitive ELISA using blood collection cards. Toxicon 2005, 45, 607–613. [Google Scholar] [CrossRef]
- Shaw, I.; O’Reilly, A.; Charleton, M.; Kane, M. Development of a High-Affinity Anti-Domoic AcidSheep scFv and its Use in Detection of the Toxin in Shellfish. Anal. Chem. 2008, 80, 3205–3212. [Google Scholar] [CrossRef]
- Kvasnicka, F.; Sevcik, R.; Voldrich, M. Determination of domoic acid by on-line coupled capillary isotachophoresis with capillary zone electrophoresis. J. Chromatogr. A 2006, 1113, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Zhang, Z.X. Quantification of domoic acid in shellfish samples by capillary electrophoresis-based enzyme immunoassay with electrochemical detection. Toxicon 2012, 59, 626–632. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Dinshaw, P.; Asif, S.; Jiang, F.; Jiang, L.; Kumar, A.; Pei, F.R.; Sylvie, N. Structure, Recognition and Adaptive Binding in RNA Aptamer Complexes. J. Mol. Biol. 1997, 272, 645–664. [Google Scholar] [CrossRef]
- Linl, C.H.; Wang, W.; Jones, R.A.; Patell, D.J. Formation of an amino-acid-binding pocket through adaptive zippering-up of a large DNA hairpin loop. Chem. Biol. 1998, 5, 555–572. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Chang, Y.; Zhang, Q.; Chang, D.; Hui, C.Y.; Brennan, J.D.; Li, Y. In Vitro Selection of a DNA Aptamer Targeting Degraded Protein Fragments for Biosensing. Angew. Chem. 2020, 132, 7780–7784. [Google Scholar] [CrossRef]
- Pleiko, K.; Saulite, L.; Parfejevs, V.; Miculis, K.; Vjaters, E.; Riekstina, U. Differential binding cell-SELEX method to identify cell-specific aptamers using high-throughput sequencing. Sci. Rep. 2019, 9, 8142. [Google Scholar] [CrossRef]
- Liu, R.; He, L.; Hu, Y.; Luo, Z.; Zhang, J. A serological aptamer-assisted proximity ligation assay for COVID-19 diagnosis and seeking neutralizing aptamers. Chem. Sci. 2020, 11, 12157–12164. [Google Scholar] [CrossRef]
- Yu, H.; Alkhamis, O.; Canoura, J.; Liu, Y.; Xiao, Y. Advances and Challenges in Small-Molecule DNA Aptamer Isolation, Characterization, and Sensor Development. Angew. Chem. Int. Ed. Engl. 2021, 60, 16800–16823. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, R.; Sinha, M.; Kumar, R.; Bhalla, V. MoS2 based digital response platform for aptamer based fluorescent detection of pathogens. Microchim. Acta 2016, 183, 1501–1506. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, P.; Zhang, H.; Cai, C. Designing activatable aptamer probes for simultaneous detection of multiple tumor-related proteins in living cancer cells. Biosens. Bioelectron. 2015, 68, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Nonaka, Y.; Futakawa, S.; Imai, H.; Akita, K.; Nishihata, T.; Fujiwara, M.; Ali, Y.; Bhisitkul, R.B.; Nakamura, Y. Anti-Angiogenic and Anti-Scarring Dual Action of an Anti-Fibroblast Growth Factor 2 Aptamer in Animal Models of Retinal Disease. Mol. Ther. Nucleic Acids 2019, 17, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.R.; Hu, G.Q.; Xue, X.H.; Li, L.; Zheng, X.X.; Gao, Y.W.; Yang, S.T.; Xia, X.Z. Selection of an aptamer against rabies virus: A new class of molecules with antiviral activity. Virus Res. 2014, 184, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xu, H.; Wu, Y.; Zhao, Y.; Meng, H.-M.; Li, Z.; Qu, L. Two-Dimension (2D) Cu-MOFs/aptamer Nanoprobe for In Situ ATP Imaging in Living Cells. J. Anal. Test. 2021, 5, 165–173. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Zhang, W.; Zhu, M.; Liu, Z.; Kong, Y.; Liu, S. Marine Toxins Detection by Biosensors Based on Aptamers. Toxins 2019, 12, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- Song, Y.; Song, J.; Wei, X.; Huang, M.; Sun, M.; Zhu, L.; Lin, B.; Shen, H.; Zhu, Z.; Yang, C. Discovery of Aptamers Targeting the Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. Anal. Chem. 2020, 92, 9895–9900. [Google Scholar] [CrossRef]
- Kim, Y.; Dennis, D.M.; Morey, T.; Yang, L.; Tan, W. Engineering dendritic aptamer assemblies as superior inhibitors of protein function. Chem. Asian J. 2010, 5, 56–59. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Ustundag, Z. Saxitoxin aptasensor based on attenuated internal reflection ellipsometry for seafood. Toxicon 2020, 187, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Reinemann, C.; Strehlitz, B. Aptamer-modified nanoparticles and their use in cancer diagnostics and treatment. Swiss Med. Wkly. 2014, 144, w13908. [Google Scholar] [CrossRef] [PubMed]
- Handy, S.M.; Yakes, B.J.; DeGrasse, J.A.; Campbell, K.; Elliott, C.T.; Kanyuck, K.M.; Degrasse, S.L. First report of the use of a saxitoxin-protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 2013, 61, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Ng, A.; Siaj, M.; Tavares, A.C.; Zourob, M. Selection and Identification of DNA Aptamers against Okadaic Acid for Biosensing Application. Anal. Chem. 2013, 85, 11794–11801. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Wu, P.; Wang, W.; Cheng, Y.; Huang, L.; Bai, J.; Peng, Y.; Ning, B.; Gao, Z.; et al. Development of a highly sensitive detection method for TTX based on a magnetic bead-aptamer competition system under triple cycle amplification. Anal. Chim. Acta 2020, 1119, 18–24. [Google Scholar] [CrossRef]
- Eissa, S.; Siaj, M.; Zourob, M. Aptamer-based competitive electrochemical biosensor for brevetoxin-2. Biosens. Bioelectron. 2015, 69, 148–154. [Google Scholar] [CrossRef]
- Ouyang, S.; Hu, B.; Zhou, R.; Liu, D.; Peng, D.; Li, Z.; Li, Z.; Jiao, B.; Wang, L. Rapid and sensitive detection of nodularin-R in water by a label-free BLI aptasensor. Analyst 2018, 143, 4316–4322. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neubock, R.; Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef]
- Miao, Z.; Adamiak, R.W.; Blanchet, M.F.; Boniecki, M.; Bujnicki, J.M.; Chen, S.J.; Cheng, C.; Chojnowski, G.; Chou, F.C.; Cordero, P.; et al. RNA-Puzzles Round II: Assessment of RNA structure prediction programs applied to three large RNA structures. RNA 2015, 21, 1066–1084. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., 3rd; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, X.; Jiao, B.; Wang, L. Post-SELEX optimization of aptamers. Anal. Bioanal. Chem. 2016, 408, 4567–4573. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.; Kuznetsov, A. Inside the Black Box: What Makes SELEX Better? Molecules 2019, 24, 3598. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.; Witte, K.; Wartchow, C.; Choo, S.; Yao, D.; Persson, H.; Wei, J.; Li, P.; Heidecker, B.; Ma, W.; et al. Label-Free Detection of Biomolecular Interactions Using BioLayer Interferometry for Kinetic Characterization. Comb. Chem. High Throughput Screen. 2009, 12, 791–800. [Google Scholar] [CrossRef]
- Kamat, V.; Rafique, A. Designing binding kinetic assay on the bio-layer interferometry (BLI) biosensor to characterize antibody-antigen interactions. Anal. Biochem. 2017, 536, 16–31. [Google Scholar] [CrossRef]
- Zhang, D.; Hamdoun, S.; Chen, R.; Yang, L.; Ip, C.K.; Qu, Y.; Li, R.; Jiang, H.; Yang, Z.; Chung, S.K.; et al. Identification of natural compounds as SARS-CoV-2 entry inhibitors by molecular docking-based virtual screening with bio-layer interferometry. Pharmacol. Res. 2021, 172, 105820. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Tobias, R. Label-free kinetic analysis of an antibody-antigen interaction using biolayer interferometry. Methods Mol. Biol. 2015, 1278, 165–182. [Google Scholar] [CrossRef]
- Miczi, M.; Dios, A.; Bozoki, B.; Tozser, J.; Motyan, J.A. Development of a Bio-Layer Interferometry-Based Protease Assay Using HIV-1 Protease as a Model. Viruses 2021, 13, 1183. [Google Scholar] [CrossRef]
- Ciesielski, G.L.; Hytonen, V.P.; Kaguni, L.S. Biolayer Interferometry: A Novel Method to Elucidate Protein-Protein and Protein-DNA Interactions in the Mitochondrial DNA Replisome. Methods Mol. Biol. 2016, 1351, 223–231. [Google Scholar] [CrossRef]
- Traynor, I.M.; Plumpton, L.; Fodey, T.L.; Higgins, C.; Elliott, C.T. Immunobiosensor Detection of Domoic Acid as a Screening Test in Bivalve Molluscs: Comparison with Liquid Chromatography-Based Analysis. J. AOAC Int. 2006, 89, 868–872. [Google Scholar] [CrossRef]
- Stevens, R.C.; Soelberg, S.D.; Eberhart, B.-T.L.; Spencer, S.; Wekell, J.C.; Chinowsky, T.M.; Trainer, V.L.; Furlong, C.E. Detection of the toxin domoic acid from clam extracts using a portable surface plasmon resonance biosensor. Harmful Algae 2007, 6, 166–174. [Google Scholar] [CrossRef]
- Lotierzo, M.; Henry, O.Y.; Piletsky, S.; Tothill, I.; Cullen, D.; Kania, M.; Hock, B.; Turner, A.P. Surface plasmon resonance sensor for domoic acid based on grafted imprinted polymer. Biosens. Bioelectron. 2004, 20, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pardo, O.; Yusa, V.; Leon, N.; Pastor, A. Development of a pressurised liquid extraction and liquid chromatography with electrospray ionization-tandem mass spectrometry method for the determination of domoic acid in shellfish. J. Chromatogr. A 2007, 1154, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; King, K.L.; Ramsdell, J.S.; Doucette, G.J. Determination of domoic acid in seawater and phytoplankton by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1163, 169–176. [Google Scholar] [CrossRef]
- de la Iglesia, P.; Gimenez, G.; Diogene, J. Determination of dissolved domoic acid in seawater with reversed-phase extraction disks and rapid resolution liquid chromatography tandem mass spectrometry with head-column trapping. J. Chromatogr. A 2008, 1215, 116–124. [Google Scholar] [CrossRef] [PubMed]



| No. | Aptamer Sequence (5′–3′) | KD (M) |
|---|---|---|
| C1/htC1 | ATTGGCACTCCACGCATAGGCCAACATGATGTTCCGTCATTTTGAGGTGTGTACACCGTGCCTATGCGTGCTACCGTGAA | 1.69 × 10−6 |
| C2 | ATTGGCACTCCACGCATAGGGGATAACGGGTTGATGGTACTTCTATCTATCGCGTTGTGCCCTATGCGTGCTACCGTGAA | 2.03 × 10−5 |
| C12/htC2 | ATTGGCACTCCACGCATAGGGACATCGAGAAGAATCCTGATACGACTTGGCTTTGCTGGCCCTATGCGTGCTACCGTGAA | 5.21 × 10−6 |
| C53 | ATTGGCACTCCACGCATAGGGTAAAGTACGTATGTCATGCACATGCCGTATTCTCTTTGCCCTATGCGTGCTACCGTGAA | NB |
| C58/htC3 | ATTGGCACTCCACGCATAGGCCAAGGTGTCAATCTTGAATAGCTGTGTAACGTCTATGTGCCTATGCGTGCTACCGTGAA | 6.33 × 10−6 |
| C66 | ATTGGCACTCCACGCATAGGGGTGGTCTTCTGGGTAATCCCCGACTACCCTTATGCCGGCCCTATGCGTGCTACCGTGAA | 2.27 × 10−5 |
| C87 | ATTGGCACTCCACGCATAGGGGAAGCTGCTCTCATCAATAAAAAATGAGACGGAGGTTACCCTATGCGTGCTACCGTGAA | 5.04 × 10−6 |
| C100/htC4 | ATTGGCACTCCACGCATAGGGAATGGACCCGGTATAATTCCCTCAAGAGTG CCAATTTCACCTATGCGTGCTACCGTGAA | 3.25 × 10−6 |
| C116 | ATTGGCACTCCACGCATAGGGATCTCATAACCAGTCTCTTTGACTGATGTTAGTAAGGTCCCTATGCGTGCTACCGTGAA | 1.17 × 10−5 |
| htC5 | ATTGGCACTCCACGCATAGGGGAGGGCCGGTTGAACTGTAATGTGTAAACCACGCGCTACCCTATGCGTGCTACCGTGAA | NB |
| Random Sequence | ATTGGCACTCCACGCATAGGATTCCGGCTAATCGACTGACTGCCGGTACGATGCAGTCAGCCTATGCGTGCTACCGTGAA | NB |
| DA (μM) | 2.5 | 3.75 | 5.0 | 6.25 | 7.5 | 10 | 20 | 30 |
| CV (%) | 4.63 | 1.97 | 1.50 | 1.12 | 1.30 | 4.71 | 4.47 | 1.39 |
| Sample | DA (μM) | Detection Value (μM) | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Seawater | 5 | 5.48 | 109.6 | 3.28 |
| 7.5 | 7.125 | 95 | 1.07 | |
| 10 | 10.89 | 108.9 | 3.98 | |
| Shellfish | 5 | 4.95 | 98.9 | 2.3 |
| 7.5 | 7.468 | 99.6 | 1.83 | |
| 10 | 11.03 | 110.3 | 1.45 |
| Detection Method | Detection Object | LOD | Linear Range | Recovery Rate (%) | Reference |
|---|---|---|---|---|---|
| LC | mussel | 20–30 μg/kg | over 104 | 93 | [13] |
| HPLC-UV | shellfish | 25 ng/mL | 50–5000 ng/mL | 96–104 | [15] |
| HPLC-UV | shellfish | 500 μg/kg | — | 72–92 | [14] |
| PLE-LC–ESI-MS–MS | shellfish | 200 μg/kg | 50–5000 ng/mL | 81–95 | [63] |
| LC-MS | seawater and phytoplankton | 0.03 ng/mL | 0.05–400 ng/mL | 95–104 | [64] |
| RRLC-MS | seawater | 0.02 ng/mL | — | 92.1–110.6 | [65] |
| cdELISA | shellfish | <25 μg/kg | — | 73.8–92.8 | [18] |
| ic-ELISA | shellfish | 0.006 ng/mL | 0.006–0.2 ng/mL | 100.56 ± 2.8 | [16] |
| Biosense Direct Competitive ELISA | shellfish | 3.3 μg/kg | 1.1–5.3 μg/kg | 85.5–106.6 | [17] |
| cELISA | blood | 0.01 ng/mL | 0.5–4.5 ng/mL | 80 | [19] |
| cITP-CZE | shellfish and algae | 1.5 ng/mL | 0–200 ng/mL | 101 ± 3 | [21] |
| CE-EIA | shellfish | 0.02 ng/mL | 0.1–50 ng/mL | 89.6–105.8 | [22] |
| Immuno-biosensor | shellfish | 20,000 μg/kg | — | — | [60] |
| SPR biosensor | shellfish | 3 μg/kg | 4–60 μg/kg | — | [61] |
| MIP-SPR sensor | — | 5 ng/mL | 5–100 ng/mL | — | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Guo, H.; Chen, H.; Zou, B.; Yang, C.; Zhang, X.; Gao, Y.; Sun, M.; Wang, L. A Rapid and Sensitive Aptamer-Based Biosensor for Amnesic Shellfish Toxin Domoic Acid. Bioengineering 2022, 9, 684. https://doi.org/10.3390/bioengineering9110684
Zhao L, Guo H, Chen H, Zou B, Yang C, Zhang X, Gao Y, Sun M, Wang L. A Rapid and Sensitive Aptamer-Based Biosensor for Amnesic Shellfish Toxin Domoic Acid. Bioengineering. 2022; 9(11):684. https://doi.org/10.3390/bioengineering9110684
Chicago/Turabian StyleZhao, Luming, Han Guo, Han Chen, Bin Zou, Chengfang Yang, Xiaojuan Zhang, Yun Gao, Mingjuan Sun, and Lianghua Wang. 2022. "A Rapid and Sensitive Aptamer-Based Biosensor for Amnesic Shellfish Toxin Domoic Acid" Bioengineering 9, no. 11: 684. https://doi.org/10.3390/bioengineering9110684
APA StyleZhao, L., Guo, H., Chen, H., Zou, B., Yang, C., Zhang, X., Gao, Y., Sun, M., & Wang, L. (2022). A Rapid and Sensitive Aptamer-Based Biosensor for Amnesic Shellfish Toxin Domoic Acid. Bioengineering, 9(11), 684. https://doi.org/10.3390/bioengineering9110684








