Finite Element Analysis and Experimental Validation of the Anterior Cruciate Ligament and Implications for the Injury Mechanism
Abstract
1. Introduction
2. Materials and Methods
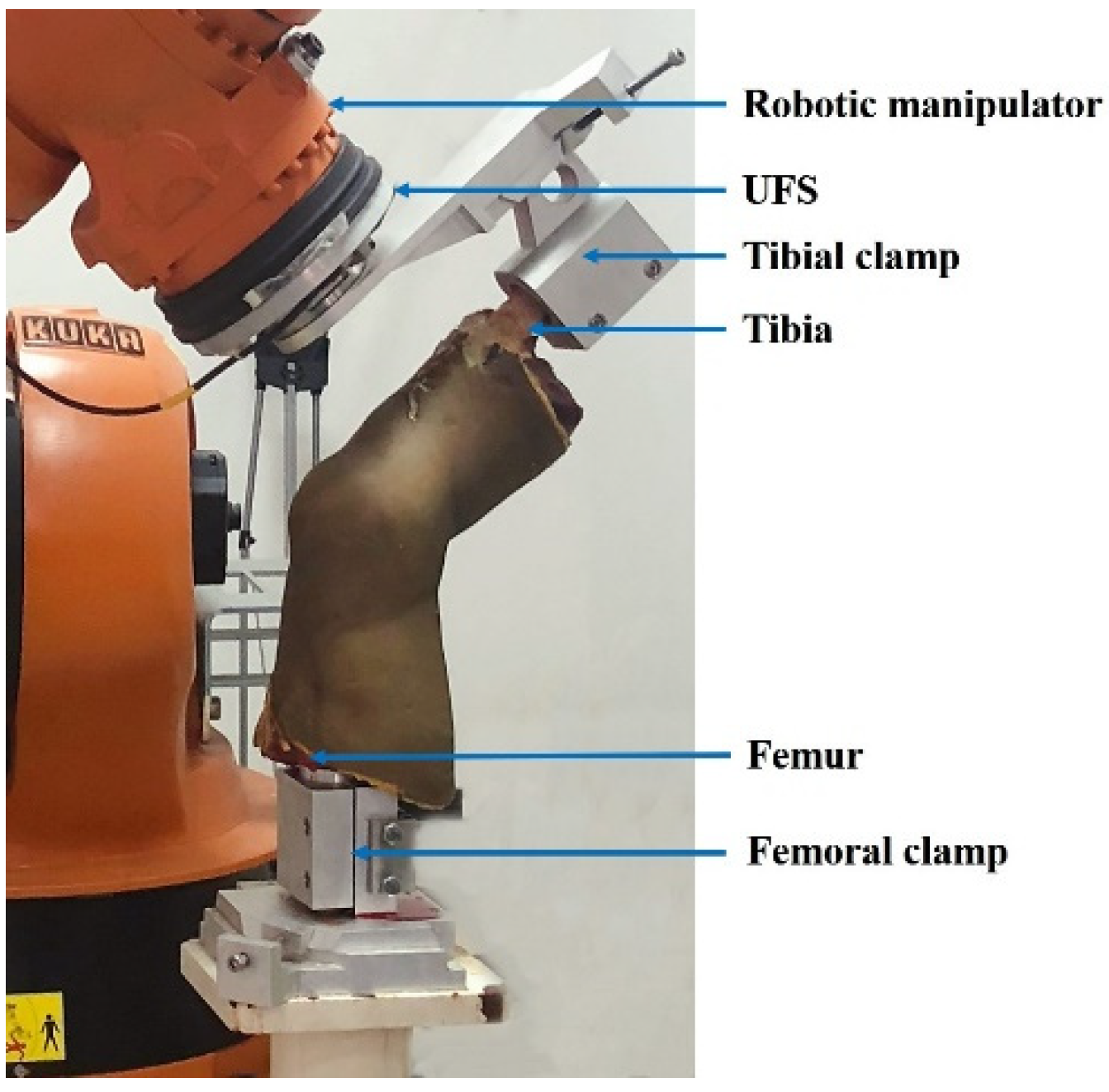
2.1. Specimen Preparation
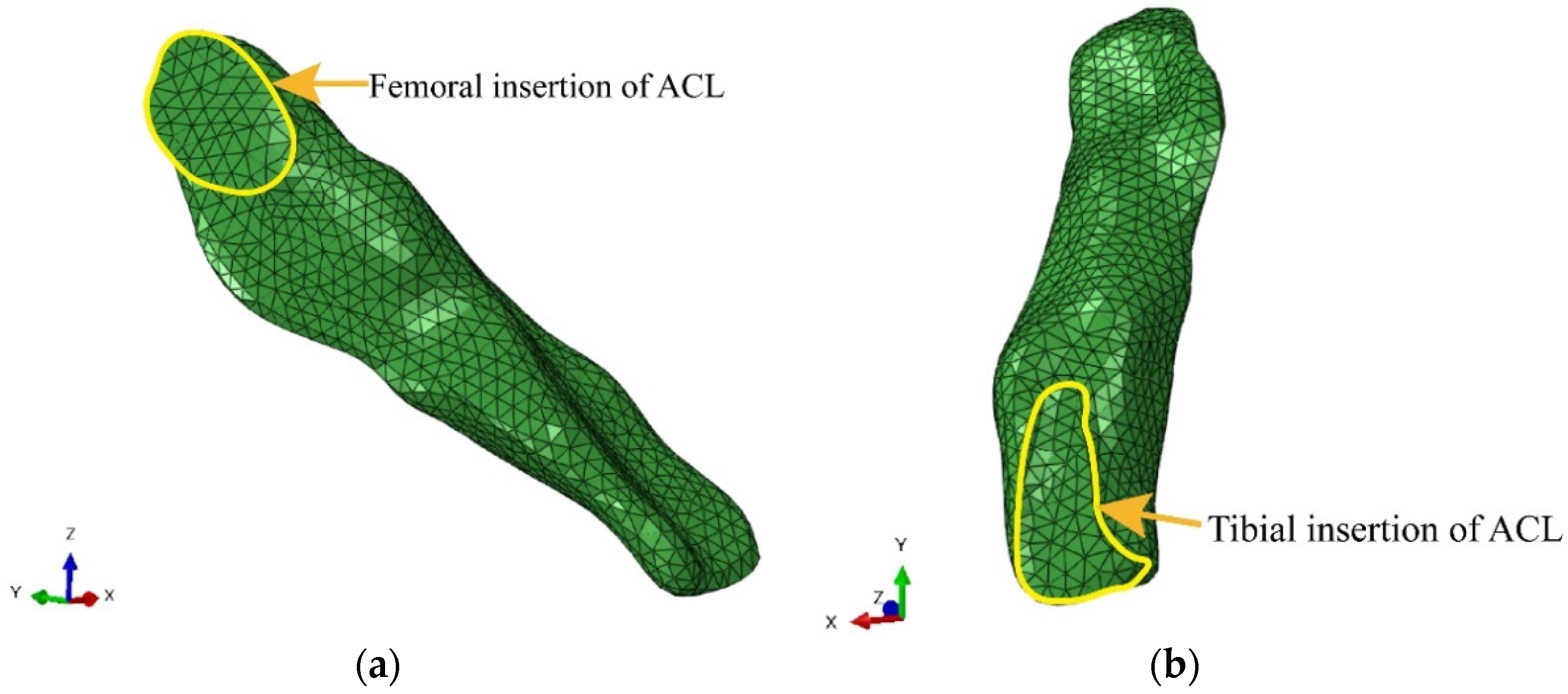
2.2. Generation of the Finite Element Model of the ACL
2.3. Simulations
2.4. Biomechanical Test for Finite Element Model Validation
3. Results
3.1. Model Establishment
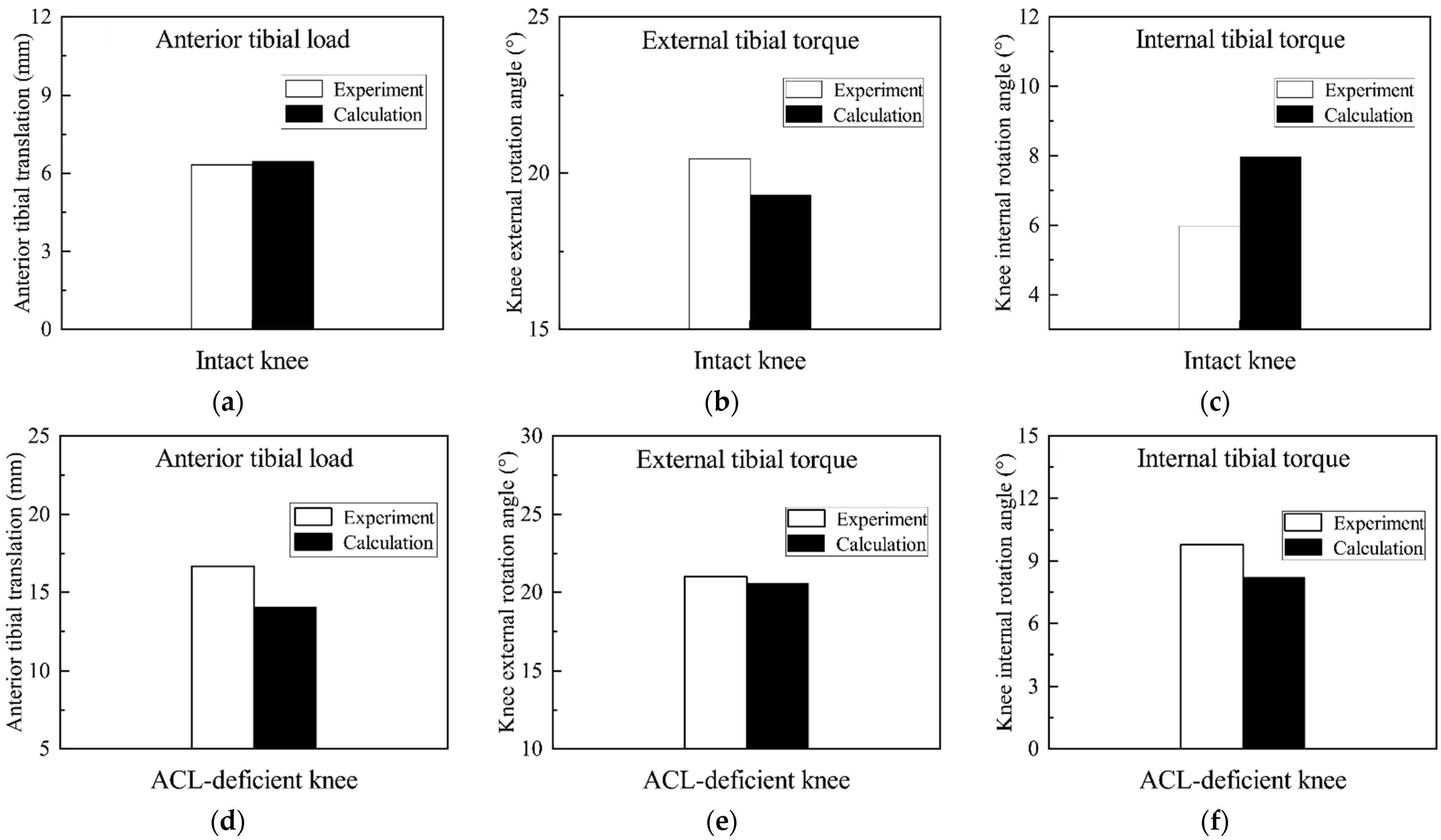
3.2. Validation of the Finite Element Model
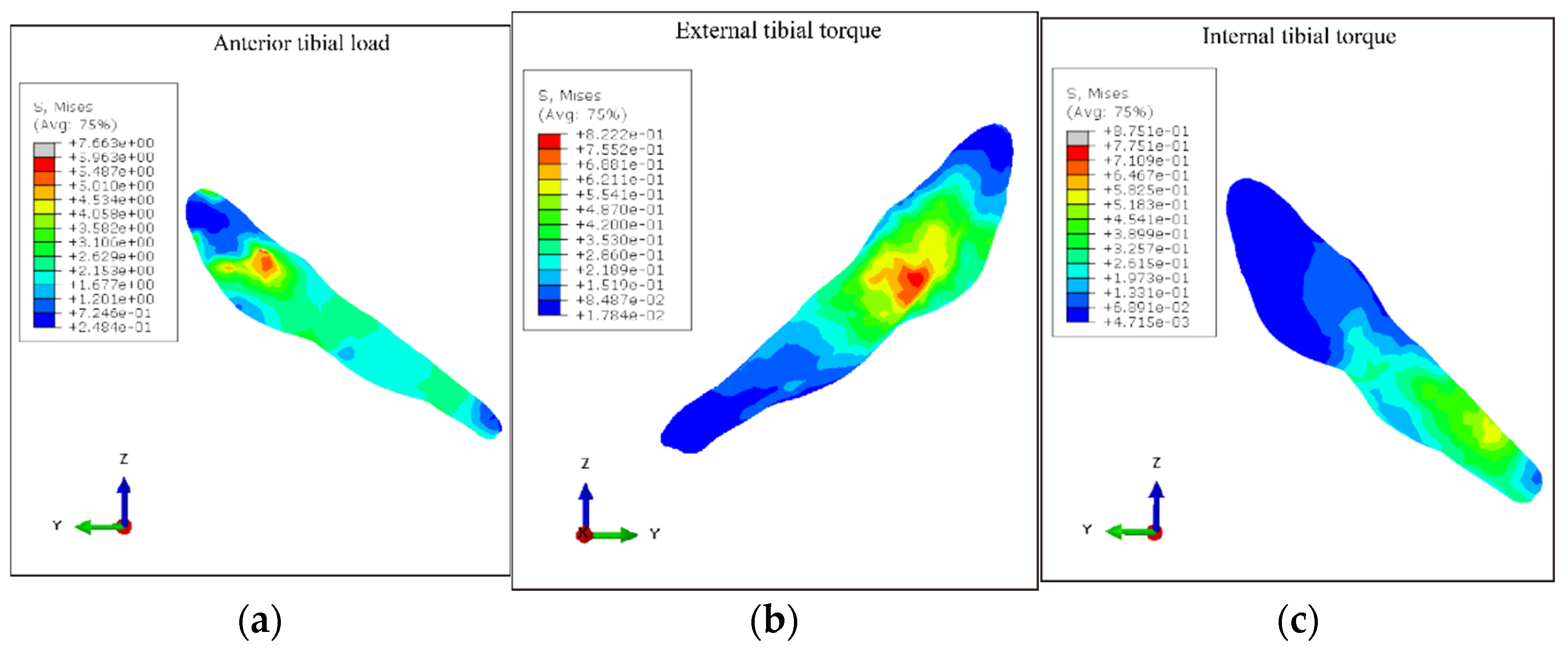
3.3. ACL Stress under Different Loading Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prodromos, C.C.; Han, Y.; Rogowski, J.; Joyce, B.; Shi, K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury—Reduction regimen. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Kostogiannis, I.; Ageberg, E.; Neuman, P.; Dahlberg, L.; Friden, T.; Roos, H. Activity Level and Subjective Knee Function 15 Years after Anterior Cruciate Ligament Injury: A Prospective, Longitudinal Study of Nonreconstructed Patients. Am. J. Sport Med. 2007, 35, 1135–1143. [Google Scholar] [CrossRef]
- Lie, M.M.; Risberg, M.A.; Storheim, K.; Engebretsen, L.; Øiestad, B.E. What’s the rate of knee osteoarthritis 10 years after anterior cruciate ligament injury? An updated systematic review. Br. J. Sport Med. 2019, 53, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- von Porat, A.; Roos, E.M.; Roos, H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: A study of radiographic and patient relevant outcomes. Ann. Rheum. Dis. 2004, 63, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Chia, L.; De Oliveira Silva, D.; Whalan, M.; McKay, M.J.; Sullivan, J.; Fuller, C.W.; Pappas, E. Non-contact Anterior Cruciate Ligament Injury Epidemiology in Team-Ball Sports: A Systematic Review with Meta-analysis by Sex, Age, Sport, Participation Level, and Exposure Type. Sport Med. 2022, 52, 2447–2467. [Google Scholar] [CrossRef] [PubMed]
- Della Villa, F.; Tosarelli, F.; Ferrari, R.; Grassi, A.; Ciampone, L.; Nanni, G.; Zaffagnini, S.; Buckthorpe, M. Systematic Video Analysis of Anterior Cruciate Ligament Injuries in Professional Male Rugby Players: Pattern, Injury Mechanism, and Biomechanics in 57 Consecutive Cases. Orthop. J. Sport Med. 2021, 9, 23259671211048182. [Google Scholar] [CrossRef]
- Della Villa, F.; Buckthorpe, M.; Grassi, A.; Nabiuzzi, A.; Tosarelli, F.; Zaffagnini, S.; Della Villa, S. Systematic video analysis of ACL injuries in professional male football (soccer): Injury mechanisms, situational patterns and biomechanics study on 134 consecutive cases. Br. J. Sport Med. 2020, 54, 1423–1432. [Google Scholar] [CrossRef]
- Lucarno, S.; Zago, M.; Buckthorpe, M.; Grassi, A.; Tosarelli, F.; Smith, R.; Della Villa, F. Systematic Video Analysis of Anterior Cruciate Ligament Injuries in Professional Female Soccer Players. Am. J. Sport Med. 2021, 49, 1794–1802. [Google Scholar] [CrossRef]
- Schilaty, N.D.; Bates, N.A.; Krych, A.J.; Hewett, T.E. Frontal Plane Loading Characteristics of Medial Collateral Ligament Strain Concurrent with Anterior Cruciate Ligament Failure. Am. J. Sport Med. 2019, 47, 2143–2150. [Google Scholar] [CrossRef]
- Kiapour, A.M.; Demetropoulos, C.K.; Kiapour, A.; Quatman, C.E.; Wordeman, S.C.; Goel, V.K.; Hewett, T.E. Strain Response of the Anterior Cruciate Ligament to Uniplanar and Multiplanar Loads During Simulated Landings: Implications for Injury Mechanism. Am. J. Sport Med. 2016, 44, 2087–2096. [Google Scholar] [CrossRef]
- Nedergaard, N.J.; Dalbø, S.; Petersen, S.V.; Zebis, M.K.; Bencke, J. Biomechanical and neuromuscular comparison of single- and multi-planar jump tests and a side-cutting maneuver: Implications for ACL injury risk assessment. Knee 2020, 27, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Peel, S.A.; Schroeder, L.E.; Sievert, Z.A.; Weinhandl, J.T. Comparing Anterior Cruciate Ligament Injury Risk Variables Between Unanticipated Cutting and Decelerating Tasks. J. Appl. Biomech. 2019, 35, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kristianslund, E.; Faul, O.; Bahr, R.; Myklebust, G.; Krosshaug, T. Sidestep cutting technique and knee abduction loading: Implications for ACL prevention exercises. Br. J. Sport Med. 2014, 48, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.S.; DeFrate, L.E.; Nha, K.W.; Papannagari, R.; Gill, T.J.; Li, G. The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion. Am. J. Sport Med. 2007, 35, 547–554. [Google Scholar] [CrossRef]
- Englander, Z.A.; Baldwin, E.L., 3rd; Smith, W.A.R.; Garrett, W.E.; Spritzer, C.E.; DeFrate, L.E. In Vivo Anterior Cruciate Ligament Deformation During a Single-Legged Jump Measured by Magnetic Resonance Imaging and High-Speed Biplanar Radiography. Am. J. Sport Med. 2019, 47, 3166–3172. [Google Scholar] [CrossRef]
- Handrik, M.; Kopas, P.; Baniari, V.; Vasko, M.; Saga, M. Analysis of Stress and Strain of Fatigue Specimens Localised in the Cross-sectional Area of the Gauge Section Testing on Bi-axial Fatigue Machine Loaded in the High-cycle Fatigue Region. Procedia Eng. 2017, 177, 516–519. [Google Scholar] [CrossRef]
- Sága, M.; Vaško, M.; Ságová, Z.; Kuric, I.; Kopas, P.; Handrik, M. FEM Simulation of Non-proportional Multiaxial Fatigue Damage. MATEC Web of Conferences. In Proceedings of the 25th Polish-Slovak Scientific Conference on Machine Modelling and Simulations (MMS 2020), Tleń, Poland, 8–11 September 2020; Volume 357, p. 02006. [Google Scholar]
- Beidokhti, H.N.; Janssen, D.; van de Groes, S.; Hazrati, J.; Van den Boogaard, T.; Verdonschot, N. The influence of ligament modelling strategies on the predictive capability of finite element models of the human knee joint. J. Biomech. 2017, 65, 1–11. [Google Scholar] [CrossRef]
- Li, G.; Gil, J.; Kanamori, A.; Woo, S.L. A validated three-dimensional computational model of a human knee joint. J. Biomech. Eng. 1999, 121, 657–662. [Google Scholar] [CrossRef]
- Homyk, A.; Orsi, A.; Wibby, S.; Yang, N.; Nayeb-Hashemi, H.; Canavan, P.K. Failure locus of the anterior cruciate ligament: 3D finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 865–874. [Google Scholar] [CrossRef]
- Orsi, A.D.; Chakravarthy, S.; Canavan, P.K.; Peña, E.; Goebel, R.; Vaziri, A.; Nayeb-Hashemi, H. The effects of knee joint kinematics on anterior cruciate ligament injury and articular cartilage damage. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 493–506. [Google Scholar]
- Xie, F.; Yang, L.; Guo, L.; Wang, Z.-j.; Dai, G. A Study on Construction Three-Dimensional Nonlinear Finite Element Model and Stress Distribution Analysis of Anterior Cruciate Ligament. J. Biomech. Eng. 2009, 131, 121007. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Sun, H.; Fan, Y.; Li, F.; Wang, Y.; Ge, C. Three dimensional finite element analysis of the influence of posterior tibial slope on the anterior cruciate ligament and knee joint forward stability. J. Back Musculoskelet. Rehabil. 2018, 31, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.; Calvo, B.; Martínez, M.A.; Doblaré, M. A three-dimensional finite element analysis of the combined behavior of ligaments and menisci in the healthy human knee joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Debski, R.E.; Musahl, V.; Thomas, M.; Woo, S.L. A three-dimensional finite element model of the human anterior cruciate ligament: A computational analysis with experimental validation. J. Biomech. 2004, 37, 383–390. [Google Scholar] [CrossRef]
- Mochizuki, T.; Fujishiro, H.; Nimura, A.; Mahakkanukrauh, P.; Yasuda, K.; Muneta, T.; Akita, K. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg. Sport Traumatol. Arthrosc. 2014, 22, 336–344. [Google Scholar] [CrossRef]
- Śmigielski, R.; Zdanowicz, U.; Drwięga, M.; Ciszek, B.; Ciszkowska-Łysoń, B.; Siebold, R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: A cadaveric study including 111 knees. Knee Surg. Sport Traumatol. Arthrosc. 2015, 23, 3143–3150. [Google Scholar] [CrossRef]
- Siebold, R.; Schuhmacher, P.; Fernandez, F.; Śmigielski, R.; Fink, C.; Brehmer, A.; Kirsch, J. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site. Knee Surg. Sport Traumatol. Arthrosc. 2015, 23, 3136–3142. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Liu, Z.; Zhao, F.; Ma, Y.; Ao, Y. Anatomical single bundle anterior cruciate ligament reconstruction with rounded rectangle tibial tunnel and oval femoral tunnel: A prospective comparative study versus conventional surgery. Am. J. Transl. Res. 2019, 11, 1908–1918. [Google Scholar]
- He, C.; He, W.; Li, Y.; Wang, F.; Tong, L.; Zhang, Z.; Jia, D.; Wang, G.; Zheng, J.; Chen, G. Biomechanics of Knee Joints after Anterior Cruciate Ligament Reconstruction. J. Knee Surg. 2018, 31, 352–358. [Google Scholar] [CrossRef]
- Kiapour, A.; Kiapour, A.M.; Kaul, V.; Quatman, C.E.; Wordeman, S.C.; Hewett, T.E.; Demetropoulos, C.K.; Goel, V.K. Finite element model of the knee for investigation of injury mechanisms: Development and validation. J. Biomech. Eng. 2014, 136, 011002. [Google Scholar] [CrossRef]
- LeRoux, M.A.; Setton, L.A. Experimental and biphasic FEM determinations of the material properties and hydraulic permeability of the meniscus in tension. J. Biomech. Eng. 2002, 124, 315–321. [Google Scholar] [CrossRef]
- Vairis, A.; Stefanoudakis, G.; Petousis, M.; Vidakis, N.; Tsainis, A.-M.; Kandyla, B. Evaluation of an intact, an ACL-deficient, and a reconstructed human knee joint finite element model. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 263–270. [Google Scholar] [CrossRef]
- Liu, P.; Wang, J.; Zhao, F.; Xu, Y.; Ao, Y. Anatomic, arthroscopically assisted, mini-open fibular collateral ligament reconstruction: An in vitro biomechanical study. Am. J. Sport Med. 2014, 42, 373–381. [Google Scholar] [CrossRef]
- Liu, P.; Gong, X.; Zhang, J.; Ao, Y. Anatomic, All-Arthroscopic Reconstruction of Posterolateral Corner of the Knee: A Cadaveric Biomechanical Study. Arthroscopy 2020, 36, 1121–1131. [Google Scholar] [CrossRef]
- Musahl, V.; Plakseychuk, A.; VanScyoc, A.; Sasaki, T.; Debski, R.E.; McMahon, P.J.; Fu, F.H. Varying femoral tunnels between the anatomical footprint and isometric positions: Effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am. J. Sport Med. 2005, 33, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Maeyama, A.; Lertwanich, P.; Wang, J.H.; Ingham, S.J.; Kramer, S.; Martins, C.Q.; Smolinski, P.; Fu, F.H. Biomechanical comparison of different graft positions for single-bundle anterior cruciate ligament reconstruction. Knee Surg. Sport Traumatol. Arthrosc. 2013, 21, 816–823. [Google Scholar] [CrossRef]
- Yuping, Y.; Yingfang, A.; Xiaopeng, L. Position of Acute Completely Substantial Tears of the Anterior Cruciate Ligament and Related Factors. Chin. J. Minim. Invasive Surg. 2011, 11, 837–840. [Google Scholar]
- Quatman, C.E.; Hewett, T.E. The anterior cruciate ligament injury controversy: Is “valgus collapse” a sex-specific mechanism? Br. J. Sport Med. 2009, 43, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ding, L.; Ren, S.; Jiang, Y.; Zhang, H.; Hu, X.; Huang, H.; Ao, Y. Prediction of Knee Kinematics at the Time of Noncontact Anterior Cruciate Ligament Injuries Based on the Bone Bruises. Ann. Biomed. Eng. 2021, 49, 162–170. [Google Scholar] [CrossRef]
- Grassi, A.; Agostinone, P.; Di Paolo, S.; Zaffagnini, S. Letter to the Editor on “Prediction of Knee Kinematics at Time of Noncontact Anterior Cruciate Ligament Injuries Based on Bone Bruises”. Ann. Biomed. Eng. 2021, 49, 1–3. [Google Scholar] [CrossRef]
- Van Der Bracht, H.; Tampere, T.; Beekman, P.; Schepens, A.; Devriendt, W.; Verdonk, P.; Victor, J. Peak stresses shift from femoral tunnel aperture to tibial tunnel aperture in lateral tibial tunnel ACL reconstructions: A 3D graft-bending angle measurement and finite-element analysis. Knee Surg. Sport Traumatol. Arthrosc. 2018, 26, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Brandon, S.C.E.; Thelen, D.G. Can altered neuromuscular coordination restore soft tissue loading patterns in anterior cruciate ligament and menisci deficient knees during walking? J. Biomech. 2019, 82, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Myller, K.A.H.; Korhonen, R.K.; Töyräs, J.; Salo, J.; Jurvelin, J.S.; Venäläinen, M.S. Computational evaluation of altered biomechanics related to articular cartilage lesions observed in vivo. J. Orthop. Res. 2019, 37, 1042–1051. [Google Scholar] [CrossRef] [PubMed]

| Ligament | Material Parameters |
|---|---|
| Bone tissues | E = 17,000 MPa, ν = 0.36 |
| Cartilage | E = 20 MPa, ν = 0.45 |
| Meniscus | E = 59 MPa, ν = 0.49 |
| Anterior cruciate ligament | C1 = 1.95, D = 0.00683, ν = 0.49 |
| Posterior cruciate ligament | C1 = 3.25, D = 0.0041 |
| Medial collateral ligament | C1 = 1.44, D = 0.00126 |
| Lateral collateral ligament | C1 = 1.44, D = 0.00126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Shi, H.; Liu, Z.; Zhang, J.; Li, H.; Huang, H.; Ao, Y. Finite Element Analysis and Experimental Validation of the Anterior Cruciate Ligament and Implications for the Injury Mechanism. Bioengineering 2022, 9, 590. https://doi.org/10.3390/bioengineering9100590
Ren S, Shi H, Liu Z, Zhang J, Li H, Huang H, Ao Y. Finite Element Analysis and Experimental Validation of the Anterior Cruciate Ligament and Implications for the Injury Mechanism. Bioengineering. 2022; 9(10):590. https://doi.org/10.3390/bioengineering9100590
Chicago/Turabian StyleRen, Shuang, Huijuan Shi, Zhenlong Liu, Jiahao Zhang, Hanjun Li, Hongshi Huang, and Yingfang Ao. 2022. "Finite Element Analysis and Experimental Validation of the Anterior Cruciate Ligament and Implications for the Injury Mechanism" Bioengineering 9, no. 10: 590. https://doi.org/10.3390/bioengineering9100590
APA StyleRen, S., Shi, H., Liu, Z., Zhang, J., Li, H., Huang, H., & Ao, Y. (2022). Finite Element Analysis and Experimental Validation of the Anterior Cruciate Ligament and Implications for the Injury Mechanism. Bioengineering, 9(10), 590. https://doi.org/10.3390/bioengineering9100590







