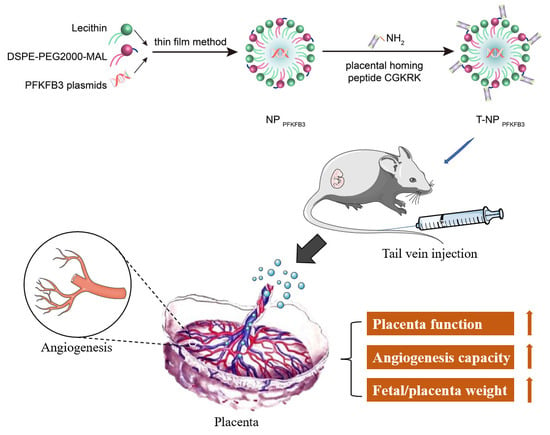
Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedures
2.2. Synthesis of Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Nanoparticle Treatment Study
2.5. Immunohistochemistry
2.6. RT-qPCR
2.7. Western Blot Analysis
2.8. Measurements of ALT, AST, and BUN Levels
2.9. Statistical Analysis
3. Results
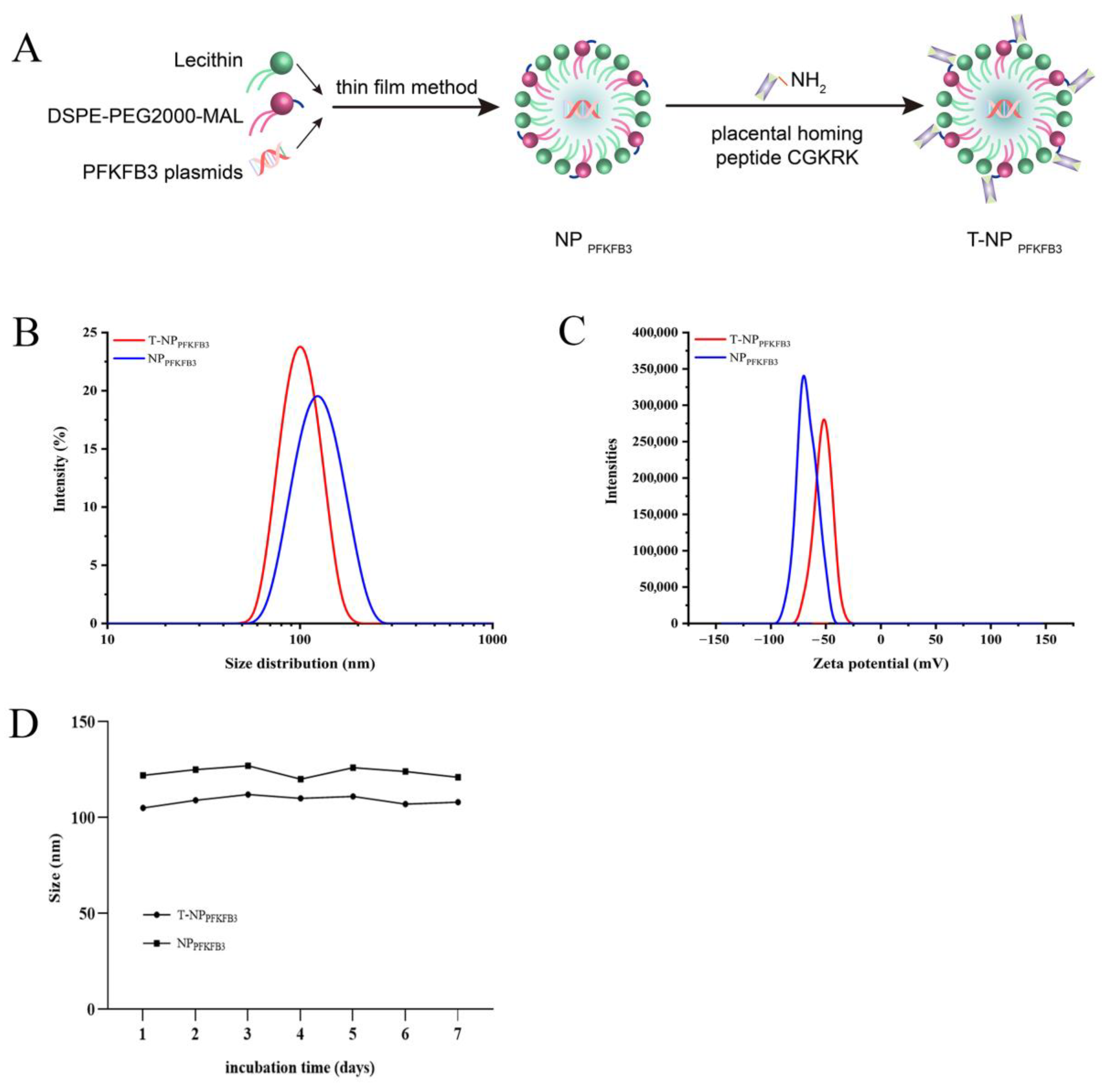
3.1. Synthesis and Characterization of Placenta-Targeted Nanoparticles Loaded with PFKFB3 Plasmids
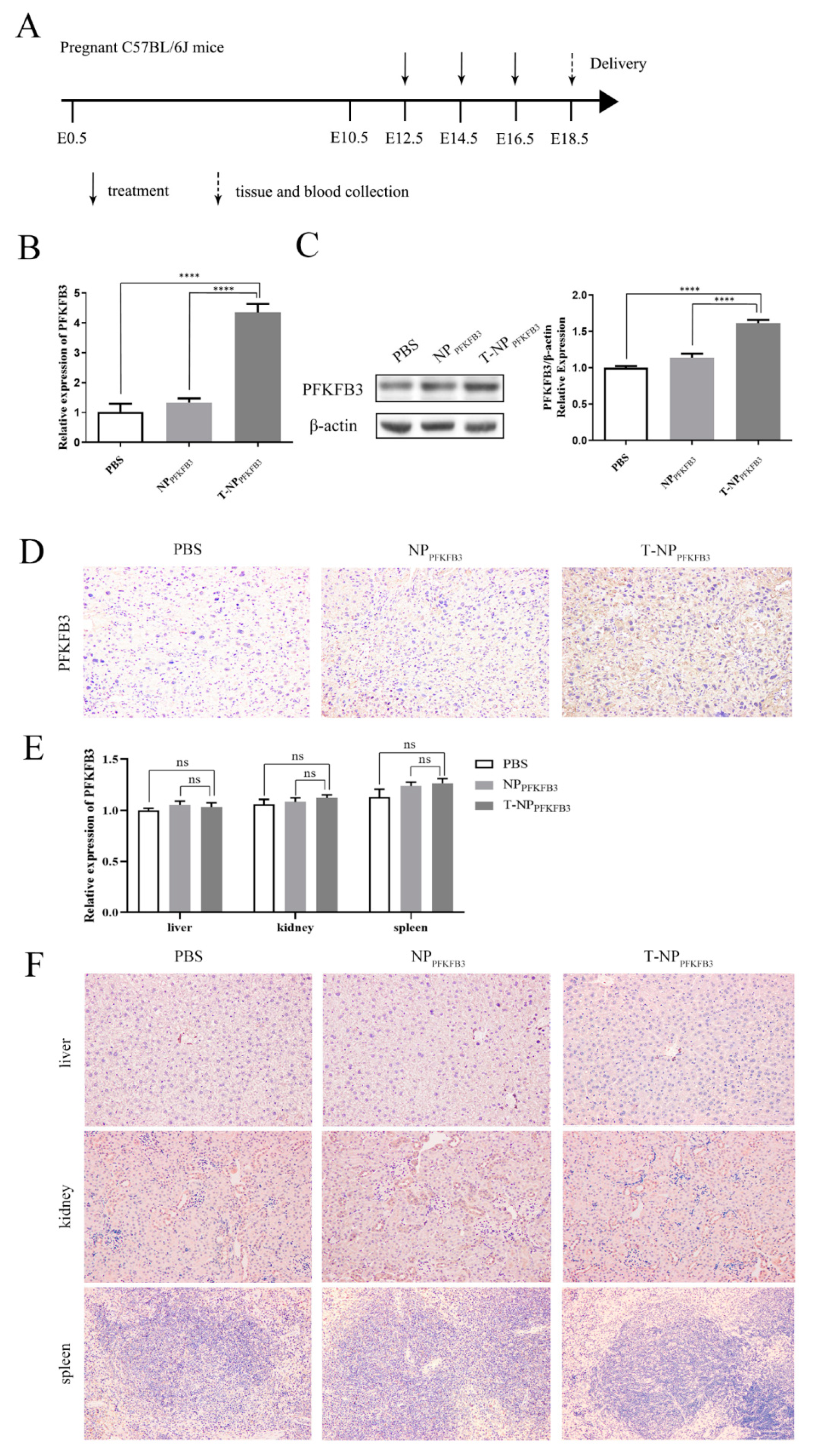
3.2. T-NPPFKFB3 Selectively Accumulates in the Mouse Placenta and Upregulates PFKFB3 Expression
3.3. T-NPPFKFB3 Promotes Placental Angiogenesis and Increases the Placental and Fetal Weights in Mice
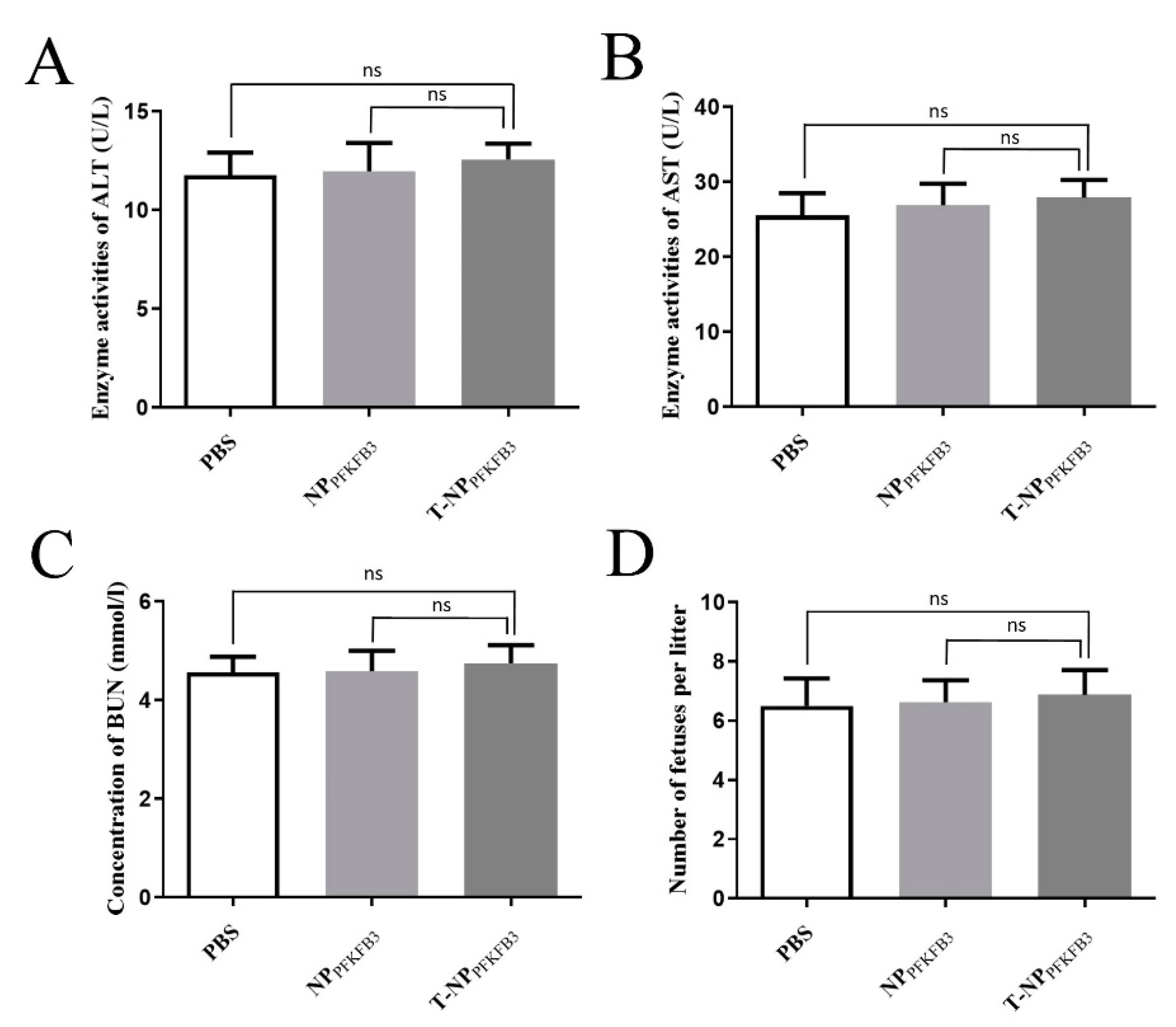
3.4. T-NPPFKFB3 Has No Obvious Side Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Liang, R.; Zheng, M.; Cai, L.; Fan, X. Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications. Int. J. Mol. Sci. 2019, 20, 3642. [Google Scholar] [CrossRef]
- Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011, 306, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Charnock, J.C.; Dilworth, M.R.; Aplin, J.D.; Sibley, C.P.; Westwood, M.; Crocker, I.P. The impact of a human IGF-II analog ([Leu27]IGF-II) on fetal growth in a mouse model of fetal growth restriction. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E24–E31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stanley, J.L.; Andersson, I.J.; Poudel, R.; Rueda-Clausen, C.F.; Sibley, C.P.; Davidge, S.T.; Baker, P.N. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension 2012, 59, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Fisk, N.M.; Atun, R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008, 5, e22. [Google Scholar] [CrossRef]
- Fisk, N.M.; McKee, M.; Atun, R. Relative and absolute addressability of global disease burden in maternal and perinatal health by investment in R&D. Trop. Med. Int. Health 2011, 16, 662–668. [Google Scholar] [CrossRef]
- He, B.; Yang, X.; Li, Y.; Huang, D.; Xu, X.; Yang, W.; Dai, Y.; Zhang, H.; Chen, Z.; Cheng, W. TLR9 (Toll-Like Receptor 9) Agonist Suppresses Angiogenesis by Differentially Regulating VEGFA (Vascular Endothelial Growth Factor A) and sFLT1 (Soluble Vascular Endothelial Growth Factor Receptor 1) in Preeclampsia. Hypertension 2018, 71, 671–680. [Google Scholar] [CrossRef]
- Dakouane-Giudicelli, M.; Brouillet, S.; Traboulsi, W.; Torre, A.; Vallat, G.; Si Nacer, S.; Vallee, M.; Feige, J.J.; Alfaidy, N.; de Mazancourt, P. Inhibition of human placental endothelial cell proliferation and angiogenesis by netrin-4. Placenta 2015, 36, 1260–1265. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial cell metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquiere, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Schoors, S.; De Bock, K.; Cantelmo, A.R.; Georgiadou, M.; Ghesquiere, B.; Cauwenberghs, S.; Kuchnio, A.; Wong, B.W.; Quaegebeur, A.; Goveia, J.; et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014, 19, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Carmeliet, P. The link between angiogenesis and endothelial metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef]
- Van Schaftingen, E.; Lederer, B.; Bartrons, R.; Hers, H.G. A kinetic study of pyrophosphate: Fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur. J. Biochem. 1982, 129, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Pan, H.; Liu, Z.; Xie, J.; Han, W. Roles of PFKFB3 in cancer. Signal Transduct. Target Ther. 2017, 2, 17044. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Liu, W.; Zhang, Y.; Wu, M.; Chen, Z.; Zhao, Y.; Zou, L. MALAT1 sponges miR-26a and miR-26b to regulate endothelial cell angiogenesis via PFKFB3-driven glycolysis in early-onset preeclampsia. Mol. Ther. Nucleic Acids 2021, 23, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Zheng, Q.; Bao, C.; He, J.; Chen, B.; Lyu, D.; Zheng, B.; Xu, Y.; Long, Z.; et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017, 388, 208–219. [Google Scholar] [CrossRef]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-based therapeutics and theranostics drug delivery systems for next generation of liver cancer nanodrug modalities. Int. J. Nanomed. 2020, 15, 1437–1456. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Harris, L.K. Could peptide-decorated nanoparticles provide an improved approach for treating pregnancy complications? Nanomedicine 2016, 11, 2235–2238. [Google Scholar] [CrossRef]
- Irvin-Choy, N.S.; Nelson, K.M.; Gleghorn, J.P.; Day, E.S. Design of nanomaterials for applications in maternal/fetal medicine. J. Mater. Chem. B 2020, 8, 6548–6561. [Google Scholar] [CrossRef]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef] [PubMed]
- Beards, F.; Jones, L.E.; Charnock, J.; Forbes, K.; Harris, L.K. Placental Homing Peptide-microRNA Inhibitor Conjugates for Targeted Enhancement of Intrinsic Placental Growth Signaling. Theranostics 2017, 7, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, X.; Guo, X.; Habtetsion, T.G.; Wang, Y.; Xu, X.; Kandala, S.; Li, Q.; Li, H.; Zhang, C.; et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Wang, L.; Wang, Y.S.; Dou, G.R. PFKFB3: A potential key to ocular angiogenesis. Front. Cell Dev. Biol. 2021, 9, 628317. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Alanine and aspartate aminotransferase and glutamine-cycling pathway: Their roles in pathogenesis of metabolic syndrome. World J. Gastroenterol. 2012, 18, 3775–3781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.E.; Niu, M.; Li, R.Y.; Feng, W.W.; Ma, X.; Dong, Q.; Ma, Z.J.; Li, G.Q.; Meng, Y.K.; Wang, Y.; et al. Untargeted metabolomics reveals dose-response characteristics for effect of rhubarb in a rat model of cholestasis. Front. Pharmacol. 2016, 7, 85. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Souza, A.C.; Street, J.M.; Hsu, V.; Tsuji, T.; Doi, K.; Li, L.; Hu, X.; Zhou, H.; Kumar, P.; et al. Comparison of serum creatinine and serum cystatin C as biomarkers to detect sepsis-induced acute kidney injury and to predict mortality in CD-1 mice. Am. J. Physiol. Renal. Physiol. 2014, 307, F939–F948. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Zhang, Y.; Yu, X.; Sun, X.; Zhu, T.; Li, X.; Liang, W.; Han, Y.; Qin, C. PINK1 deficiency ameliorates cisplatin-induced acute kidney injury in rats. Front. Physiol. 2019, 10, 1225. [Google Scholar] [CrossRef]
- Roset Bahmanyar, E.; Out, H.J.; van Duin, M. Women and babies are dying from inertia: A collaborative framework for obstetrical drug development is urgently needed. Am. J. Obstet. Gynecol. 2021, 225, 43–50. [Google Scholar] [CrossRef]
- Refuerzo, J.S.; Longo, M.; Godin, B. Targeted nanoparticles in pregnancy: A new frontier in perinatal therapeutics. Am. J. Obstet. Gynecol. 2017, 216, 204–205. [Google Scholar] [CrossRef]
- Ali, I.; Alsehli, M.; Scotti, L.; Tullius Scotti, M.; Tsai, S.T.; Yu, R.S.; Hsieh, M.F.; Chen, J.C. Progress in polymeric nano-medicines for theranostic cancer treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Tierney, E.G.; Curtin, C.M.; Cryan, S.A.; O’Brien, F.J. Development of a gene-activated scaffold platform for tissue engineering applications using chitosan-pDNA nanoparticles on collagen-based scaffolds. J. Control. Release 2015, 210, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Montemagno, C.; Leary, J.F.; Ritch, R. Regenerative nanomedicine and the treatment of degenerative retinal diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 113–137. [Google Scholar] [CrossRef]
- Campagnolo, L.; Massimiani, M.; Vecchione, L.; Piccirilli, D.; Toschi, N.; Magrini, A.; Bonanno, E.; Scimeca, M.; Castagnozzi, L.; Buonanno, G.; et al. Silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus. Nanotoxicology 2017, 11, 687–698. [Google Scholar] [CrossRef]
- Mottola, F.; Iovine, C.; Santonastaso, M.; Romeo, M.L.; Pacifico, S.; Cobellis, L.; Rocco, L. NPs-TiO2 and lincomycin coexposure induces DNA damage in cultured human amniotic cells. Nanomaterials 2019, 9, 1511. [Google Scholar] [CrossRef]
- Keelan, J.A.; Leong, J.W.; Ho, D.; Iyer, K.S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015, 10, 2229–2247. [Google Scholar] [CrossRef]
- Whigham, C.A.; MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta 2019, 84, 28–31. [Google Scholar] [CrossRef]
- Furth, P.A.; Hennighausen, L.; Baker, C.; Beatty, B.; Woychick, R. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res. 1991, 19, 6205–6208. [Google Scholar] [CrossRef]
- Schmidt, E.V.; Christoph, G.; Zeller, R.; Leder, P. The cytomegalovirus enhancer: A pan-active control element in transgenic mice. Mol. Cell. Biol. 1990, 10, 4406–4411. [Google Scholar] [CrossRef]
- Arita, E.; Kondoh, M.; Isoda, K.; Nishimori, H.; Yoshida, T.; Mizuguchi, H.; Yagi, K. Evaluation of promoter strength in mouse and rat primary hepatocytes using adenovirus vectors. Eur. J. Pharm. Biopharm. 2008, 70, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, N.; Kaitu’u-Lino, T.; Harris, L.; Tong, S.; Hannan, N. Nanoparticles in pregnancy: The next frontier in reproductive therapeutics. Hum. Reprod. Update 2021, 27, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, M.R.; Sibley, C.P. Review: Transport across the placenta of mice and women. Placenta 2013, 34, S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.P. Treating the dysfunctional placenta. J. Endocrinol. 2017, 234, R81–R97. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, X.; Liu, W.; Zhang, Y.; Liu, W.; Wu, M.; Chen, Z.; Zhao, Y.; Zou, L. Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function. Bioengineering 2022, 9, 652. https://doi.org/10.3390/bioengineering9110652
Li Q, Liu X, Liu W, Zhang Y, Liu W, Wu M, Chen Z, Zhao Y, Zou L. Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function. Bioengineering. 2022; 9(11):652. https://doi.org/10.3390/bioengineering9110652
Chicago/Turabian StyleLi, Qi, Xiaoxia Liu, Weifang Liu, Yang Zhang, Wen Liu, Mengying Wu, Zhirui Chen, Yin Zhao, and Li Zou. 2022. "Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function" Bioengineering 9, no. 11: 652. https://doi.org/10.3390/bioengineering9110652
APA StyleLi, Q., Liu, X., Liu, W., Zhang, Y., Liu, W., Wu, M., Chen, Z., Zhao, Y., & Zou, L. (2022). Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function. Bioengineering, 9(11), 652. https://doi.org/10.3390/bioengineering9110652