A Narrative Review on Means to Promote Oxygenation and Angiogenesis in Oral Wound Healing
Abstract
1. Introduction
2. Oral Wounds
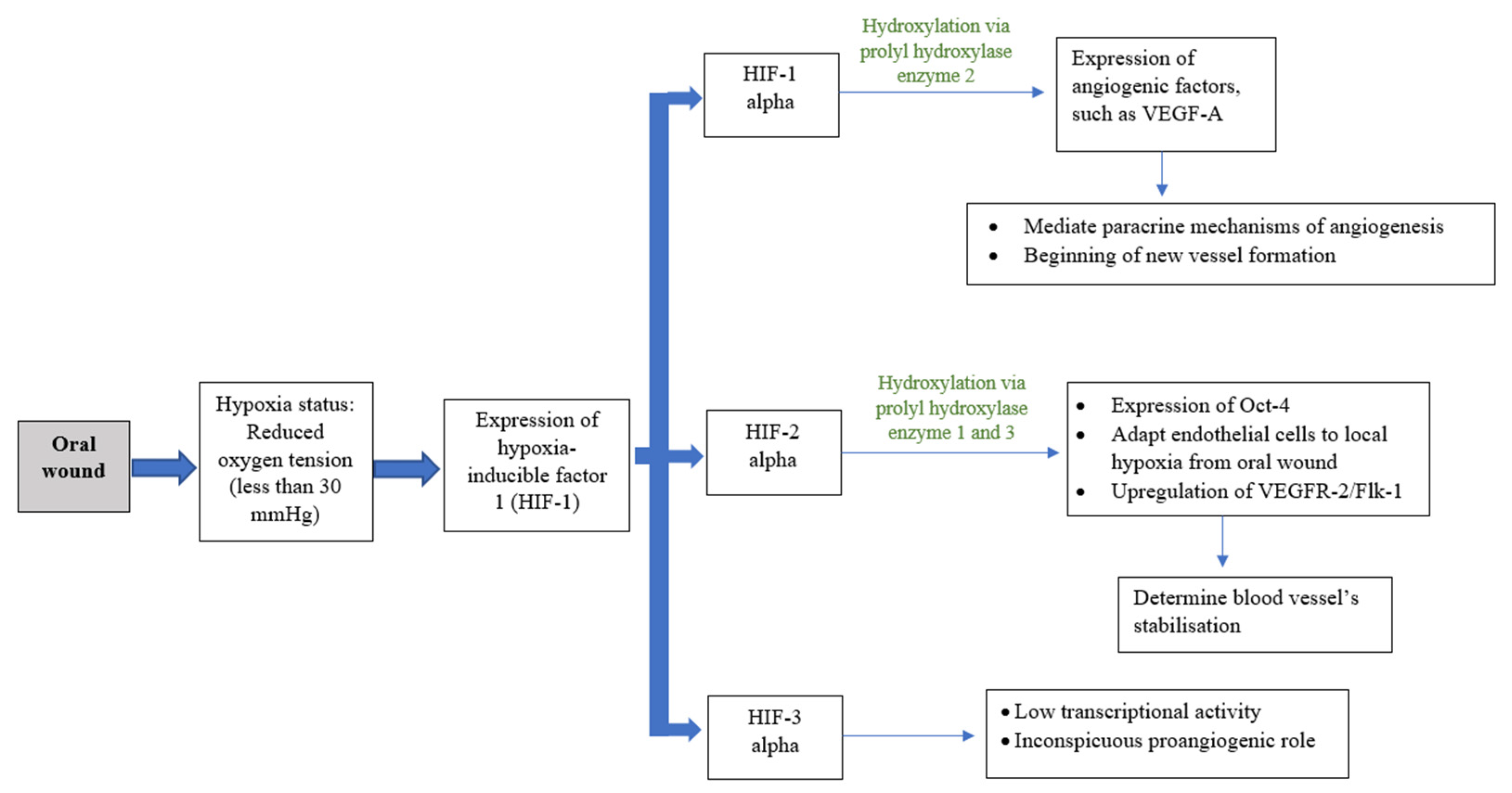
Angiogenesis in Oral Wound Healing
3. Oxygen Therapy
3.1. Ozone Therapy
3.2. Hyperbaric Oxygen Therapy
3.2.1. Osteoradionecrosis (ORN)
3.2.2. Surgical Flap/Graft
3.2.3. Dental Implant Therapy
3.2.4. Periodontal Disease
3.3. Topical Oxygen Therapy
3.3.1. Hydrogen Peroxide
3.3.2. Oxygen-Releasing Gel (blue®m)
| Method | Concentration/Examples | Mechanism of Actions | Oral Conditions | References |
|---|---|---|---|---|
| Ozone | 95–99.95% oxygen and 0.05–5% pure ozone (gas, water, or oil form) | Ulcers, gingival graft surgery, peri-implantitis | [23,24,25,26,27,28] | |
| Hyperbaric | Hyperbaric chamber and breathing in one hundred percent oxygen with a pressure higher than that at sea level (>1.0 atmosphere absolute (ATA)). | Based on concept theory of ”hypoxic-hypocellular-hypoxia” | Osteoradionecrosis | [34,35,36,37,38,39,40,41,42,43] |
| 2.0–2.5 ATA, 90–120 min, twice daily | Improve neovascularization | Enhance outcomes of surgical flaps and grafts | [48,49,50,51,52,53,54,55,56] | |
| Reduce the post-ischemic tissue failure rate; improve flap survival | Implant therapy | [57,58,59] | ||
| Periodontitis | [63,64] | |||
| Topical oxygen | H2O2 blue®m (gel, mouthwash, toothpaste) | Upregulate the body’s defence mechanisms | Post-periodontal surgery, peri-implantitis | [74,75,76,79,84,85,86,87,91,92,93,94,95] |
| Bacteriostatic/bactericidal with anti-inflammatory effects |
3.4. Gas Plasma Therapy
4. Other Means (Sound, Light, Biological, and Chemical Derivatives) to Promote Angiogenesis in Oral Wound Healing
4.1. Sound—Ultrasound
4.2. Light—Photobiomodulation Laser
4.3. Biological Stimulants—Platelet-Derived Products
4.4. Chemical Stimulants—Hyaluronic Acid, Astaxanthin, and Centella Asiatica Extract
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Szpaderska, A.M.; Walsh, C.G.; Steinberg, M.J.; DiPietro, L.A. Distinct Patterns of Angiogenesis in Oral and Skin Wounds. J. Dent. Res. 2005, 84, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Martínez, C. Wound Healing in the Oral Mucosa. In Oral Mucosa in Health and Disease; Springer Science: Cham, Switzerland, 2018; pp. 77–90. [Google Scholar]
- Politis, C.; Schoenaers, J.; Jacobs, R.; Agbaje, O.J. Wound Healing Problems in the Mouth. Front. Physiol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of Oral Mucositis in Patients Who Have Cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; La Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, S.; Udupa, E.G.P.; Kumar, U.; Rao, P.; Honnegowda, T.M. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthetic Res. 2015, 2, 243–249. [Google Scholar] [CrossRef]
- Pettet, G.; Chaplain, M.; McElwain, D.L.S.; Byrne, H. On the rôle of angiogenesis in wound healing. Proc. R. Soc. B Boil. Sci. 1996, 263, 1487–1493. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in Wound Healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Castilla, D.M.; Liu, Z.-J.; Velazquez, O.C. Oxygen: Implications for Wound Healing. Adv. Wound Care 2012, 1, 225–230. [Google Scholar] [CrossRef]
- Hashimoto, T.; Shibasaki, F. Hypoxia-Inducible Factor as an Angiogenic Master Switch. Front. Pediatr. 2015, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2015, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J. Reactive oxygen species (ROS)—A family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef]
- LaVan, F.B.; Hunt, T.K. Oxygen and wound healing. Clin. Plast. Surg. 1990, 17, 463–472. [Google Scholar] [CrossRef]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Sjöberg, F.; Singer, M. The medical use of oxygen: A time for critical reappraisal. J. Intern. Med. 2013, 274, 505–528. [Google Scholar] [CrossRef]
- Nogales, C.G.; Ferrari, P.H.; Kantorovich, E.O.; Lage-Marques, J.L. Ozone Therapy in Medicine and Dentistry. J. Contemp. Dent. Pr. 2008, 9, 75–84. [Google Scholar] [CrossRef]
- Filipovic-Zore, I.; Divic, Z.; Duski, R.; Gnjatovic, N.; Galic, N.; Prebeg, D. Impact of ozone on healing after alveolectomy of impacted lower third molars. Saudi Med. J. 2011, 32, 642–644. [Google Scholar]
- Imamura, Y.M.Y.; Tamura, K.M.I.; Makita, Y.; Masuno, K.; Fujiwara, S.-I.; Shiota, G.; Shiba, A.; Wang, P.-L. The Effect of Ozone on Collagen Type-1 and Inflammatory Cytokine Production in Human Gingival Fibroblasts. Dentistry 2015, 5, 339. [Google Scholar] [CrossRef]
- AlZarea, B.K. Management of denture-related traumatic ulcers using ozone. J. Prosthet. Dent. 2019, 121, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Taşdemir, Z.; Alkan, B.A.; Albayrak, H. Effects of Ozone Therapy on the Early Healing Period of Deepithelialized Gingival Grafts: A Randomized Placebo-Controlled Clinical Trial. J. Periodontol. 2016, 87, 663–671. [Google Scholar] [CrossRef]
- Patel, P.V.; Kumar, V.; Kumar, S.; Gd, V.; Patel, A. Therapeutic effect of topical ozonated oil on the epithelial healing of palatal wound sites: A planimetrical and cytological study. J. Investig. Clin. Dent. 2011, 2, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.V.; Kumar, S.; Vidya, G.; Patel, A.; Holmes, J.C.; Kumar, V. Cytological Assessment of Healing Palatal Donor Site Wounds and Grafted Gingival Wounds after Application of Ozonated Oil: An Eighteen-Month Randomized Controlled Clinical Trial. Acta Cytol. 2012, 56, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, A.; Srivastava, S.; Bhati, L.K.; Chaturvedi, A.; Singh, S.; Agarwal, B.; Arora, K. An evaluation of the effect of ozone therapy on tissues surrounding dental implants. Int. Immunopharmacol. 2021, 96, 107588. [Google Scholar] [CrossRef] [PubMed]
- Isler, S.C.; Unsal, B.; Soysal, F.; Ozcan, G.; Peker, E.; Karaca, I.R. The effects of ozone therapy as an adjunct to the surgical treatment of peri-implantitis. J. Periodontal Implant Sci. 2018, 48, 136–151. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Edwards, M.L. Hyperbaric oxygen therapy. Part 1: History and principles. J. Vet. Emerg. Crit. Care 2010, 20, 284–288. [Google Scholar] [CrossRef]
- Leopardi, L.N.; Metcalfe, M.S.; Maddern, G.J. Ite Boerema—Surgeon and engineer with a double-Dutch legacy to medical technology. Surgery 2004, 135, 99–103. [Google Scholar] [CrossRef]
- Marx, R.E.; Johnson, R.P.; Kline, S.N. Prevention of osteoradionecrosis: A randomized prospective clinical trial of hyperbaric oxygen versus penicillin. J. Am. Dent. Assoc. 1985, 111, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.J.; Koom, W.S.; Lee, C.G.; Kim, Y.B.; Yoo, S.W.; Keum, K.C.; Kim, G.E.; Choi, E.C.; Cha, I. Risk Factors and Dose–Effect Relationship for Mandibular Osteoradionecrosis in Oral and Oropharyngeal Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1084–1091. [Google Scholar] [CrossRef]
- Ceponis, P.; Keilman, C.; Guerry, C.; Freiberger, J. Hyperbaric oxygen therapy and osteonecrosis. Oral Dis. 2017, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Nabil, S.; Samman, N. Risk factors for osteoradionecrosis after head and neck radiation: A systematic review. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Hoff, A.O.; Toth, B.; Hu, M.; Hortobagyi, G.N.; Gagel, R.F. Epidemiology and risk factors for osteonecrosis of the jaw in cancer patients. Ann. N. Y. Acad. Sci. 2011, 1218, 47–54. [Google Scholar] [CrossRef]
- Chen, J.-A.; Wang, C.-C.; Wong, Y.-K.; Wang, C.-P.; Jiang, R.-S.; Lin, J.-C.; Chen, C.-C.; Liu, S.-A. Osteoradionecrosis of mandible bone in patients with oral cancer-associated factors and treatment outcomes. Head Neck 2016, 38, 762–768. [Google Scholar] [CrossRef]
- Meyer, I. Infectious diseases of the jaws. J. Oral Surg. 1970, 28, 17–26. [Google Scholar]
- Marx, R.E. A new concept in the treatment of osteoradionecrosis. J. Oral Maxillofac. Surg. 1983, 41, 351–357. [Google Scholar] [CrossRef]
- Bras, J.; de Jonge, H.; van Merkesteyn, J. Osteoradionecrosis of the mandible: Pathogenesis. Am. J. Otolaryngol. 1990, 11, 244–250. [Google Scholar] [CrossRef]
- Lyons, A.; Ghazali, N. Osteoradionecrosis of the jaws: Current understanding of its pathophysiology and treatment. Br. J. Oral Maxillofac. Surg. 2008, 46, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Undersea and Hyperbaric Medical Society; Hyperbaric Oxygen Committee; Weaver, L.K. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report; Best Publishing Company: North Palm Beach, FL, USA, 2014. [Google Scholar]
- Feldmeier, J.J.; Hampson, N.B. A systematic review of the literature reporting the application of hyperbaric oxygen prevention and treatment of delayed radiation injuries: An evidence based approach. Undersea Hyperb. Med. 2002, 29, 4–30. [Google Scholar] [PubMed]
- Bennett, M.H.; Feldmeier, J.; Hampson, N.B.; Smee, R.; Milross, C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst. Rev. 2016, 2018, CD005005. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zeng, W.; Jing, W.; Tang, W.; Guo, W.H. Evaluation of hyperbaric oxygen therapy for the osteoradionecrosis of the jaws: Meta-analysis. West China J. Stomatol. 2021, 39, 690–697. [Google Scholar] [CrossRef]
- Nabil, S.; Samman, N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 229–243. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The Surgically Induced Stress Response. J. Parenter. Enter. Nutr. 2013, 37 (Suppl. 5), 21S–29S. [Google Scholar] [CrossRef]
- Gutierrez, T.; Hornigold, R.; Pearce, A. The systemic response to surgery. Surgery 2011, 29, 93–96. [Google Scholar] [CrossRef]
- Camporesi, E.M.; Bosco, G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb. Med. 2014, 41, 247–252. [Google Scholar]
- Francis, A.; Baynosa, R.C. Hyperbaric Oxygen Therapy for the Compromised Graft or Flap. Adv. Wound Care 2017, 6, 23–32. [Google Scholar] [CrossRef]
- Renner, G.; McClane, S.D.; Early, E.; Bell, P.; Shaw, B. Enhancement of Auricular Composite Graft Survival with Hyperbaric Oxygen Therapy. Arch. Facial Plast. Surg. 2002, 4, 102–104. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, C.; Gerlach, T.; Kim, D.-Y.; Lineaweaver, W.C.; Buncke, H.J. Effect of Hyperbaric Oxygen on Survival of the Composite Ear Graft in Rats. Ann. Plast. Surg. 1998, 41, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Helmers, R.; Milstein, D.M.; van Hulst, R.A.; de Lange, J. Hyperbaric oxygen therapy accelerates vascularization in keratinized oral mucosal surgical flaps. Head Neck 2014, 36, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Shulman, A.G.; Krohn, H.L. Influence of hyperbaric oxygen and multiple skin allografts on the healing of skin wounds. Surgery 1967, 62, 1051–1058. [Google Scholar] [PubMed]
- Boet, S.; Martin, L.; Cheng-Boivin, O.; Etherington, N.; Louge, P.; Pignel, R.; Pellégrini, M.; Magnan, M.-A.; Bennett, M. Can preventive hyperbaric oxygen therapy optimise surgical outcome?: A systematic review of randomised controlled trials. Eur. J. Anaesthesiol. 2020, 37, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.N.; Chauhan, C.J.; Solanki, J.S. Effectiveness of hyperbaric oxygen therapy in irradiated maxillofacial dental implant patients: A systematic review with meta-analysis. J. Indian Prosthodont. Soc. 2017, 17, 109–119. [Google Scholar] [CrossRef]
- Condezo, A.B.; Araujo, R.Z.; Koga, D.H.; Curi, M.M.; Cardoso, C.L. Hyperbaric oxygen therapy for the placement of dental implants in irradiated patients: Systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2020, 59, 625–632. [Google Scholar] [CrossRef]
- Coulthard, P.; Patel, S.; Grusovin, G.M.; Worthington, H.V.; Esposito, M. Hyperbaric oxygen therapy for irradiated patients who require dental implants: A Cochrane review of randomised clinical trials. Eur. J. Oral Implant. 2008, 9 (Suppl. 1), 105–110. [Google Scholar]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162 Pt A, 22–38. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef]
- Robo, I.; Heta, S.; Karkanaqe, L.; Ostreni, V. HBOT application at cases of gingival inflammation. J. Dent. Oral. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Lombardo, G.; Pardo, A.; Signoretto, C.; Signoriello, A.; Simeoni, E.; Rovera, A.; Nocini, P.F. Hyperbaric oxygen therapy for the treatment of moderate to severe periodontitis: A clinical pilot study. Undersea Hyperb. Med. 2020, 47, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Whelan, H.T.; Buchmann, E.V.; Dhokalia, A.; Kane, M.P.; Whelan, N.T.; Wong-Riley, M.T.; Eells, J.T.; Gould, L.J.; Hammamieh, R.; Das, R.; et al. Effect of NASA Light-Emitting Diode Irradiation on Molecular Changes for Wound Healing in Diabetic Mice. J. Clin. Laser Med. Surg. 2003, 21, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, R.; Yang, X.; Yuan, L. Efficacy and safety of topical oxygen therapy for diabetic foot ulcers: An updated systematic review and meta-analysis. Int. Wound J. 2022; ahead of print. [Google Scholar] [CrossRef]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 2022, 134, 112560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, P.; Yang, R.; Tan, X.; Shi, T.; Ma, J.; Xue, W.; Chi, B. Bio-fabricated nanocomposite hydrogel with ROS scavenging and local oxygenation accelerates diabetic wound healing. J. Mater. Chem. B 2022, 10, 4083–4095. [Google Scholar] [CrossRef]
- Soleimanpour, M.; Mirhaji, S.S.; Jafari, S.; Derakhshankhah, H.; Mamashli, F.; Nedaei, H.; Karimi, M.R.; Motasadizadeh, H.; Fatahi, Y.; Ghasemi, A.; et al. Designing a new alginate-fibrinogen biomaterial composite hydrogel for wound healing. Sci. Rep. 2022, 12, 7213. [Google Scholar] [CrossRef]
- Lei, H.; Zhao, J.; Li, H.; Fan, D. Paramylon hydrogel: A bioactive polysaccharides hydrogel that scavenges ROS and promotes angiogenesis for wound repair. Carbohydr. Polym. 2022, 289, 119467. [Google Scholar] [CrossRef]
- Vulakh, G.M.; Hingorani, A.P.; Ascher, E.; Marks, N. Adjunctive topical oxygen therapy for wound healing in patients with peripheral arterial disease. Vascular 2022, 17085381221080270. [Google Scholar] [CrossRef]
- Velding, K.; Klis, S.-A.; Abass, K.M.; Tuah, W.; Stienstra, Y.; van der Werf, T. Wound Care in Buruli Ulcer Disease in Ghana and Benin. Am. J. Trop. Med. Hyg. 2014, 91, 313–318. [Google Scholar] [CrossRef]
- Gold, S.I. Early Origins of Hydrogen Peroxide Use in Oral Hygiene: A Historical Note. J. Periodontol. 1983, 54, 247. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Cancro, L.P.; Fischman, S.L. Hydrogen Peroxide: A Review of Its Use in Dentistry. J. Periodontol. 1995, 66, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.; Mirza, K. The relative effectiveness of sodium peroxyborate and hydrogen peroxide in treating acute ulcerative gingivitis. Dent. Prac. 1964, 14, 185–189. [Google Scholar]
- Rees, T.D.; Orth, C.F. Oral Ulcerations with Use of Hydrogen Peroxide. J. Periodontol. 1986, 57, 689–692. [Google Scholar] [CrossRef]
- Tombes, M.B.; Gallucci, B. The effects of hydrogen peroxide rinses on the normal oral mucosa. Nurs. Res. 1993, 42, 332–337. [Google Scholar] [CrossRef]
- Hasturk, H.; Nunn, M.; Warbington, M.; Van Dyke, T.E. Efficacy of a Fluoridated Hydrogen Peroxide-Based Mouthrinse for the Treatment of Gingivitis: A Randomized Clinical Trial. J. Periodontol. 2004, 75, 57–65. [Google Scholar] [CrossRef]
- Niveda, R.; Kaarthikeyan, G. Effect of Oxygen Releasing Oral Gel Compared to Chlorhexidine Gel in the Treatment of Periodontitis. J. Pharm. Res. Int. 2020, 32, 75–82. [Google Scholar] [CrossRef]
- Sy, K.; Flamme, J.; Maquet, H.; Chai, F.; Nuet, C.; Siepmann, F.; Agossa, K. Antimicrobial effect and physical properties of an injectable “active oxygen” gel for the treatment of periodontitis. Am. J. Dent. 2020, 33, 305–309. [Google Scholar]
- Shibli, J.A.; Rocha, T.F.; Coelho, F.; de Oliveira Capote, T.S.; Saska, S.; Melo, M.A.; Pingueiro, J.M.S.; de Faveri, M.; Bueno-Silva, B. Metabolic activity of hydro-carbon-oxo-borate on a multispecies subgingival periodontal biofilm: A short communication. Clin. Oral Investig. 2021, 25, 5945–5953. [Google Scholar] [CrossRef]
- Deliberador, T.M.; Weiss, S.G.; Rychuv, F.; Cordeiro, G.; Ten Cate, M.C.L.; Leonardi, L.; Brancher, J.A.; Scariot, R. Comparative Analysis in Vitro of the Application of blue®m Oral Gel versus Chlorhexidine on Porphyromonas gingivalis: A Pilot Study. Adv. Appl. Microbiol. 2020, 10, 194–201. [Google Scholar] [CrossRef]
- Juliana, H.; Tarek, S. Comparative study of the effect of BlueM active oxygen gel and coe-pack dressing on postoperative surgical depigmentation healing. Saudi Dent. J. 2022, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Deliberador, T.M.; Macalossi, J.M.S.; Tenorio, C.; Dall’Agnol, G.S.; Boia, M.F.; Zielak, J.C. Oxygen-releasing agent promotes healing of skin wounds in rats. J. Wound Care, 2022; accepted for publication. [Google Scholar]
- Grootveld, M.; Lynch, E.; Page, G.; Chan, W.; Percival, B.; Anagnostaki, E.; Mylona, V.; Bordin-Aykroyd, S.; Grootveld, K.L. Potential Advantages of Peroxoborates and Their Ester Adducts Over Hydrogen Peroxide as Therapeutic Agents in Oral Healthcare Products: Chemical/Biochemical Reactivity Considerations In Vitro, Ex Vivo And In Vivo. Dent. J. 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, R.; Saita, M.; Sakaue, S.; Okada, R.; Sato, T.; Kawamata, R.; Sakurai, T.; Hamada, N.; Kimoto, K.; Nagasaki, Y. Redox injectable gel protects osteoblastic function against oxidative stress and suppresses alveolar bone loss in a rat peri-implantitis model. Acta Biomater. 2020, 110, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.-T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis—Consensus report of working group 4. Int. Dent. J. 2019, 69, 18–22. [Google Scholar] [CrossRef]
- Furiya-Sato, S.; Fukushima, A.; Mayanagi, G.; Sasaki, K.; Takahashi, N. Electrochemical evaluation of the hydrogen peroxide- and fluoride-induced corrosive property and its recovery on the titanium surface. J. Prosthodont. Res. 2020, 64, 307–312. [Google Scholar] [CrossRef]
- Mattei, B.M.; Imanishi, S.A.W.; de Oliveira Ramos, G.; de Campos, P.S.; Weiss, S.G.; Deliberador, T.M. Mouthwash with Active Oxygen (blue®m) Reduces Postoperative Inflammation and Pain. Case Rep. Dent. 2021, 2021, 5535807. [Google Scholar] [CrossRef]
- Mattei, B.M.; Imanishi, S.A.W.; de Oliveira Ramos, G.; de Campos, P.S.; Weiss, S.G.; Deliberador, T.M. Mouthwash with Active Oxygen (blue®m) Induces Keratinocytes Proliferation. Open J. Stomatol. 2020, 10, 107–114. [Google Scholar] [CrossRef]
- Hossainian, N.; Slot, D.; Afennich, F.; Van Der Weijden, G. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: A systematic review. Int. J. Dent. Hyg. 2011, 9, 171–181. [Google Scholar] [CrossRef]
- Muniz, F.W.M.G.; Cavagni, J.; Langa, G.P.J.; Stewart, B.; Malheiros, Z.; Rösing, C.K. A Systematic Review of the Effect of Oral Rinsing with H2O2 on Clinical and Microbiological Parameters Related to Plaque, Gingivitis, and Microbes. Int. J. Dent. 2020, 2020, 8841722. [Google Scholar] [CrossRef]
- Cunha, E.J.; Auersvald, C.M.; Deliberador, T.M.; Gonzaga, C.C.; Florez, F.L.E.; Correr, G.M.; Storrer, C.L.M. Effects of Active Oxygen Toothpaste in Supragingival Biofilm Reduction: A Randomized Controlled Clinical Trial. Int. J. Dent. 2019, 2019, 3938214. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Ida, Y.; Fukushima, N.; Matsumura, H. Topical application of oxygen nano-bubble water enhances the healing process of ischaemic skin wound healing in an animal model. Int. Wound J. 2022; ahead of print. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, E.; Kieft, I.E.; Sladek, R.E.; van der Laan, E.P.; Slaaf, D.W. Gas plasma treatment: A new approach to surgery? Cri. Rev. Biomed. Eng. 2004, 32, 427–460. [Google Scholar] [CrossRef]
- Harley, J.C.; Suchowerska, N.; McKenzie, D.R. Cancer treatment with gas plasma and with gas plasma-activated liquid: Positives, potentials and problems of clinical translation. Biophys. Rev. 2020, 12, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Abonti, T.R.; Kaku, M.; Kojima, S.; Sumi, H.; Kojima, S.; Yamamoto, T.; Yashima, Y.; Miyahara, H.; Okino, A.; Kawata, T.; et al. Irradiation effects of low temperature multi gas plasma jet on oral bacteria. Dent. Mater. J. 2016, 35, 822–828. [Google Scholar] [CrossRef][Green Version]
- Feril, L.B., Jr.; Tachibana, K.; Ogawa, K.; Yamaguchi, K.; Solano, I.G.; Irie, Y. Therapeutic potential of low-intensity ultrasound (part 1): Thermal and sonomechanical effects. J. Med. Ultrason. 2008, 35, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ramli, R.; Reher, P.; Harris, M.; Meghji, S. The effect of ultrasound on angiogenesis: An in vivo study using the chick chori-oallantoic membrane. Int. J. Oral Maxillofac. Implants 2009, 24, 591–596. [Google Scholar] [PubMed]
- Maddi, A.; Hai, H.; Ong, S.-T.; Sharp, L.; Harris, M.; Meghji, S. Long wave ultrasound may enhance bone regeneration by altering OPG/RANKL ratio in human osteoblast-like cells. Bone 2006, 39, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Harris, M. The conservative management of osteoradionecrosis of the mandible with ultrasound therapy. Br. J. Oral Maxillofac. Surg. 1992, 30, 313–318. [Google Scholar] [CrossRef]
- Reher, P.; Doan, N.; Bradnock, B.; Meghji, S.; Harris, M. Therapeutic ultrasound for osteoradionecrosis: An in vitro comparison between 1 MHz and 45 kHz machines. Eur. J. Cancer 1998, 34, 1962–1968. [Google Scholar] [CrossRef]
- Haffey, P.R.; Bansal, N.; Kaye, E.; Ottestad, E.; Aiyer, R.; Noori, S.; Gulati, A. The Regenerative Potential of Therapeutic Ultrasound on Neural Tissue: A Pragmatic Review. Pain Med. 2020, 21, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Acheta, J.; Stephens, S.B.Z.; Belin, S.; Poitelon, Y. Therapeutic Low-Intensity Ultrasound for Peripheral Nerve Regeneration—A Schwann Cell Perspective. Front. Cell. Neurosci. 2021, 15, 812588. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, Y.; Zhou, J.; Li, J.; Deng, F.; Wang, Z.; Song, J. Low-Intensity Pulsed Ultrasound Stimulation Facilitates Osteogenic Differentiation of Human Periodontal Ligament Cells. PLoS ONE 2014, 9, e95168. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, L.; Deng, F.; Wang, Z.; Song, J. Low-Intensity Pulsed Ultrasound Induces Osteogenic Differentiation of Human Periodontal Ligament Cells Through Activation of Bone Morphogenetic Protein-Smad Signaling. J. Ultrasound Med. 2014, 33, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation Directly Benefits Primary Neurons Functionally Inactivated by Toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef]
- Alghamdi, K.M.; Kumar, A.; Moussa, N.A. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef]
- Zand, N.; Fateh, M.; Ataie-Fashtami, L.; Djavid, G.E.; Fatemi, S.-M.; Shirkavand, A. Promoting Wound Healing in Minor Recurrent Aphthous Stomatitis by Non-Thermal, Non-Ablative CO2Laser Therapy: A Pilot Study. Photomed. Laser Surg. 2012, 30, 719–723. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Tunér, J.; Fekrazad, R. Photobiomodulation in Oral Surgery: A Review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 814–825. [Google Scholar] [CrossRef]
- Daigo, Y.; Daigo, E.; Hasegawa, A.; Fukuoka, H.; Ishikawa, M.; Takahashi, K. Utility of High-Intensity Laser Therapy Combined with Photobiomodulation Therapy for Socket Preservation After Tooth Extraction. Photobiomodul. Photomed. Laser Surg. 2020, 38, 75–83. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Postiglione, F.; Mastrangelo, F.; Petrini, M. Photobiomodulation Enhances the Healing of Postextraction Alveolar Sockets: A Randomized Clinical Trial with Histomorphometric Analysis and Immunohistochemistry. J. Oral Maxillofac. Surg. 2021, 79, 57.e1–57.e12. [Google Scholar] [CrossRef]
- dos Santos, J.A.; Normando, A.G.C.; de Toledo, I.P.; Melo, G.; De Luca Canto, G.; Santos-Silva, A.R.; Guerra, E.N.S. Laser therapy for recurrent aphthous stomatitis: An overview. Clin. Oral Investig. 2020, 24, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Suter, V.G.A.; Sjölund, S.; Bornstein, M.M. Effect of laser on pain relief and wound healing of recurrent aphthous stomatitis: A systematic review. Lasers Med. Sci. 2017, 32, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Uslu, K.; Tansuker, H.D.; Tabaru, A.; Egeren, S.E.; Kulahci, K.K.; Bulut, P.; Emre, F.; Oktay, M.F. Investigation of the effects of thrombocyte-rich plasma, systemic ozone and hyperbaric oxygen treatment on intraoral wound healing in rats: Experimental study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Isler, S.C.; Uraz, A.; Guler, B.; Ozdemir, Y.; Cula, S.; Cetiner, D. Effects of Laser Photobiomodulation and Ozone Therapy on Palatal Epithelial Wound Healing and Patient Morbidity. Photomed. Laser Surg. 2018, 36, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Caccianiga, G.; Rey, G.; Baldoni, M.; Caccianiga, P.; Porcaro, G.; Baldoni, A.; Ceraulo, S. Laser Decontamination and LED Photobiomodulation Promote Bone Regeneration and Wound Healing by Secondary Intention, in Alveolar Ridge Preservation—Clinical and Radiographic Evaluation: A Pilot Experience. Photobiomodul. Photomed. Laser Surg. 2022, 40, 343–354. [Google Scholar] [CrossRef]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef]
- KonRoberto, E.; Buda, R.; Filardo, G.; Di Martino, A.; Timoncini, A.; Cenacchi, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma: Intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg. Sport. Traumatol. Arthrosc. 2010, 18, 472–479. [Google Scholar] [CrossRef]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. The opportunity in perio-implantology: The PRF. Implantodontie 2000, 42, 55–62. [Google Scholar]
- Blatt, S.; Thiem, D.G.E.; Pabst, A.; Al-Nawas, B.; Kämmerer, P.W. Does Platelet-Rich Fibrin Enhance the Early Angiogenetic Potential of Different Bone Substitute Materials? An In Vitro and In Vivo Analysis. Biomedicines 2021, 9, 61. [Google Scholar] [CrossRef]
- Rengarajoo, J.; Ngeow, W.C.; Ibrahim, N.B. The effects of lyophilised platelet-rich plasma in third molar extraction sockets and its surrounding tissues. J. Taibah Univ. Med. Sci. 2022, 17, 289–296. [Google Scholar] [CrossRef]
- Shu, X.Z.; Ghosh, K.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Clark, R.A.; Prestwich, G.D. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J. Biomed. Mater. Res. Part A 2004, 68, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Dalessandri, D.; Zotti, F.; Laffranchi, L.; Migliorati, M.; Isola, G.; Bonetti, S.; Visconti, L. Treatment of recurrent aphthous stomatitis (RAS; aphthae; canker sores) with a barrier forming mouth rinse or topical gel formulation containing hyaluronic acid: A retrospective clinical study. BMC Oral Health 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Aras-Tosun, D.; Önder, C.; Akdoğan, N.; Kurgan, Ş.; Aktay, I.; Tuncay, E.; Orhan, K. Astaxanthin Enhances Gingival Wound Healing following High Glucose-Induced Oxidative Stress. BioMed. Res. Int. 2022, 2022, 4043105. [Google Scholar] [CrossRef] [PubMed]
- Damkerngsuntorn, W.; Rerknimitr, P.; Panchaprateep, R.; Tangkijngamvong, N.; Kumtornrut, C.; Kerr, S.J.; Asawanonda, P.; Tantisira, M.H.; Khemawoot, P. The Effects of a Standardized Extract of Centella asiatica on Postlaser Resurfacing Wound Healing on the Face: A Split-Face, Double-Blind, Randomized, Placebo-Controlled Trial. J. Altern. Complement Med. 2020, 26, 529–536. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef]
- Sukmawan, Y.P.; Alifiar, I.; Nurdianti, L.; Ningsih, W.R. Wound Healing Effectivity of the Ethanolic Extracts of Ageratum conyzoides L. Leaf (White and Purple Flower Type) and Centella asiatica and Astaxanthin Combination Gel Preparation in Animal Model. Turk. J. Pharm. Sci. 2021, 18, 609–615. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Torralba-Ruiz, M.R.; García-Carrillo, N.; Lacal-Luján, J.; Martínez-Díaz, F.; Sánchez-Siles, M. Effects of topical applications of porcine acellular urinary bladder matrix and Centella asiatica extract on oral wound healing in a rat model. Clin. Oral Investig. 2018, 23, 2083–2095. [Google Scholar] [CrossRef]
- Younis, I. Role of oxygen in wound healing. J. Wound Care 2020, 29, S4–S10. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngeow, W.C.; Tan, C.C.; Goh, Y.C.; Deliberador, T.M.; Cheah, C.W. A Narrative Review on Means to Promote Oxygenation and Angiogenesis in Oral Wound Healing. Bioengineering 2022, 9, 636. https://doi.org/10.3390/bioengineering9110636
Ngeow WC, Tan CC, Goh YC, Deliberador TM, Cheah CW. A Narrative Review on Means to Promote Oxygenation and Angiogenesis in Oral Wound Healing. Bioengineering. 2022; 9(11):636. https://doi.org/10.3390/bioengineering9110636
Chicago/Turabian StyleNgeow, Wei Cheong, Chuey Chuan Tan, Yet Ching Goh, Tatiana Miranda Deliberador, and Chia Wei Cheah. 2022. "A Narrative Review on Means to Promote Oxygenation and Angiogenesis in Oral Wound Healing" Bioengineering 9, no. 11: 636. https://doi.org/10.3390/bioengineering9110636
APA StyleNgeow, W. C., Tan, C. C., Goh, Y. C., Deliberador, T. M., & Cheah, C. W. (2022). A Narrative Review on Means to Promote Oxygenation and Angiogenesis in Oral Wound Healing. Bioengineering, 9(11), 636. https://doi.org/10.3390/bioengineering9110636







