Recent Advances in Drug Delivery System Fabricated by Microfluidics for Disease Therapy
Abstract
1. Introduction
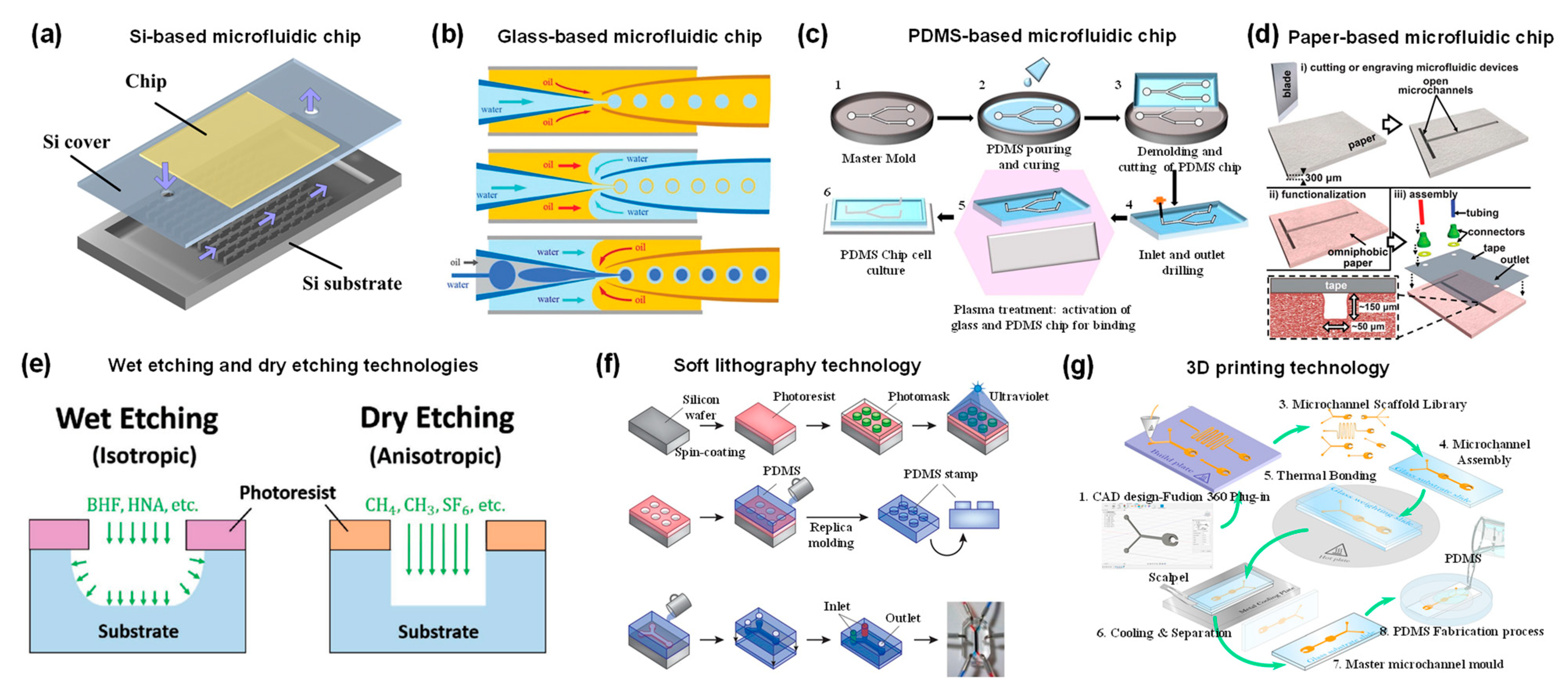
2. Microfluidic Device
3. Drug Delivery System Fabricated by Microfluidics
3.1. Micro/Nanogels for Drug Delivery
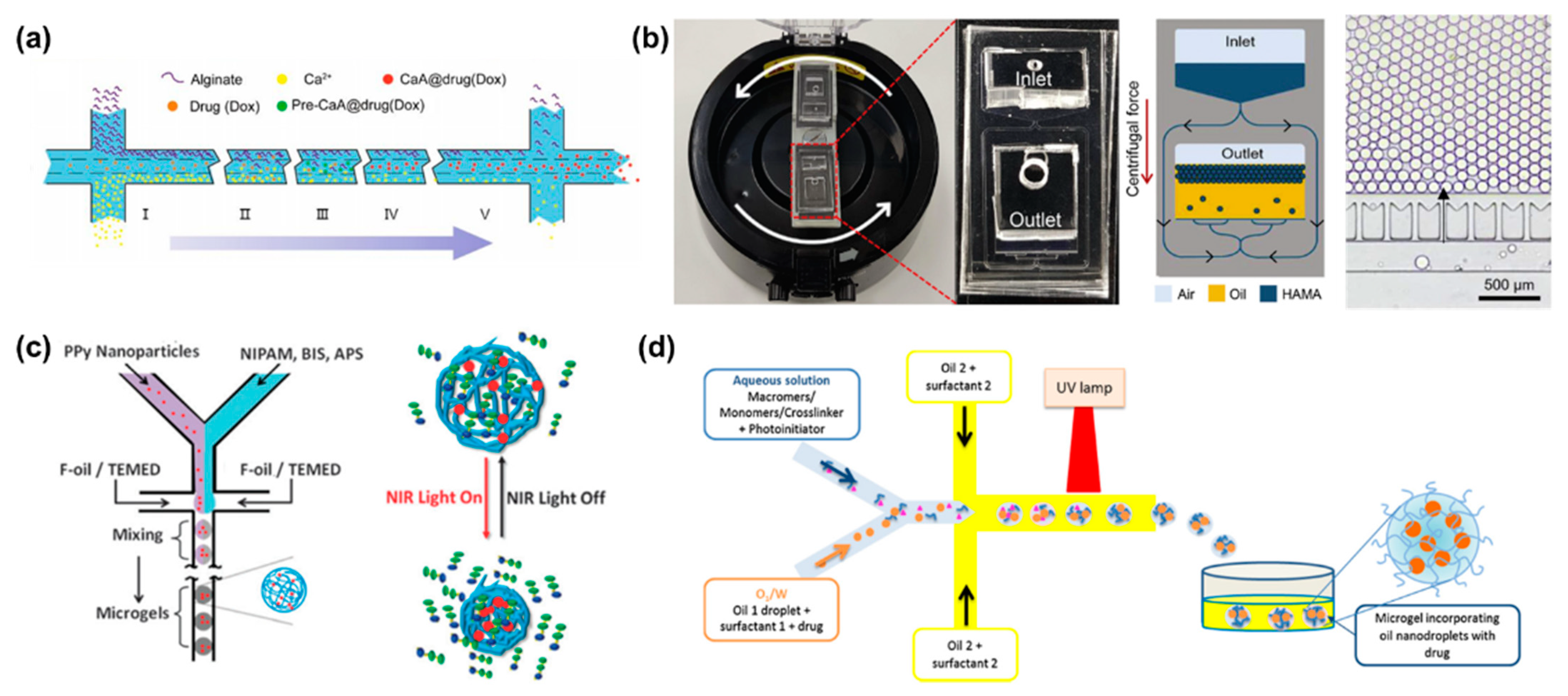
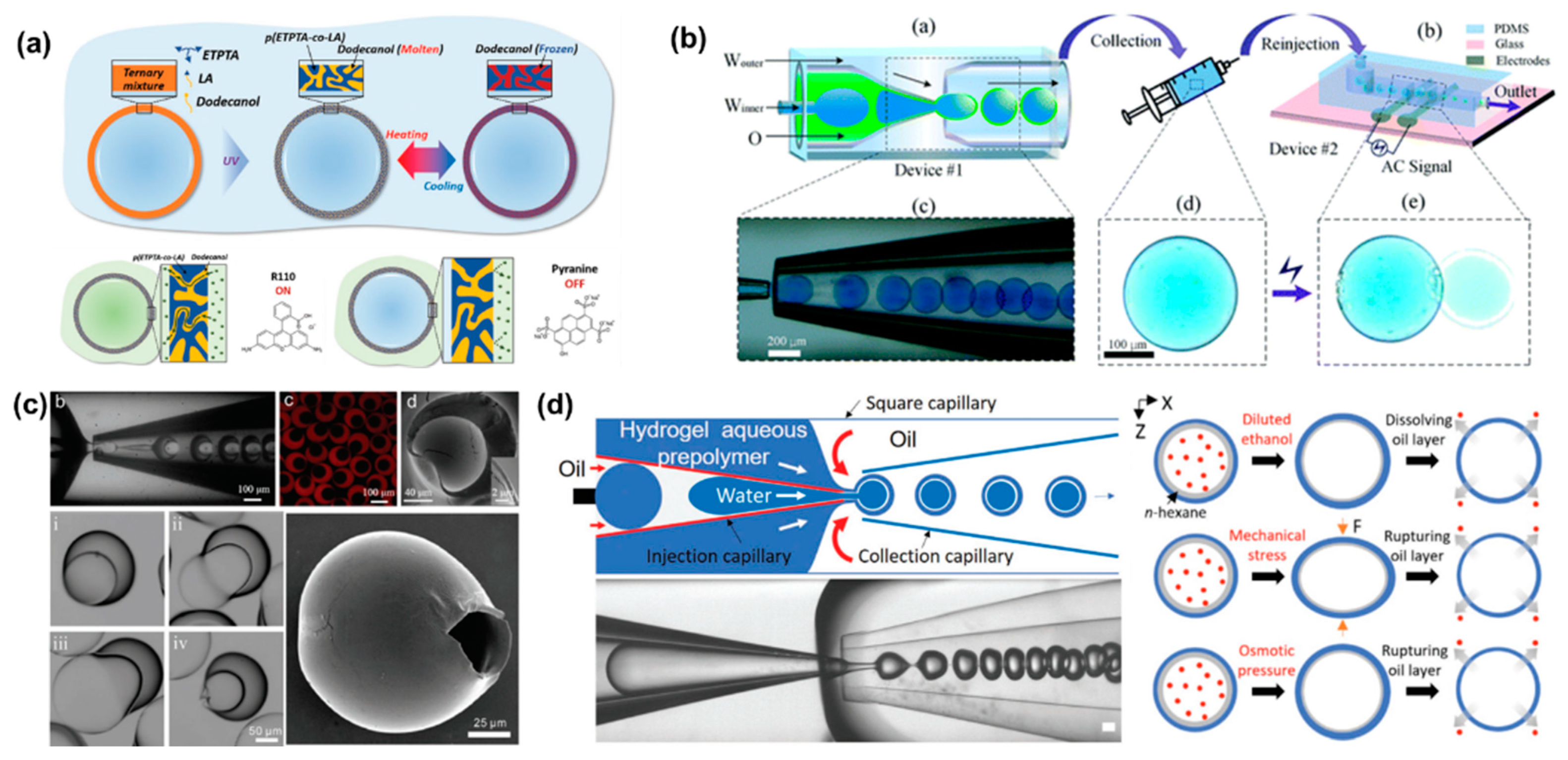
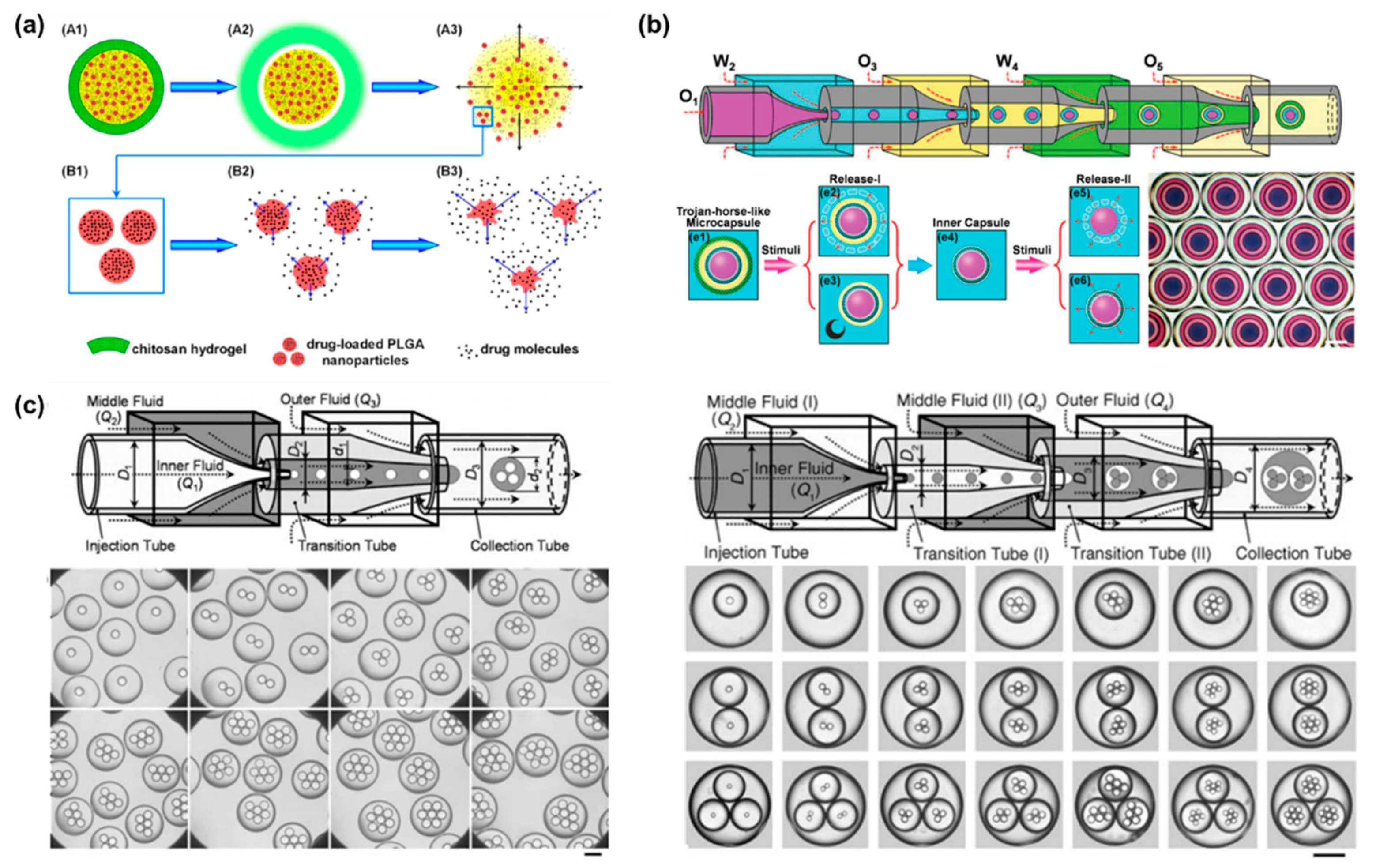
3.2. Microcapsules for Drug Delivery
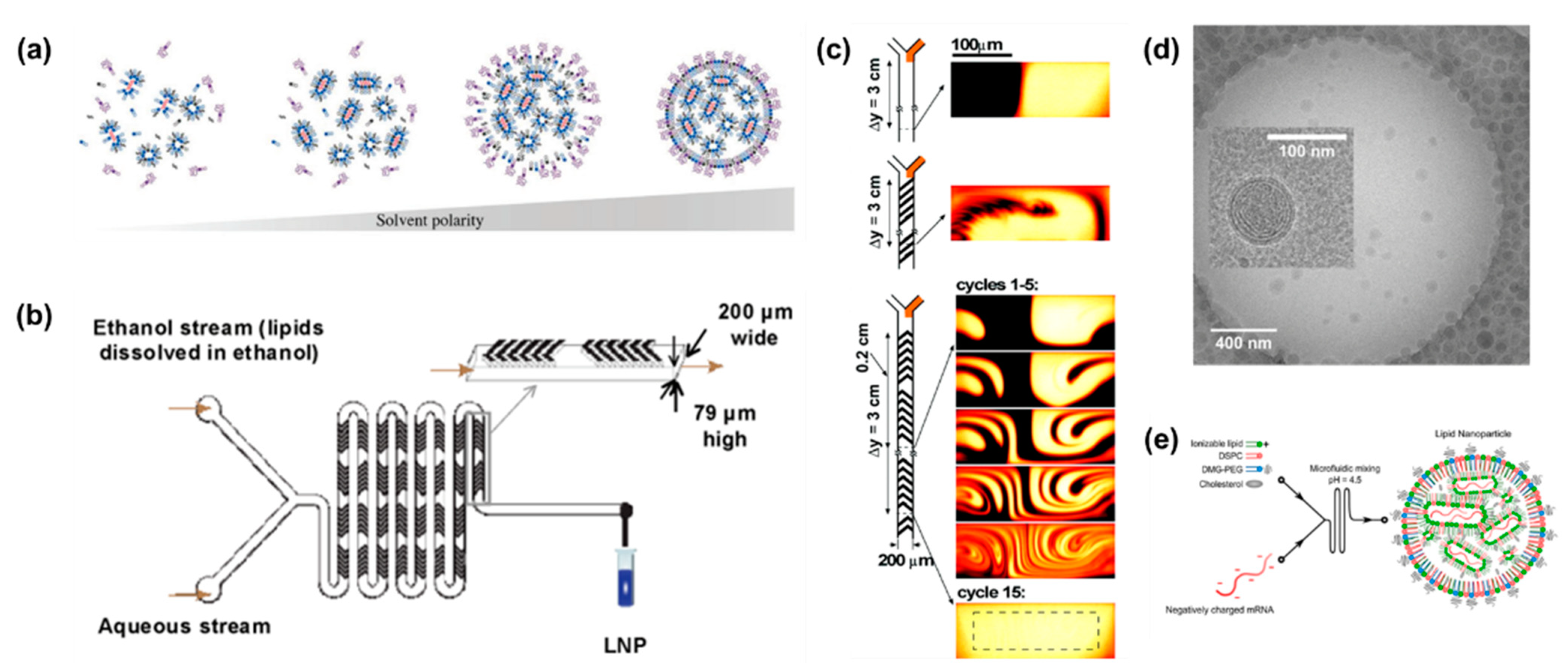
3.3. Lipid Nanoparticles for Drug Delivery
3.4. Other Micro/Nanocarriers for Drug Delivery

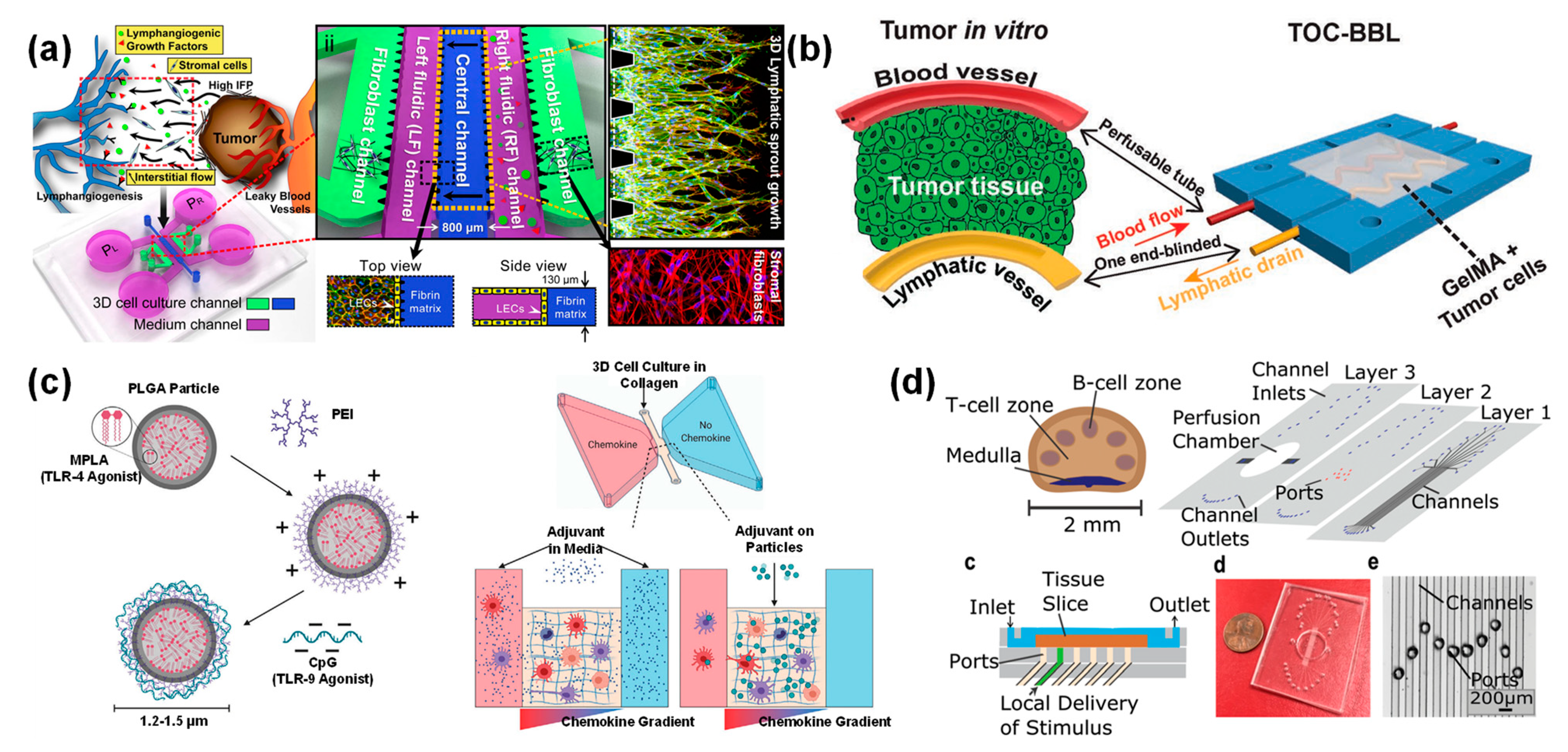
3.5. Lymphatic Chip for Drug Delivery
4. The Challenges of Microfluidic Technology
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.C.H. Microfluidic DNA microarray analysis: A review. Anal. Chim. Acta 2011, 687, 12–27. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Lee, D.; Tice, T.R.; Schwendeman, S.P.; Prausnitz, M.R. Clinical translation of long-acting drug delivery formulations. Nat. Rev. Mater. 2022, 7, 406–420. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 2017, 17, 1856–1883. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Kearney, C.J.; Mooney, D.J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater. 2013, 12, 1004–1017. [Google Scholar] [CrossRef]
- Tao, Z.; Ghoroghchian, P.P. Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotechnol. 2014, 32, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, J.; Kong, X.; Hyeon, T.; Ling, D. Dynamic Nanoparticle Assemblies for Biomedical Applications. Adv. Mater. 2017, 29, 1605897. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Feldmann, C. Microemulsions: Options To Expand the Synthesis of Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 15728–15752. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Tan, S.H.; Gañán-Calvo, A.M.; Tor, S.B.; Loh, N.H.; Nguyen, N.-T. Active droplet generation in microfluidics. Lab Chip 2016, 16, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Choi, T.M.; Shim, T.S.; Frijns, R.A.M.; Kim, S.-H. Microfluidic production of multiple emulsions and functional microcapsules. Lab Chip 2016, 16, 3415–3440. [Google Scholar] [CrossRef]
- Song, R.; Cho, S.; Shin, S.; Kim, H.; Lee, J. From shaping to functionalization of micro-droplets and particles. Nanoscale Adv. 2021, 3, 3395–3416. [Google Scholar] [CrossRef]
- Sticker, D.; Geczy, R.; Häfeli, U.O.; Kutter, J.P. Thiol–Ene Based Polymers as Versatile Materials for Microfluidic Devices for Life Sciences Applications. ACS Appl. Mater. Interfaces 2020, 12, 10080–10095. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Leung, A.; Soleymani, L.; Didar, T.F. Biofunctionalization of Glass- and Paper-Based Microfluidic Devices: A Review. Adv. Mater. Interfaces 2019, 6, 1900940. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Wang, G.-L.; Yang, D.-W.; Wang, Y.; Niu, D.; Zhao, X.-L.; Ding, G.-F. Heat Transfer and Friction Characteristics of the Microfluidic Heat Sink with Variously-Shaped Ribs for Chip Cooling. Sensors 2015, 15, 9547–9562. [Google Scholar] [CrossRef] [PubMed]
- Ofner, A.; Moore, D.G.; Rühs, P.A.; Schwendimann, P.; Eggersdorfer, M.; Amstad, E.; Weitz, D.A.; Studart, A.R. High-Throughput Step Emulsification for the Production of Functional Materials Using a Glass Microfluidic Device. Macromol. Chem. Phys. 2017, 218, 1600472. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.-H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Funano, S.-i.; Ota, N.; Tanaka, Y. A simple and reversible glass–glass bonding method to construct a microfluidic device and its application for cell recovery. Lab Chip 2021, 21, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.A.; Vladisavljević, G.T.; Gu, S.; Ekanem, E.E. Double emulsion production in glass capillary microfluidic device: Parametric investigation of droplet generation behaviour. Chem. Eng. Sci. 2015, 130, 183–196. [Google Scholar] [CrossRef]
- Leister, N.; Vladisavljević, G.T.; Karbstein, H.P. Novel glass capillary microfluidic devices for the flexible and simple production of multi-cored double emulsions. J. Colloid Interface Sci. 2022, 611, 451–461. [Google Scholar] [CrossRef]
- Yu, Y.; Shang, L.; Guo, J.; Wang, J.; Zhao, Y. Design of capillary microfluidics for spinning cell-laden microfibers. Nat. Protoc. 2018, 13, 2557–2579. [Google Scholar] [CrossRef]
- Ter Schiphorst, J.; Saez, J.; Diamond, D.; Benito-Lopez, F.; Schenning, A.P.H.J. Light-responsive polymers for microfluidic applications. Lab Chip 2018, 18, 699–709. [Google Scholar] [CrossRef]
- Akther, F.; Yakob, S.B.; Nguyen, N.-T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Rhyou, J.; Youn, J.; Eom, S.; Kim, D.S. Facile Fabrication of Electrospun Nanofiber Membrane-Integrated PDMS Microfluidic Chip via Silver Nanowires-Uncured PDMS Adhesive Layer. ACS Macro Lett. 2021, 10, 965–970. [Google Scholar] [CrossRef]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A practical guide to rapid-prototyping of PDMS-based microfluidic devices: A tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Wu, N.; Zhu, Y.; Brown, S.; Oakeshott, J.; Peat, T.S.; Surjadi, R.; Easton, C.; Leech, P.W.; Sexton, B.A. A PMMA microfluidic droplet platform for in vitroprotein expression using crude E. coli S30 extract. Lab Chip 2009, 9, 3391–3398. [Google Scholar] [CrossRef]
- Voicu, D.; Lestari, G.; Wang, Y.; DeBono, M.; Seo, M.; Cho, S.; Kumacheva, E. Thermoplastic microfluidic devices for targeted chemical and biological applications. RSC Adv. 2017, 7, 2884–2889. [Google Scholar] [CrossRef]
- Riche, C.T.; Zhang, C.; Gupta, M.; Malmstadt, N. Fluoropolymer surface coatings to control droplets in microfluidic devices. Lab Chip 2014, 14, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Alsharhan, A.T.; Acevedo, R.; Warren, R.; Sochol, R.D. 3D microfluidics via cyclic olefin polymer-based in situ direct laser writing. Lab Chip 2019, 19, 2799–2810. [Google Scholar] [CrossRef]
- Lepowsky, E.; Ghaderinezhad, F.; Knowlton, S.; Tasoglu, S. Paper-based assays for urine analysis. Biomicrofluidics 2017, 11, 051501. [Google Scholar] [CrossRef]
- Glavan, A.C.; Martinez, R.V.; Maxwell, E.J.; Subramaniam, A.B.; Nunes, R.M.D.; Soh, S.; Whitesides, G.M. Rapid fabrication of pressure-driven open-channel microfluidic devices in omniphobic RF paper. Lab Chip 2013, 13, 2922–2930. [Google Scholar] [CrossRef]
- Akyazi, T.; Tudor, A.; Diamond, D.; Basabe-Desmonts, L.; Florea, L.; Benito-Lopez, F. Driving flows in microfluidic paper-based analytical devices with a cholinium based poly(ionic liquid) hydrogel. Sens. Actuators B Chem. 2018, 261, 372–378. [Google Scholar] [CrossRef]
- Anbari, A.; Chien, H.-T.; Datta, S.S.; Deng, W.; Weitz, D.A.; Fan, J. Microfluidic Model Porous Media: Fabrication and Applications. Small 2018, 14, 1703575. [Google Scholar] [CrossRef]
- Nady, E.; Nagy, G.; Huszánk, R. Improvement in mixing efficiency of microfluidic passive mixers functionalized by microstructures created with proton beam lithography. Chem. Eng. Sci. 2022, 247, 117006. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Felton, H.; Hughes, R.; Diaz-Gaxiola, A. Negligible-cost microfluidic device fabrication using 3D-printed interconnecting channel scaffolds. PLoS ONE 2021, 16, e0245206. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Monia Kabandana, G.K.; Zhang, T.; Chen, C. Emerging 3D printing technologies and methodologies for microfluidic development. Anal. Methods 2022, 14, 2885–2906. [Google Scholar] [CrossRef]
- Chan, H.N.; Tan, M.J.A.; Wu, H. Point-of-care testing: Applications of 3D printing. Lab Chip 2017, 17, 2713–2739. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of Nanoparticle Mechanical Properties in Cancer Drug Delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Batalov, I.; Stevens, K.R.; DeForest, C.A. Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels. Proc. Natl. Acad. Sci. USA 2021, 118, e2014194118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Dong, S.; Cui, J.; Hao, J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019, 266, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Mao, A.S.; Shin, J.-W.; Utech, S.; Wang, H.; Uzun, O.; Li, W.; Cooper, M.; Hu, Y.; Zhang, L.; Weitz, D.A.; et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat. Mater. 2017, 16, 236–243. [Google Scholar] [CrossRef]
- Cai, S.; Shi, H.; Li, G.; Xue, Q.; Zhao, L.; Wang, F.; Hu, B. 3D-Printed Concentration-Controlled Microfluidic Chip with Diffusion Mixing Pattern for the Synthesis of Alginate Drug Delivery Microgels. Nanomaterials 2019, 9, 1451. [Google Scholar] [CrossRef]
- Maeda, K.; Onoe, H.; Takinoue, M.; Takeuchi, S. Controlled Synthesis of 3D Multi-Compartmental Particles with Centrifuge-Based Microdroplet Formation from a Multi-Barrelled Capillary. Adv. Mater. 2012, 24, 1340–1346. [Google Scholar] [CrossRef]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef]
- Kim, S.; Yim, S.-G.; Chandrasekharan, A.; Seong, K.-Y.; Lee, T.W.; Kim, B.; Kim, K.; Choi, S.; Yang, S.Y. On-site fabrication of injectable 131I-labeled microgels for local radiotherapy. J. Control. Release 2020, 322, 337–345. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Kubota, R.; Nakamura, K.; Matsuzaki, T.; Minami, S.; Aoyama, T.; Urayama, K.; Hamachi, I. Protein-responsive protein release of supramolecular/polymer hydrogel composite integrating enzyme activation systems. Nat. Commun. 2020, 11, 3859. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Rijpkema, S.J.; Luan, J.; Zhang, S.; Wilson, D.A. Generating biomembrane-like local curvature in polymersomes via dynamic polymer insertion. Nat. Commun. 2021, 12, 2235. [Google Scholar] [CrossRef] [PubMed]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012. [Google Scholar] [CrossRef]
- Luo, R.-C.; Ranjan, S.; Zhang, Y.; Chen, C.-H. Near-infrared photothermal activation of microgels incorporating polypyrrole nanotransducers through droplet microfluidics. Chem. Commun. 2013, 49, 7887–7889. [Google Scholar] [CrossRef]
- Busatto, C.A.; Labie, H.; Lapeyre, V.; Auzely-Velty, R.; Perro, A.; Casis, N.; Luna, J.; Estenoz, D.A.; Ravaine, V. Oil-in-microgel strategy for enzymatic-triggered release of hydrophobic drugs. J. Colloid Interface Sci. 2017, 493, 356–364. [Google Scholar] [CrossRef]
- Foster, G.A.; Headen, D.M.; González-García, C.; Salmerón-Sánchez, M.; Shirwan, H.; García, A.J. Protease-degradable microgels for protein delivery for vascularization. Biomaterials 2017, 113, 170–175. [Google Scholar] [CrossRef]
- Vericella, J.J.; Baker, S.E.; Stolaroff, J.K.; Duoss, E.B.; Hardin, J.O.; Lewicki, J.; Glogowski, E.; Floyd, W.C.; Valdez, C.A.; Smith, W.L.; et al. Encapsulated liquid sorbents for carbon dioxide capture. Nat. Commun. 2015, 6, 6124. [Google Scholar] [CrossRef]
- Song, Y.; Jeong, Y.; Kwon, T.; Lee, D.; Oh, D.Y.; Park, T.-J.; Kim, J.; Kim, J.; Kwon, S. Liquid-capped encoded microcapsules for multiplex assays. Lab Chip 2017, 17, 429–437. [Google Scholar] [CrossRef]
- Keidel, R.; Ghavami, A.; Lugo, D.M.; Lotze, G.; Virtanen, O.; Beumers, P.; Pedersen, J.S.; Bardow, A.; Winkler, R.G.; Richtering, W. Time-resolved structural evolution during the collapse of responsive hydrogels: The microgel-to-particle transition. Sci. Adv. 2018, 4, eaao7086. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, Z.; Parker, R.M.; Wu, Y.; Abell, C.; Scherman, O.A. Interfacial assembly of dendritic microcapsules with host–guest chemistry. Nat. Commun. 2014, 5, 5772. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, C.-H.; Abbaspourrad, A.; Wesner, C.; Caggioni, M.; Zhu, T.; Weitz, D.A. Encapsulation and Enhanced Retention of Fragrance in Polymer Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Park, J.-G.; Choi, T.M.; Manoharan, V.N.; Weitz, D.A. Osmotic-pressure-controlled concentration of colloidal particles in thin-shelled capsules. Nat. Commun. 2014, 5, 3068. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Lee, S.S.; Park, J.; Ku, M.; Yang, J.; Kim, S.-H. Smart Microcapsules with Molecular Polarity- and Temperature-Dependent Permeability. Small 2019, 15, 1900434. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-S.; Kim, E.; Nam, C.; Choi, Y.; Lee, Y.-J.; Weitz, D.A.; Lee, H.; Choi, C.-H. Hydrogel Microcapsules with a Thin Oil Layer: Smart Triggered Release via Diverse Stimuli. Adv. Funct. Mater. 2021, 31, 2009553. [Google Scholar] [CrossRef]
- Jia, Y.; Ren, Y.; Hou, L.; Liu, W.; Jiang, T.; Deng, X.; Tao, Y.; Jiang, H. Electrically controlled rapid release of actives encapsulated in double-emulsion droplets. Lab Chip 2018, 18, 1121–1129. [Google Scholar] [CrossRef]
- Mirvakili, S.M.; Langer, R. Wireless on-demand drug delivery. Nat. Electron. 2021, 4, 464–477. [Google Scholar] [CrossRef]
- Neumann, S.E.; Chamberlayne, C.F.; Zare, R.N. Electrically controlled drug release using pH-sensitive polymer films. Nanoscale 2018, 10, 10087–10093. [Google Scholar] [CrossRef]
- Lee, S.; Lee, T.Y.; Kim, D.J.; Kim, B.; Kim, S.-H. Osmotic-Stress-Mediated Control of Membrane Permeability of Polymeric Microcapsules. Chem. Mater. 2018, 30, 7211–7220. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, L.; Pei, H.; Qin, Z.; Didier, J.; Wu, Z.; Bobe, F.; Ingber, D.E.; Weitz, D.A. Controllable Fabrication of Inhomogeneous Microcapsules for Triggered Release by Osmotic Pressure. Small 2019, 15, 1903087. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Ju, X.-J.; Mu, X.-T.; Wang, W.; Xie, R.; Liu, Z.; Chu, L.-Y. Core–Shell Chitosan Microcapsules for Programmed Sequential Drug Release. ACS Appl. Mater. Interfaces 2016, 8, 10524–10534. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.-L.; Wang, W.; Li, Z.-L.; Ju, X.-J.; Xie, R.; Deng, N.-N.; Wei, J.; Liu, Z.; Chu, L.-Y. Trojan-Horse-Like Stimuli-Responsive Microcapsules. Adv. Sci. 2018, 5, 1700960. [Google Scholar] [CrossRef] [PubMed]
- Okushima, S.; Nisisako, T.; Torii, T.; Higuchi, T. Controlled Production of Monodisperse Double Emulsions by Two-Step Droplet Breakup in Microfluidic Devices. Langmuir 2004, 20, 9905–9908. [Google Scholar] [CrossRef]
- Chu, L.-Y.; Utada, A.S.; Shah, R.K.; Kim, J.-W.; Weitz, D.A. Controllable Monodisperse Multiple Emulsions. Angew. Chem. Int. Ed. 2007, 46, 8970–8974. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Evers, M.J.W.; Kulkarni, J.A.; van der Meel, R.; Cullis, P.R.; Vader, P.; Schiffelers, R.M. State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery. Small Methods 2018, 2, 1700375. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled Vesicle Self-Assembly in Microfluidic Channels with Hydrodynamic Focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Belliveau, N.; Hafez, I.; Leung, A.K.K.; Huft, J.; Hansen, C.; Cullis, P.R. Bottom-Up Design and Synthesis of Limit Size Lipid Nanoparticle Systems with Aqueous and Triglyceride Cores Using Millisecond Microfluidic Mixing. Langmuir 2012, 28, 3633–3640. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.C.; Chen, S.; Leung, A.K.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, Q.; Chen, L.; Chang, J.; Jiang, T.; Zhao, J.; Wang, M.; Chen, P.R. Cationic Lipid-based Intracellular Delivery of Bacterial Effectors for Rewiring Malignant Cell Signaling. Angew. Chem. Int. Ed. 2020, 59, 18087–18094. [Google Scholar] [CrossRef]
- Chen, D.; Love, K.T.; Chen, Y.; Eltoukhy, A.A.; Kastrup, C.; Sahay, G.; Jeon, A.; Dong, Y.; Whitehead, K.A.; Anderson, D.G. Rapid Discovery of Potent siRNA-Containing Lipid Nanoparticles Enabled by Controlled Microfluidic Formulation. J. Am. Chem. Soc. 2012, 134, 6948–6951. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Amiji, M. Clinical approval of nanotechnology-based SARS-CoV-2 mRNA vaccines: Impact on translational nanomedicine. Drug Deliv. Transl. Res. 2021, 11, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Elia, U.; Ramishetti, S.; Rosenfeld, R.; Dammes, N.; Bar-Haim, E.; Naidu, G.S.; Makdasi, E.; Yahalom-Ronen, Y.; Tamir, H.; Paran, N.; et al. Design of SARS-CoV-2 hFc-Conjugated Receptor-Binding Domain mRNA Vaccine Delivered via Lipid Nanoparticles. ACS Nano 2021, 15, 9627–9637. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Gu, X.; Liu, Y.; Chen, G.; Wang, H.; Shao, C.; Chen, Z.; Lu, P.; Zhao, Y. Mesoporous Colloidal Photonic Crystal Particles for Intelligent Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 33936–33944. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Yu, Y.; Huang, Q.; Ji, W.; Li, J.; Zhao, Y. Hierarchically porous composite microparticles from microfluidics for controllable drug delivery. Nanoscale 2018, 10, 12595–12604. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; He, J.; Hourwitz, M.J.; Yang, Y.; Fourkas, J.T.; Han, X.; Nie, Z. Continuous Microfluidic Self-Assembly of Hybrid Janus-Like Vesicular Motors: Autonomous Propulsion and Controlled Release. Small 2015, 11, 3762–3767. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Choi, D.G.; Lee, H.; Shim, M.S.; Bong, K.W. Fabrication of dual stimuli-responsive multicompartmental drug carriers for tumor-selective drug release. Lab Chip 2018, 18, 754–764. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, W.; Achazi, K.; Cuellar-Camacho, J.L.; Melzig, M.F.; Chen, W.; Haag, R. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 77, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, J.; Wang, H.; Wang, Q.; Zhu, J.; Yang, Y. Microfluidic Fabrication and Thermoreversible Response of Core/Shell Photonic Crystalline Microspheres Based on Deformable Nanogels. Langmuir 2012, 28, 17186–17192. [Google Scholar] [CrossRef] [PubMed]
- Ulker, D.; Barut, I.; Şener, E.; Bütün, V. Advanced liposome based PEGylated microgel as a novel release system for 5-fluorouracil against MCF-7 cancer cell. Eur. Polym. J. 2021, 146, 110270. [Google Scholar] [CrossRef]
- Wang, C.X.; Utech, S.; Gopez, J.D.; Mabesoone, M.F.J.; Hawker, C.J.; Klinger, D. Non-Covalent Microgel Particles Containing Functional Payloads: Coacervation of PEG-Based Triblocks via Microfluidics. ACS Appl. Mater. Interfaces 2016, 8, 16914–16921. [Google Scholar] [CrossRef]
- Hsu, M.N.; Luo, R.; Kwek, K.Z.; Por, Y.C.; Zhang, Y.; Chen, C.-H. Sustained release of hydrophobic drugs by the microfluidic assembly of multistage microgel/poly (lactic-co-glycolic acid) nanoparticle composites. Biomicrofluidics 2015, 9, 052601. [Google Scholar] [CrossRef]
- Bai, X.; Sun, Q.; Cui, H.; Guerzoni, L.P.B.; Wuttke, S.; Kiessling, F.; De Laporte, L.; Lammers, T.; Shi, Y. Controlled Covalent Self-Assembly of a Homopolymer for Multiscale Materials Engineering. Adv. Mater. 2022, 34, 2109701. [Google Scholar] [CrossRef]
- Sui, X.; Shui, L.; Cui, J.; Xie, Y.; Song, J.; van den Berg, A.; Hempenius, M.A.; Julius Vancso, G. Redox-responsive organometallic microgel particles prepared from poly(ferrocenylsilane)s generated using microfluidics. Chem. Commun. 2014, 50, 3058–3060. [Google Scholar] [CrossRef]
- Kim, B.; Jeon, T.Y.; Oh, Y.-K.; Kim, S.-H. Microfluidic Production of Semipermeable Microcapsules by Polymerization-Induced Phase Separation. Langmuir 2015, 31, 6027–6034. [Google Scholar] [CrossRef]
- Navi, M.; Kieda, J.; Tsai, S.S.H. Magnetic polyelectrolyte microcapsules via water-in-water droplet microfluidics. Lab Chip 2020, 20, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Zieringer, M.A.; Carroll, N.J.; Abbaspourrad, A.; Koehler, S.A.; Weitz, D.A. Microcapsules for Enhanced Cargo Retention and Diversity. Small 2015, 11, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hou, L.; Hou, J.; Yu, J.; Hu, Q. Generation of Ultra-Thin-Shell Microcapsules Using Osmolarity-Controlled Swelling Method. Micromachines 2020, 11, 444. [Google Scholar] [CrossRef]
- Lee, H.; Choi, C.-H.; Abbaspourrad, A.; Wesner, C.; Caggioni, M.; Zhu, T.; Nawar, S.; Weitz, D.A. Fluorocarbon Oil Reinforced Triple Emulsion Drops. Adv. Mater. 2016, 28, 8425–8430. [Google Scholar] [CrossRef]
- Lee, T.Y.; Ku, M.; Kim, B.; Lee, S.; Yang, J.; Kim, S.-H. Microfluidic Production of Biodegradable Microcapsules for Sustained Release of Hydrophilic Actives. Small 2017, 13, 1700646. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, X.; Huang, R.; Wang, H.; Lan, P.; Zhao, Y. Prebiotics and Postbiotics Synergistic Delivery Microcapsules from Microfluidics for Treating Colitis. Adv. Sci. 2022, 9, 2104089. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.-P.; Ju, X.-J.; Xie, R.; Liu, Y.-M.; Wang, W.; Zhang, J.-J.; Niu, C.H.; Chu, L.-Y. Monodisperse core-shell chitosan microcapsules for pH-responsive burst release of hydrophobic drugs. Soft Matter 2011, 7, 4821–4827. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, C.; Eggersdorfer, M.; Cui, J.; Li, Y.; Hai, M.; Chen, D.; Weitz, D.A. A general strategy for one-step fabrication of biocompatible microcapsules with controlled active release. Chin. Chem. Lett. 2020, 31, 249–252. [Google Scholar] [CrossRef]
- Liu, R.; Wu, Q.; Huang, X.; Zhao, X.; Chen, X.; Chen, Y.; Weitz, D.A.; Song, Y. Synthesis of nanomedicine hydrogel microcapsules by droplet microfluidic process and their pH and temperature dependent release. RSC Adv. 2021, 11, 37814–37823. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Gong, J.; Ma, J. Microfluidic Fabrication of Monodisperse Microcapsules for Thermo-Triggered Release of Liposoluble Drugs. Polymers 2020, 12, 2200. [Google Scholar] [CrossRef]
- Ryu, S.A.; Hwang, Y.-H.; Oh, H.; Jeon, K.; Lee, J.H.; Yoon, J.; Lee, J.B.; Lee, H. Biocompatible Wax-Based Microcapsules with Hermetic Sealing for Thermally Triggered Release of Actives. ACS Appl. Mater. Interfaces 2021, 13, 36380–36387. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sakai, Y.; Sugimori, N.; Ikeda, T.; Monzen, M.; Ono, T. Microfluidic Production of Monodisperse Biopolymer Microcapsules for Latent Heat Storage. ACS Mater. Au 2022, 2, 250–259. [Google Scholar] [CrossRef]
- Mu, X.-T.; Li, Y.; Ju, X.-J.; Yang, X.-L.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.-Y. Microfluidic Fabrication of Structure-Controlled Chitosan Microcapsules via Interfacial Cross-Linking of Droplet Templates. ACS Appl. Mater. Interfaces 2020, 12, 57514–57525. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-S.; Yang, C.-H.; Wang, Y.-C.; Wang, W.-T.; Lu, Y.-Y. Microfluidic Synthesis of Vinblastine-Loaded Multifunctional Particles for Magnetically Responsive Controlled Drug Release. Pharmaceutics 2019, 11, 212. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, C.; Wen, B.; Fu, X.; Shang, L.; Kong, W.; Zhao, Y. Oxygen-carrying microfluidic microcapsules for enhancing chemo-sonodynamic therapy on patient-derived tumor organoid models. Chem. Eng. J. 2022, 435, 134871. [Google Scholar] [CrossRef]
- Field, R.D.; Jakus, M.A.; Chen, X.; Human, K.; Zhao, X.; Chitnis, P.V.; Sia, S.K. Ultrasound-Responsive Aqueous Two-Phase Microcapsules for On-Demand Drug Release. Angew. Chem. Int. Ed. 2022, 61, e202116515. [Google Scholar] [CrossRef]
- Tang, J.; Mi, J.; Huang, W.; Zhong, H.; Li, Y.; Zhou, J.; Johri, A.M. Controlled drug release from ultrasound-visualized elastic eccentric microcapsules using different resonant modes. J. Mater. Chem. B 2018, 6, 1920–1929. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, L.-H.; Lienemann, P.S.; Rossow, T.; Polenz, I.; Vallmajo-Martin, Q.; Ehrbar, M.; Na, H.; Mooney, D.J.; Weitz, D.A. One-Step Microfluidic Fabrication of Polyelectrolyte Microcapsules in Aqueous Conditions for Protein Release. Angew. Chem. Int. Ed. 2016, 55, 13470–13474. [Google Scholar] [CrossRef]
- Luo, Z.; Zhao, G.; Panhwar, F.; Akbar, M.F.; Shu, Z. Well-designed microcapsules fabricated using droplet-based microfluidic technique for controlled drug release. J. Drug Deliv. Sci. Technol. 2017, 39, 379–384. [Google Scholar] [CrossRef]
- Hu, Y.; Pérez-Mercader, J. Microcapsules with Distinct Dual-Layer Shells and Their Applications for the Encapsulation, Preservation, and Slow Release of Hydrophilic Small Molecules. ACS Appl. Mater. Interfaces 2019, 11, 41640–41648. [Google Scholar] [CrossRef]
- Watanabe, T.; Motohiro, I.; Ono, T. Microfluidic Formation of Hydrogel Microcapsules with a Single Aqueous Core by Spontaneous Cross-Linking in Aqueous Two-Phase System Droplets. Langmuir 2019, 35, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, H.; Tang, G.; Ma, T.; Cao, X. Controllable microfluidic fabrication of Janus and microcapsule particles for drug delivery applications. RSC Adv. 2015, 5, 23181–23188. [Google Scholar] [CrossRef]
- Herranz-Blanco, B.; Arriaga, L.R.; Mäkilä, E.; Correia, A.; Shrestha, N.; Mirza, S.; Weitz, D.A.; Salonen, J.; Hirvonen, J.; Santos, H.A. Microfluidic assembly of multistage porous silicon–lipid vesicles for controlled drug release. Lab Chip 2014, 14, 1083–1086. [Google Scholar] [CrossRef]
- Khan, I.U.; Serra, C.A.; Anton, N.; Li, X.; Akasov, R.; Messaddeq, N.; Kraus, I.; Vandamme, T.F. Microfluidic conceived drug loaded Janus particles in side-by-side capillaries device. Int. J. Pharm. 2014, 473, 239–249. [Google Scholar] [CrossRef]
- He, F.; Wang, W.; He, X.-H.; Yang, X.-L.; Li, M.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Controllable Multicompartmental Capsules with Distinct Cores and Shells for Synergistic Release. ACS Appl. Mater. Interfaces 2016, 8, 8743–8754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, D.; Shahbazi, M.-A.; Mäkilä, E.; Herranz-Blanco, B.; Salonen, J.; Hirvonen, J.; Santos, H.A. Fabrication of a Multifunctional Nano-in-micro Drug Delivery Platform by Microfluidic Templated Encapsulation of Porous Silicon in Polymer Matrix. Adv. Mater. 2014, 26, 4497–4503. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Choi, D.G.; Roh, Y.H.; Shim, M.S.; Bong, K.W. Microfluidic Synthesis of pH-Sensitive Multicompartmental Microparticles for Multimodulated Drug Release. Small 2016, 12, 3463–3470. [Google Scholar] [CrossRef]
- Peng, F.; Månsson, L.K.; Holm, S.H.; Ghosh, S.; Carlström, G.; Crassous, J.J.; Schurtenberger, P.; Tegenfeldt, J.O. A Droplet-Based Microfluidics Route to Temperature-Responsive Colloidal Molecules. J. Phys. Chem. B 2019, 123, 9260–9271. [Google Scholar] [CrossRef]
- Roh, Y.H.; Moon, J.Y.; Hong, E.J.; Kim, H.U.; Shim, M.S.; Bong, K.W. Microfluidic fabrication of biocompatible poly(N-vinylcaprolactam)-based microcarriers for modulated thermo-responsive drug release. Colloids Surf. B Biointerfaces 2018, 172, 380–386. [Google Scholar] [CrossRef]
- Roh, Y.H.; Eom, J.Y.; Choi, D.G.; Moon, J.Y.; Shim, M.S.; Bong, K.W. Gold nanorods-encapsulated thermosensitive drug carriers for NIR light-responsive anticancer therapy. J. Ind. Eng. Chem. 2021, 98, 211–216. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Kobayashi, T. Fabrication and Photoresponse of Supramolecular Liquid−Crystalline Microparticles. ACS Appl. Mater. Interfaces 2011, 3, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, L.; Gu, Y.-Y.; Chu, L.-Y. Microfluidic fabrication of monodisperse microcapsules for glucose-response at physiological temperature. Soft Matter 2013, 9, 4150–4159. [Google Scholar] [CrossRef]
- Rodrigues, J.; Heinrich, M.A.; Teixeira, L.M.; Prakash, J. 3D In Vitro Model (R)evolution: Unveiling Tumor-Stroma Interactions. Trends Cancer 2021, 7, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Gough, A.; Soto-Gutierrez, A.; Vernetti, L.; Ebrahimkhani, M.R.; Stern, A.M.; Taylor, D.L. Human biomimetic liver microphysiology systems in drug development and precision medicine. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef]
- Kim, S.; Chung, M.; Jeon, N.L. Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro. Biomaterials 2016, 78, 115–128. [Google Scholar] [CrossRef]
- Cao, X.; Ashfaq, R.; Cheng, F.; Maharjan, S.; Li, J.; Ying, G.; Hassan, S.; Xiao, H.; Yue, K.; Zhang, Y.S. A Tumor-on-a-Chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv. Funct. Mater. 2019, 29, 1807173. [Google Scholar] [CrossRef]
- Atalis, A.; Dixon, J.B.; Roy, K. Soluble and Microparticle-Based Delivery of TLR4 and TLR9 Agonists Differentially Modulate 3D Chemotaxis of Bone Marrow-Derived Dendritic Cells. Adv. Healthc. Mater. 2021, 10, 2001899. [Google Scholar] [CrossRef]
- Ross, A.E.; Belanger, M.C.; Woodroof, J.F.; Pompano, R.R. Spatially resolved microfluidic stimulation of lymphoid tissue ex vivo. Analyst 2017, 142, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-h.; Huang, S.A.; Aw, W.Y.; Rathod, M.L.; Cho, C.; Ligler, F.S.; Polacheck, W.J. Multilayer microfluidic platform for the study of luminal, transmural, and interstitial flow. Biofabrication 2022, 14, 025007. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Serrano, J.C.; Kamm, R.D. Cooperative Effects of Vascular Angiogenesis and Lymphangiogenesis. Regen. Eng. Transl. Med. 2018, 4, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.R.; Ilan, I.S.; Lee, E. A bioengineered lymphatic vessel model for studying lymphatic endothelial cell-cell junction and barrier function. Microcirculation 2021, 28, e12730. [Google Scholar] [CrossRef]
- Sato, M.; Sasaki, N.; Ato, M.; Hirakawa, S.; Sato, K.; Sato, K. Microcirculation-on-a-Chip: A Microfluidic Platform for Assaying Blood- and Lymphatic-Vessel Permeability. PLoS ONE 2015, 10, e0137301. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Gong, M.M.; Skala, M.C.; Harari, P.M.; Beebe, D.J. Human Tumor-Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells. Adv. Healthc. Mater. 2020, 9, 1900925. [Google Scholar] [CrossRef]
- Lugo-Cintrón, K.M.; Ayuso, J.M.; White, B.R.; Harari, P.M.; Ponik, S.M.; Beebe, D.J.; Gong, M.M.; Virumbrales-Muñoz, M. Matrix density drives 3D organotypic lymphatic vessel activation in a microfluidic model of the breast tumor microenvironment. Lab Chip 2020, 20, 1586–1600. [Google Scholar] [CrossRef]
- Lugo-Cintrón, K.M.; Ayuso, J.M.; Humayun, M.; Gong, M.M.; Kerr, S.C.; Ponik, S.M.; Harari, P.M.; Virumbrales-Muñoz, M.; Beebe, D.J. Primary head and neck tumour-derived fibroblasts promote lymphangiogenesis in a lymphatic organotypic co-culture model. eBioMedicine 2021, 73, 103634. [Google Scholar] [CrossRef]
- Pisano, M.; Triacca, V.; Barbee, K.A.; Swartz, M.A. An in vitro model of the tumor–lymphatic microenvironment with simultaneous transendothelial and luminal flows reveals mechanisms of flow enhanced invasion. Integr. Biol. 2015, 7, 525–533. [Google Scholar] [CrossRef]
- Shim, S.; Belanger, M.C.; Harris, A.R.; Munson, J.M.; Pompano, R.R. Two-way communication between ex vivo tissues on a microfluidic chip: Application to tumor–lymph node interaction. Lab Chip 2019, 19, 1013–1026. [Google Scholar] [CrossRef]
| Drug Delivery Systems | Materials | Stimuli | Ref. |
|---|---|---|---|
| Micro/nanogels | sodium alginate; poly(vinyl alcohol); | pH | [57,103] |
| hyaluronic acid; Methacrylate-modified hyaluronic acid | hyaluronidase | [60,67] | |
| PNIPAM and Pyy; poly(N-isopropylacrylamide-coacrylic acid); PEGylated poly(2-(N-morpholino) ethyl methacrylate) | temperature | [66,104,105] | |
| PEO-based triblock copolymers | structure and ionic nature | [106] | |
| poly (ethylene glycol); N-(2-oxopropyl)methacrylamide (OPMA) | sustained release | [107,108] | |
| poly(ferrocenylsilane) | redox | [109] | |
| Microcapsules | decanol, lauryl acrylate and trimethylolpropane ethoxylate triacrylate (ETPTA); GMA, ETPTA and 1-decanol | polarity | [75,110] |
| PDMS, silicon oil and curing agent; | electric fields | [77] | |
| poly(ethylene glycol) divinyl ether and trimethylolpropane tris(3-mercaptopropionate); PEG and dextran; perfluoropolyether; PDMS, silicon oil and curing agent; | osmolarity | [81,111,112,113] | |
| poly(ethylene glycol) diacrylate (PEGDA); ethoxylated trimethylolpropane triacry-late | multifunctional responsive | [76,114] | |
| chitosan and PLGA; PLGA; alginate sodium, resistant starch and chitosan; terephthalaldehyde-crosslinked chitosan; shellac | pH | [82,115,116,117,118] | |
| PEGDA and PNIPAM; sodium alginate and PEG-g-chitosan; palm oil; cellulose acetate | temperature | [83,119,120,121,122] | |
| chitosan, terephthalaldehyde (TPA) and magnetic NPs; iron oxide NPs and chi-tosan; polyethylene glycol, polyIJdial-lyldimethylammonium chloride) and ferrofluid | magnetic | [111,123,124] | |
| perfluorocarbon and sodium alginate; PEGDA | ultrasound | [125,126,127] | |
| oppositely charged polyelectrolyte; PLGA and PCL photocurable resin | sustained release | [128,129,130] | |
| Other micro/nanocarriers | dextran-rich core and a tetra-PEG; PLGA and PCL; PLGA and MSNs; porous silicon (PSi) microparticles and lipid; poly(acrylamide) and poly(methyl acrylate) | sustained release | [100,131,132,133,134] |
| calcium alginate; poly(methyl vinyl ether-co-maleic acid) and porous silicon NPs; ketal-containing diacrylamide | pH | [135,136,137] | |
| MCPCP and PNIPAM; PNIPAM and PNIPMAM; poly(N-vinyl caprolactam) | temperature | [99,138,139] | |
| PEO-b-PS and BCP modified AuNRs/PtNPs; PVCL and Au NPs; azopyridyl polymer | light | [101,140,141] | |
| ketal linkage-containing precursor and a reducible disulfide linkage-containing precursor; poly(N-isopropylacrylamide-co-3-aminophenylboronic acid-co-acrylic acid) | multifunctional responsive | [102,142] |
| Type | Cells | Major Research | Ref. |
|---|---|---|---|
| Lymphangiogenesis models | LECs; LECs and HUVECs | interstitial flow | [148,152] |
| LECs and HUVECs | bi-directional effects between the lymphatic and vascular cells | [153] | |
| LECs | lymphatic barrier function | [154] | |
| LECs and blood vascular endothelial cells | permeability | [155] | |
| Lymphatic-tumor models | MCF7 cells, MDA-MB-231 cells and LECs | pathological changes | [156] |
| MCF-7 cells | diffusion profiles for biomolecules and drugs | [149] | |
| LECs and MDA-MB-231 cells | extracellular matrix density | [157] | |
| Tumor-derived fibroblasts and tubular lymphatic vessel | tumor progression and lymph node metastasis | [158] | |
| LECs and MDA-MB-231 | flow enhanced invasion | [159] | |
| tumor-draining lymph nodes and tumor | tumor-lymph node interaction | [160] | |
| Lymphatic-organ models | DCs | chemotaxis | [150] |
| lymph node slices | local stimulation | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, F.; Gao, Y.; Wang, H. Recent Advances in Drug Delivery System Fabricated by Microfluidics for Disease Therapy. Bioengineering 2022, 9, 625. https://doi.org/10.3390/bioengineering9110625
Jia F, Gao Y, Wang H. Recent Advances in Drug Delivery System Fabricated by Microfluidics for Disease Therapy. Bioengineering. 2022; 9(11):625. https://doi.org/10.3390/bioengineering9110625
Chicago/Turabian StyleJia, Fuhao, Yanbing Gao, and Hai Wang. 2022. "Recent Advances in Drug Delivery System Fabricated by Microfluidics for Disease Therapy" Bioengineering 9, no. 11: 625. https://doi.org/10.3390/bioengineering9110625
APA StyleJia, F., Gao, Y., & Wang, H. (2022). Recent Advances in Drug Delivery System Fabricated by Microfluidics for Disease Therapy. Bioengineering, 9(11), 625. https://doi.org/10.3390/bioengineering9110625








