Formation of Three-Dimensional Spheres Enhances the Neurogenic Potential of Stem Cells from Apical Papilla
Abstract
1. Introduction
2. Materials and Methods
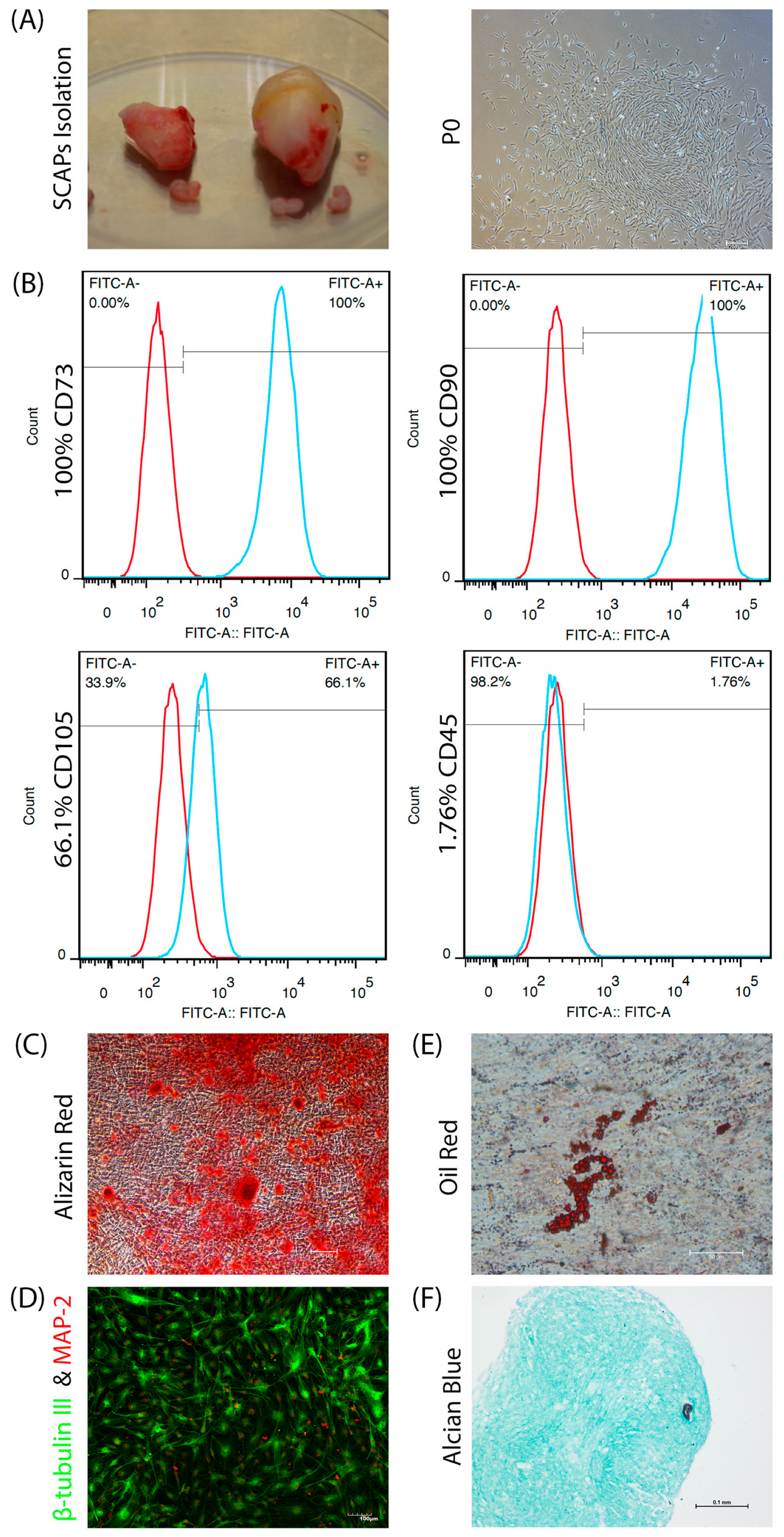
2.1. SCAPs Isolation and Characterization
2.2. Formation and Culture of 3D SCAP Spheres
2.3. Live and Dead Assay and Immunofluorescence Staining
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Image Analysis for Live and Dead Assay, Markers, and Neurites
2.6. RT-qPCR
2.7. Statistical Analysis
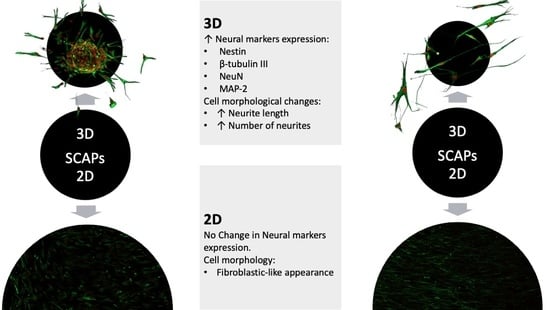
3. Results
3.1. SCAPs Characterization
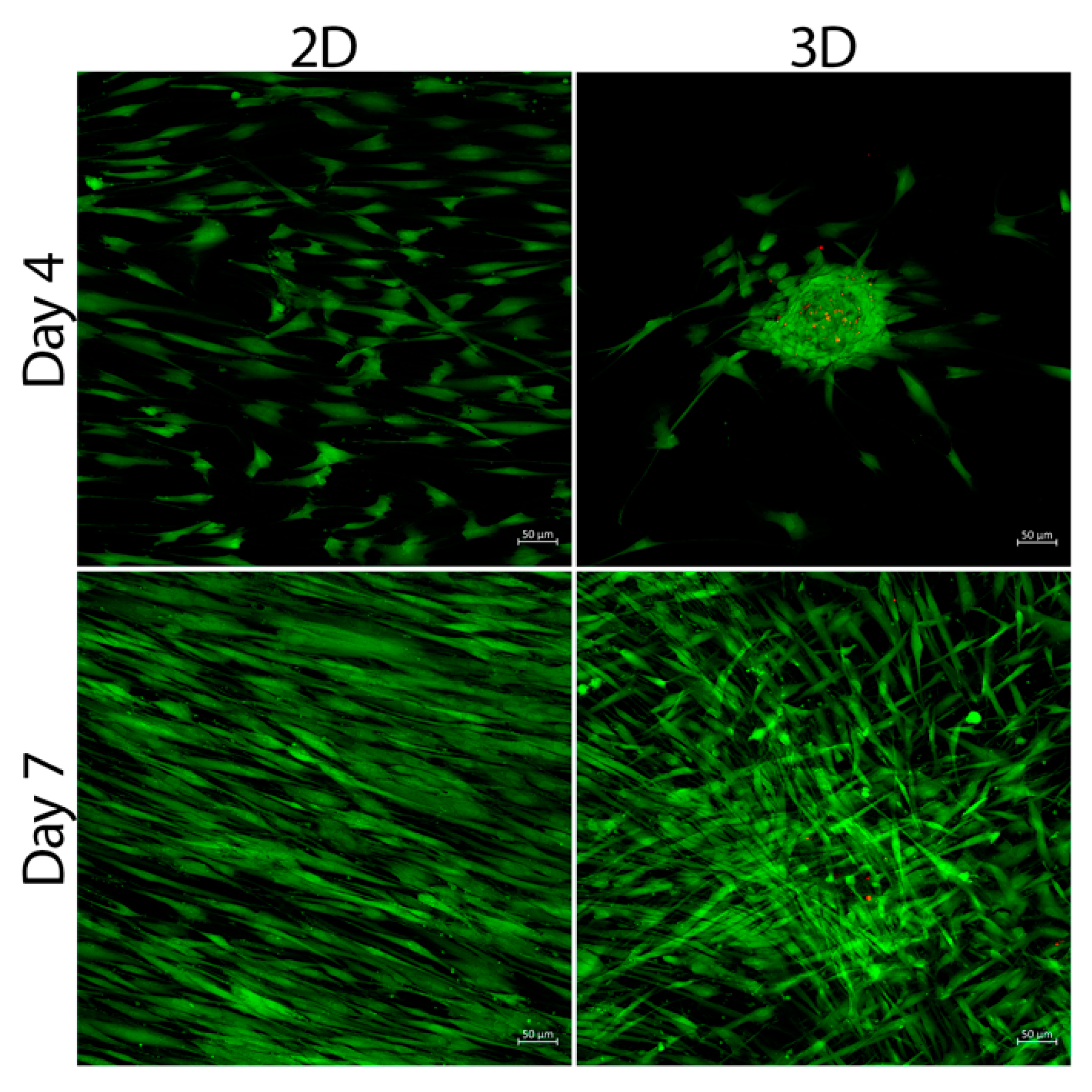
3.2. Cell Viability
3.3. SCAPs Morphological Changes and Neurites Measurement
3.4. Secretion of BDNF and NGF by SCAP Spheres
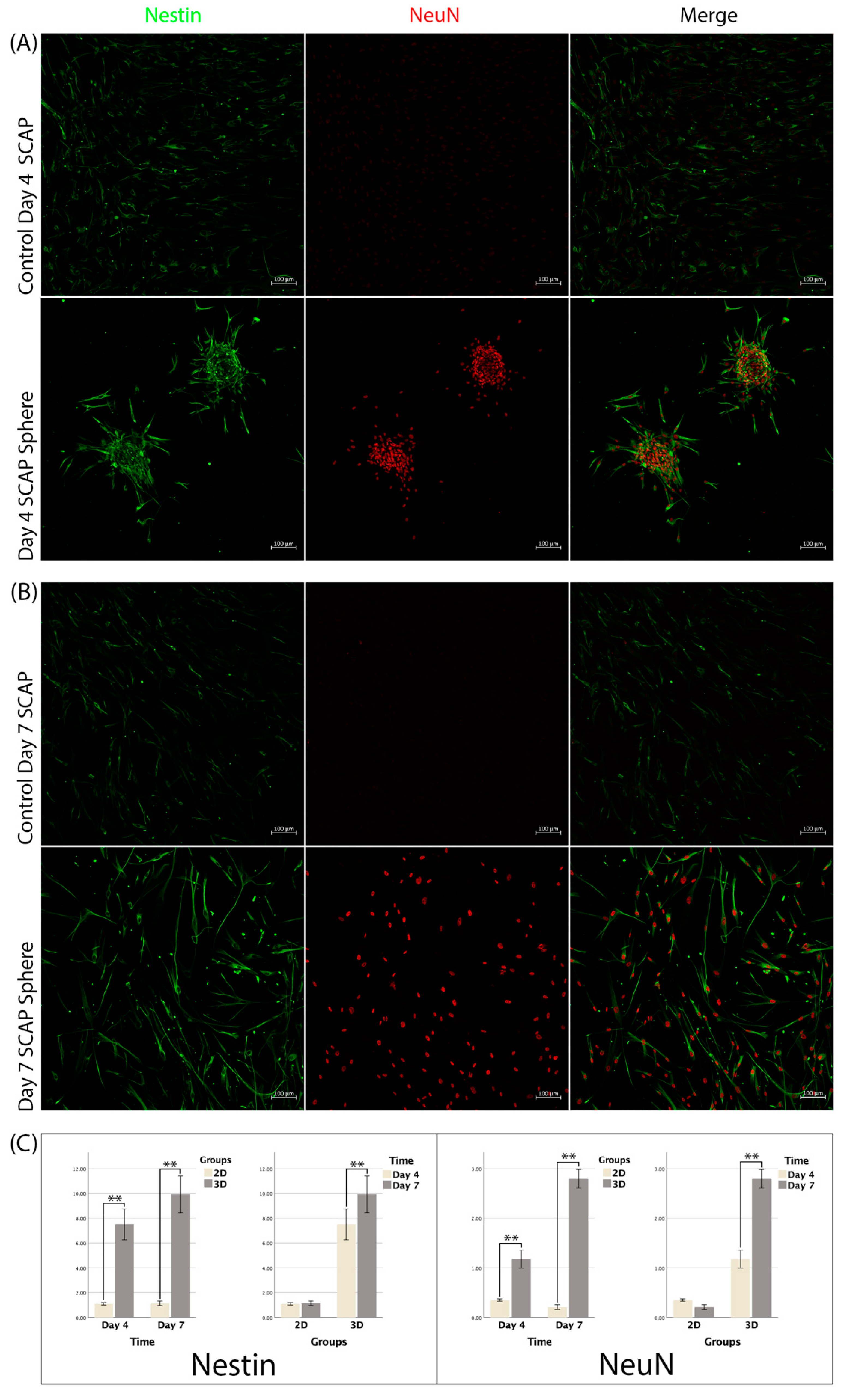
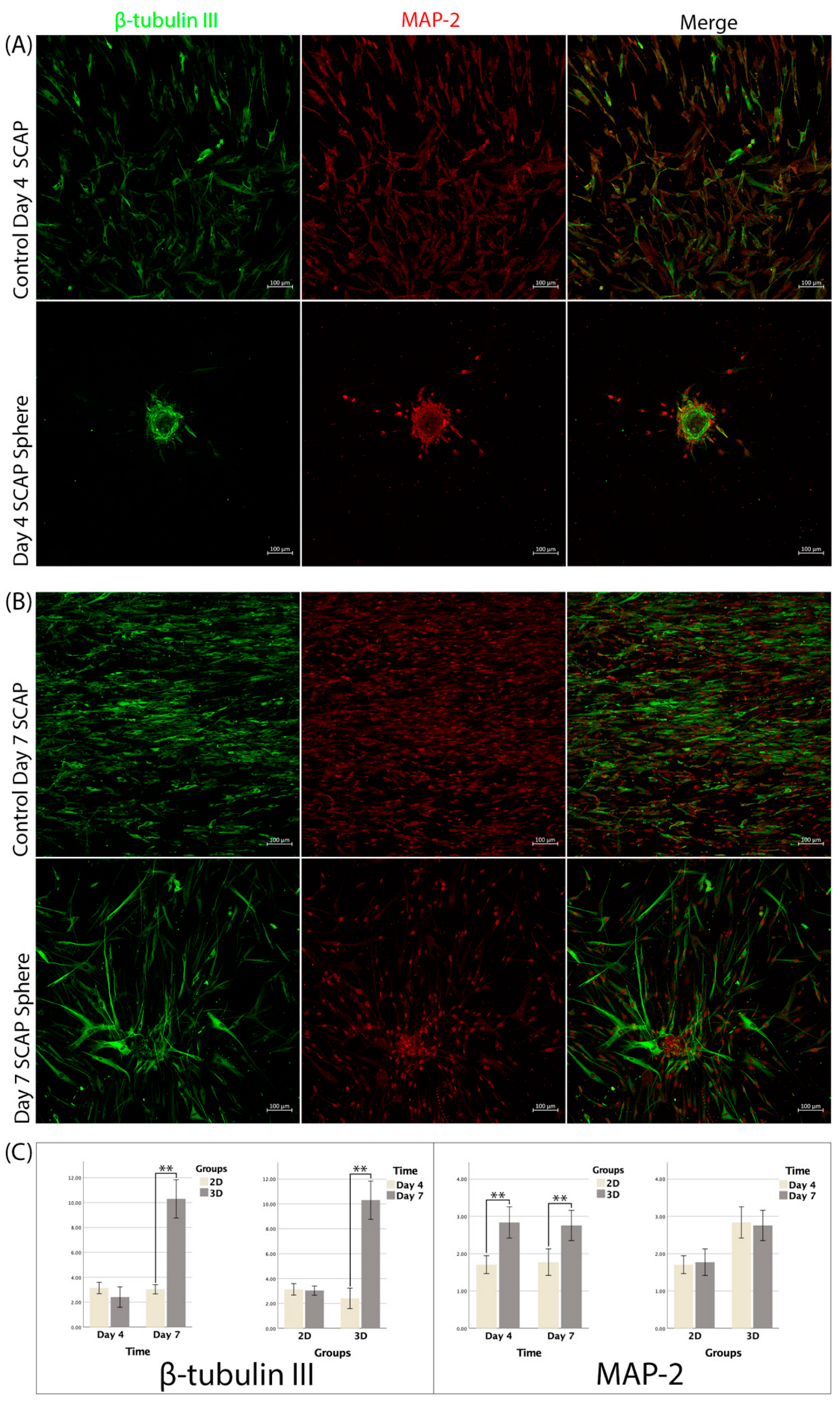
3.5. Immunofluorescence
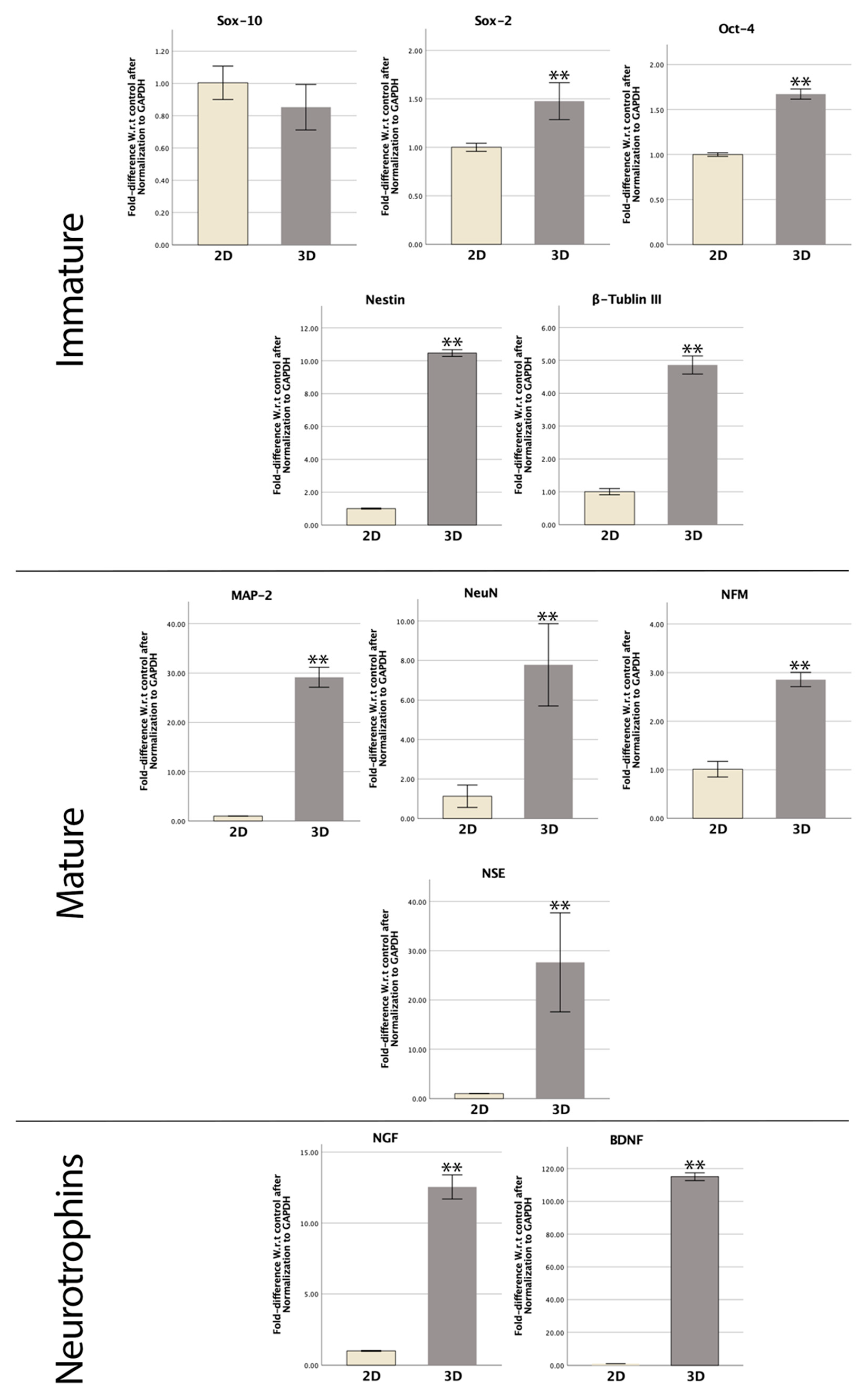
3.6. RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Najem, D.; Rennie, K.; Ribecco-Lutkiewicz, M.; Ly, D.; Haukenfrers, J.; Liu, Q.; Nzau, M.; Fraser, D.D.; Bani-Yaghoub, M. Traumatic brain injury: Classification, models, and markers. Biochem. Cell Biol. 2018, 96, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W. Overcoming Inhibition in the Damaged Spinal Cord. J. Neurotrauma 2006, 23, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Shoichet, M.S.; Tate, C.C.; Baumann, M.D.; LaPlaca, M.C. Chapter 8 Strategies for Regeneration and Repair in the Injured Central Nervous System. In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2008; ISBN 978-0-8493-9362-4. [Google Scholar]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30, 963689721989266. [Google Scholar] [CrossRef] [PubMed]
- Rosado-De-Castro, P.H.; de Carvalho, F.G.; de Freitas, G.R.; Mendez-Otero, R.; Pimentel-Coelho, P.M. Review of Preclinical and Clinical Studies of Bone Marrow-Derived Cell Therapies for Intracerebral Hemorrhage. Stem Cells Int. 2016, 2016, 4617983. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Massironi, S.M.G.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and Characterization of a Population of Immature Dental Pulp Stem Cells Expressing OCT-4 and Other Embryonic Stem Cell Markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef]
- De Berdt, P.; Vanacker, J.; Ucakar, B.; Elens, L.; Diogenes, A.; Leprince, J.G.; Deumens, R.; des Rieux, A. Dental Apical Papilla as Therapy for Spinal Cord Injury. J. Dent. Res. 2015, 94, 1575–1581. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef]
- Wu, X.; Su, J.; Wei, J.; Jiang, N.; Ge, X. Recent Advances in Three-Dimensional Stem Cell Culture Systems and Applications. Stem Cells Int. 2021, 2021, 9477332. [Google Scholar] [CrossRef]
- Su, G.; Zhao, Y.; Wei, J.; Han, J.; Chen, L.; Xiao, Z.; Chen, B.; Dai, J. The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials 2013, 34, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.C.; Chen, S.Y.; Li, J.R.; Young, T.H. Short-term spheroid formation enhances the regenerative capacity of adi-pose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl. Med. 2013, 2, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tsutsui, T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: Stem cells in the core. J. Cell. Biochem. 2013, 114, 2624–2636. [Google Scholar] [CrossRef]
- Kim, B.C.; Jun, S.M.; Kim, S.Y.; Kwon, Y.D.; Choe, S.C.; Kim, E.C.; Lee, J.H.; Kim, J.; Suh, J.F.; Hwang, Y.S. Engineering three di-mensional micro nerve tissue using postnatal stem cells from human dental apical papilla. Biotechnol. Bioeng. 2017, 114, 903–914. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, P.; Zhu, L.; Dissanayaka, W.L.; Green, D.W.; Tong, E.H.; Jin, L.; Zhang, C. Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Eng. Part A 2015, 21, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Jiang, S.; Yi, B.; Gong, T.; Lim, L.W.; Zhang, C. Small molecules enhance neurogenic differentiation of dental-derived adult stem cells. Arch. Oral Biol. 2019, 102, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Dissanayaka, W.L.; Jiang, S.; Wang, S.; Heng, B.C.; Huang, X.; Zhang, C. Semaphorin 4D Enhances Angiogenic Potential and Suppresses Osteo-/Odontogenic Differentiation of Human Dental Pulp Stem Cells. J. Endod. 2016, 43, 297–305. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. In Vitro Analysis of Scaffold-free Prevascularized Microtissue Spheroids Containing Human Dental Pulp Cells and Endothelial Cells. J. Endod. 2015, 41, 663–670. [Google Scholar] [CrossRef]
- Richardson, S.M.; Purmessur, D.; Baird, P.; Probyn, B.; Freemont, A.J.; Hoyland, J.A. Degenerate Human Nucleus Pulposus Cells Promote Neurite Outgrowth in Neural Cells. PLoS ONE 2012, 7, e47735. [Google Scholar] [CrossRef]
- Lima, R.L.; Holanda-Afonso, R.C.; Moura-Neto, V.; Bolognese, A.M.; DosSantos, M.F.; Souza, M.M. Human dental follicle cells express embryonic, mesenchymal and neural stem cells markers. Arch. Oral Biol. 2017, 73, 121–128. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward function-ally active neurons underappropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Bifari, F.; Berton, V.; Pino, A.; Kusalo, M.; Malpeli, G.; Di Chio, M.; Bersan, E.; Amato, E.; Scarpa, A.; Krampera, M.; et al. Meninges harbor cells expressing neural precursor markers during development and adulthood. Front. Cell. Neurosci. 2015, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Liu, C.; Shen, S.; Yu, Z.; Mo, J.; Chen, X.; Sun, F. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia 2015, 63, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, J.; Pineda, J.R.; Irastorza, I.; Uribe-Etxebarria, V.; García-Gallastegui, P.; Encinas, J.M.; Chamero, P.; Unda, F.; Ibarretxe, G. BDNF and NT3 Reprogram Human Ectomesenchymal Dental Pulp Stem Cells to Neurogenic and Gliogenic Neural Crest Progenitors Cultured in Serum-Free Medium. Cell. Physiol. Biochem. 2019, 52, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation 2009, 77, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Lee, Y.-C.; Hung, C.-Y.; Yang, P.-J.; Lai, P.-C.; Feng, S.-W. Three-dimensional Spheroid Culture Enhances Multipotent Differentiation and Stemness Capacities of Human Dental Pulp-derived Mesenchymal Stem Cells by Modulating MAPK and NF-kB Signaling Pathways. Stem Cell Rev. Rep. 2021, 17, 1810–1826. [Google Scholar] [CrossRef]
- Gervois, P.; Struys, T.; Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Politis, C.; Brône, B.; Lambrichts, I.; Martens, W. Neurogenic Maturation of Human Dental Pulp Stem Cells Following Neurosphere Generation Induces Morphological and Electrophysiological Characteristics of Functional Neurons. Stem Cells Dev. 2015, 24, 296–311. [Google Scholar] [CrossRef]
- Sasaki, R.; Aoki, S.; Yamato, M.; Uchiyama, H.; Wada, K.; Okano, T.; Ogiuchi, H. Neurosphere generation from dental pulp of adult rat incisor. Eur. J. Neurosci. 2008, 27, 538–548. [Google Scholar] [CrossRef]
- Abe, S.; Hamada, K.; Miura, M.; Yamaguchi, S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol. Int. 2012, 36, 927–936. [Google Scholar] [CrossRef]
- Faroni, A.; Smith, R.J.P.; Lu, L.; Reid, A.J. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur. J. Neurosci. 2015, 43, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.; Zuliani, T.; Olejnik, C.; LeRoy, H.; Obriot, H.; Kerr-Conte, J.; Formstecher, P.; Bailliez, Y.; Polakowska, R.R. Human Dental Pulp Stem Cells Differentiate into Neural Crest-Derived Melanocytes and Have Label-Retaining and Sphere-Forming Abilities. Stem Cells Dev. 2008, 17, 1175–1184. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The Interplay of Dental Pulp Stem Cells and Endothelial Cells in an Injectable Peptide Hydrogel on Angiogenesis and Pulp Regeneration In Vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Hall, W.; LaMantia, A.-S.; White, L. Neuroscience, 6th ed.; Oxford University Press: New York, NY, USA, 2018; pp. 547–550. ISBN 9781605353807. [Google Scholar]
- Solis-Castro, O.O.; Boissonade, F.M.; Rivolta, M.N. Establishment and neural differentiation of neural crest-derived stem cells from human dental pulp in serum-free conditions. Stem Cells Transl. Med. 2020, 9, 1462–1476. [Google Scholar] [CrossRef] [PubMed]
- Widera, D.; Zander, C.; Heidbreder, M.; Kasperek, Y.; Noll, T.; Seitz, O.; Saldamli, B.; Sudhoff, H.; Sader, R.; Kaltschmidt, C.; et al. Adult Palatum as a Novel Source of Neural Crest-Related Stem Cells. Stem Cells 2009, 27, 1899–1910. [Google Scholar] [CrossRef][Green Version]
- Wetmore, C.; Olson, L.; Bean, A.J. Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors. J. Neurosci. 1994, 14, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Carnahan, J.; Greenberg, M.E.; Bodmer, H.; Viville, S.; Benoist, C.; Mathis, D. Requirement for BDNF in Activity-Dependent Survival of Cortical Neurons. Science 1994, 263, 1618–1623. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences | |
|---|---|---|
| Immature | Oct-4 | F 5′-GTTGATCCTCGGACCTGGCTA-3′ R 5′-GGTTGCCTCTCACTCGGTTCT-3′ |
| Sox-2 | F 5′-AGTCTCCAAGCGACGAAAAA-3′ R 5′-GCAAGAAGCCTCTCCTTGAA-3′ | |
| Sox-10 | F 5′-CCTCACAGATCGCCTACACC-3′ R 5′-CATATAGGAGAAGGCCGAGTAGA-3′ | |
| Nestin | F 5′-CAACAGCGACGGAGGTCTC-3′ R 5′-GCCTCTACGCTCTCTTCTTTGA-3′ | |
| β-tubulin III | F 5′-AGACCTACTGCATCGACAACGAGG-3′ R 5′-GCTCATGGTGGCCGATACCAGG-3′ | |
| Mature | MAP-2 | F 5′-TTGGTGCCGAGTGAGAAGAA-3′ R 5′-GGTCTGGCAGTGGTTGGTTAA-3′ |
| NeuN | F 5′-GCGGCTACACGTCTCCAACATC-3′ R 5′-ATCGTCCCATTCAGCTTCTCCC-3′ | |
| NSE | F 5′-GTCCCACGTGTCTTCCACTT-3′ R 5′-TGGGATCTACAGCCACATGA-3′ | |
| NFM | F 5′-GTCAAGATGGCTCTGGATATAGAAATC-3′ R 5′-TACAGTGGCCCAGTGATGCTT-3′ | |
| Neurotrophins | BDNF | F 5′-TAACGGCGGCAGACAAAAAGA-3′ R 5′- TGCACTTGGTCTCGTAGAAGTAT-3′ |
| NGF | F 5′- TGTGGGTTGGGGATAAGACCA-3′ R 5′- GCTGTCAACGGGATTTGGGT-3′ | |
| Control | GAPDH | F 5′-TGTCTCCTCCGACTTCAACA-3′ R 5′-GCCATGTGGGCCATGAGGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basabrain, M.S.; Zhong, J.; Luo, H.; Liu, J.; Yi, B.; Zaeneldin, A.; Koh, J.; Zou, T.; Zhang, C. Formation of Three-Dimensional Spheres Enhances the Neurogenic Potential of Stem Cells from Apical Papilla. Bioengineering 2022, 9, 604. https://doi.org/10.3390/bioengineering9110604
Basabrain MS, Zhong J, Luo H, Liu J, Yi B, Zaeneldin A, Koh J, Zou T, Zhang C. Formation of Three-Dimensional Spheres Enhances the Neurogenic Potential of Stem Cells from Apical Papilla. Bioengineering. 2022; 9(11):604. https://doi.org/10.3390/bioengineering9110604
Chicago/Turabian StyleBasabrain, Mohammed S., Jialin Zhong, Haiyun Luo, Junqing Liu, Baicheng Yi, Ahmed Zaeneldin, Junhao Koh, Ting Zou, and Chengfei Zhang. 2022. "Formation of Three-Dimensional Spheres Enhances the Neurogenic Potential of Stem Cells from Apical Papilla" Bioengineering 9, no. 11: 604. https://doi.org/10.3390/bioengineering9110604
APA StyleBasabrain, M. S., Zhong, J., Luo, H., Liu, J., Yi, B., Zaeneldin, A., Koh, J., Zou, T., & Zhang, C. (2022). Formation of Three-Dimensional Spheres Enhances the Neurogenic Potential of Stem Cells from Apical Papilla. Bioengineering, 9(11), 604. https://doi.org/10.3390/bioengineering9110604