Comparison of Lower Extremity Joint Moment and Power Estimated by Markerless and Marker-Based Systems during Treadmill Running
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup and Procedure
2.3. Data Analysis
2.3.1. Pre-Processing
2.3.2. Visual3D Analyses
2.3.3. Discrete Measurements
2.4. Statistical Analysis
3. Results
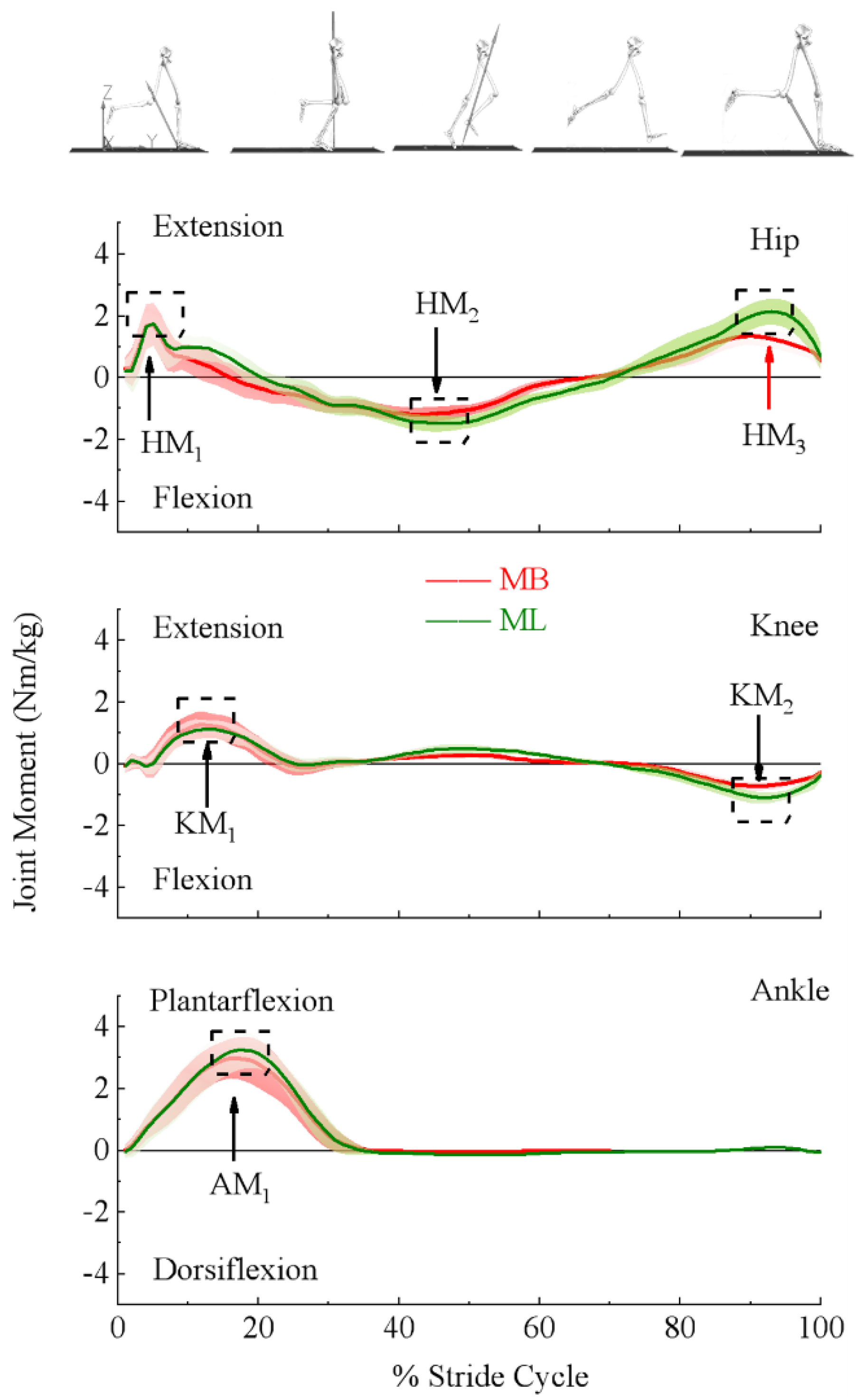
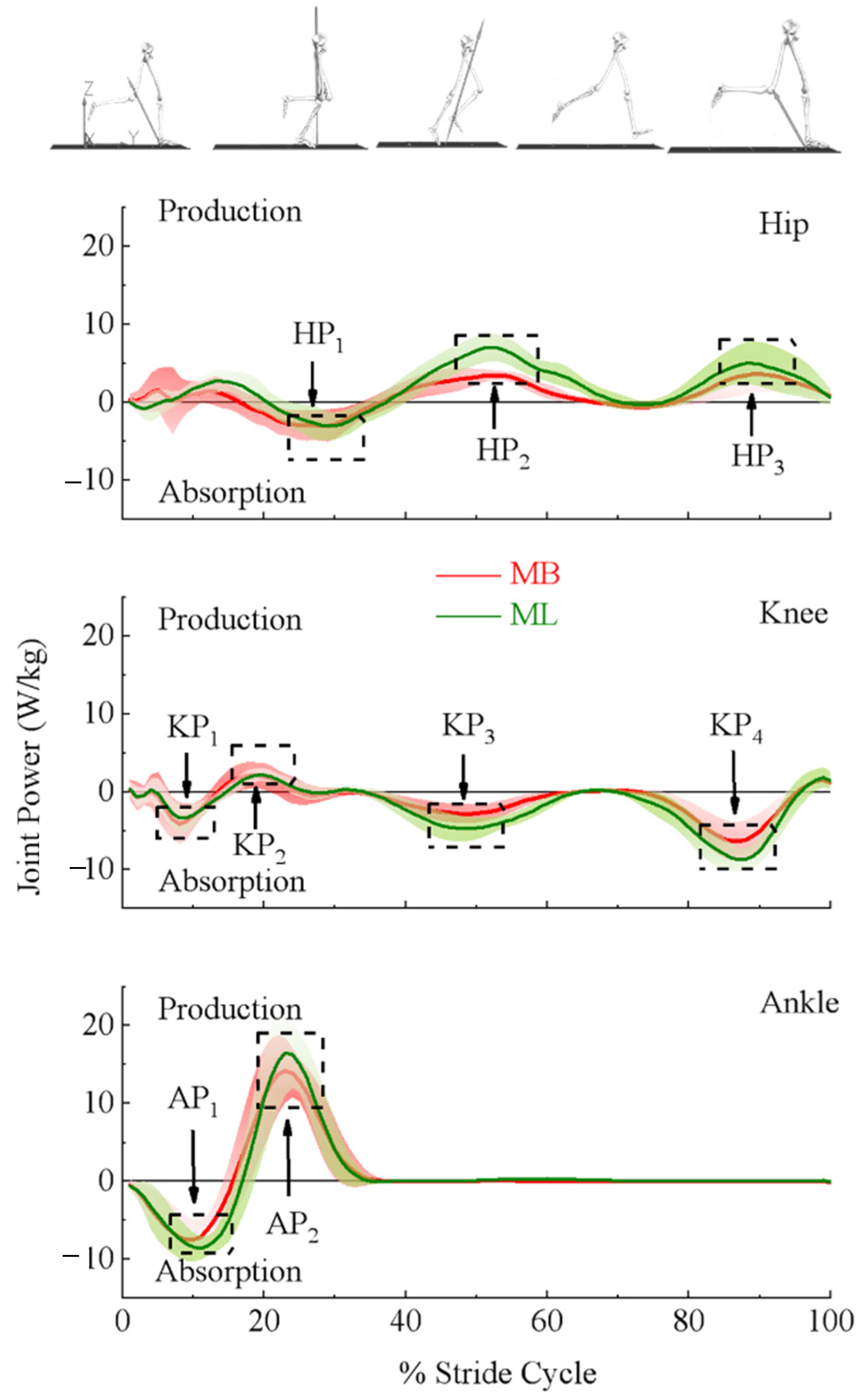
3.1. Lower Extremity Joint Moments and Powers
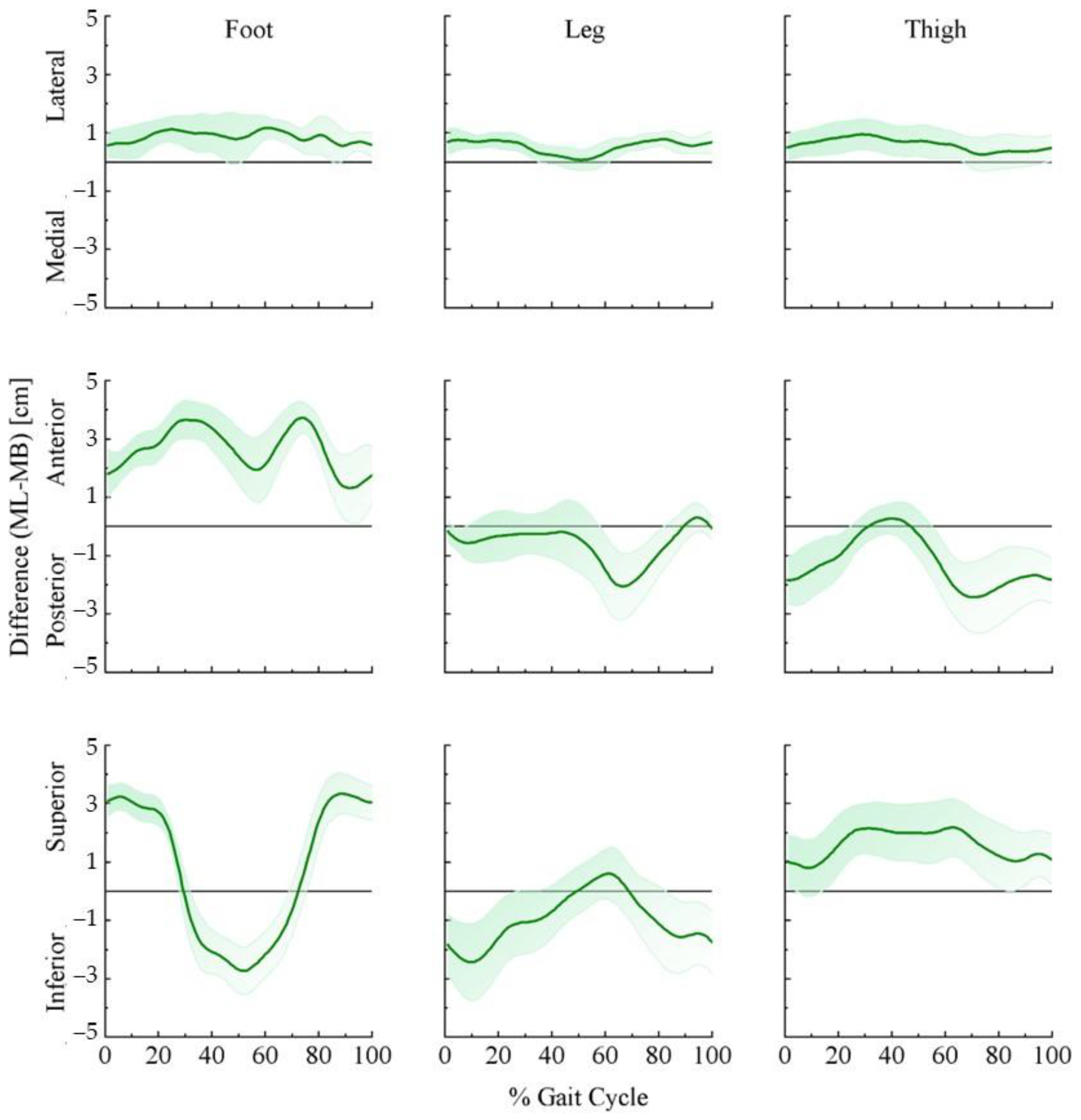
3.2. Lower Extremity Joint Center and Segment Center of Mass
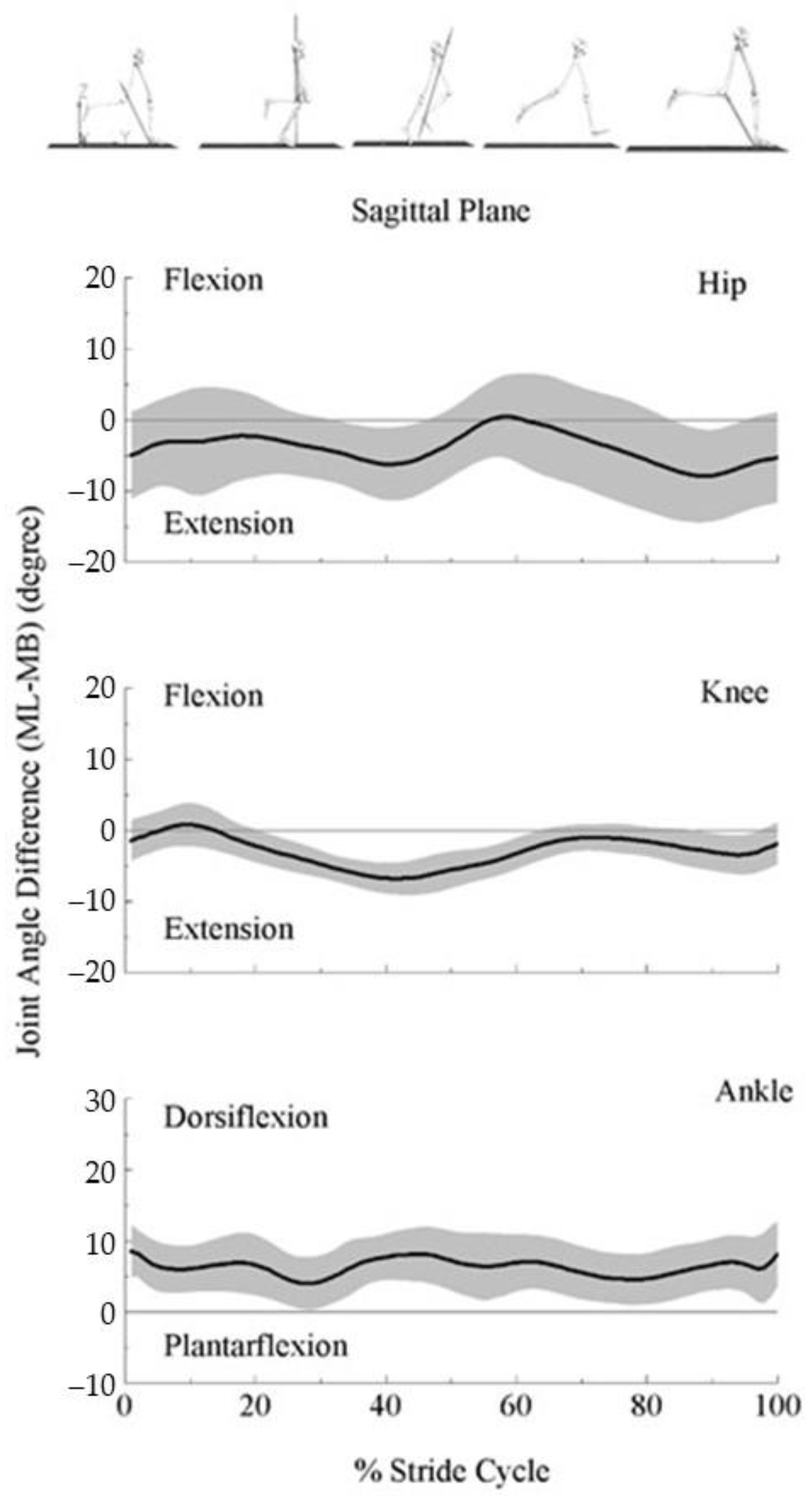
3.3. Lower Extremity Joint Angles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winter, D.A. Biomechanics and Motor Control of Human Movement; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Tate, J.J.; Milner, C. Real-Time Kinematic, Temporospatial, and Kinetic Biofeedback During Gait Retraining in Patients: A Systematic Review. Phys. Ther. 2010, 90, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.E.; Charlton, J.; Perry, C.K.L.; De Vries, C.M.; Redekopp, M.J.; White, J.A.; Hunt, M.A. The effects of shoe-worn insoles on gait biomechanics in people with knee osteoarthritis: A systematic review and meta-analysis. Br. J. Sport. Med. 2017, 52, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Hart, H.; Culvenor, A.; Collins, N.; Ackland, D.; Cowan, S.; Machotka, Z.; Crossley, K. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Br. J. Sport. Med. 2015, 50, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Riemer, R.; Hsiao-Wecksler, E.T.; Zhang, X. Uncertainties in inverse dynamics solutions: A comprehensive analysis and an application to gait. Gait Posture 2008, 27, 578–588. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.G.; Walker, K.; Ford, K.R.; Myer, G.D.; E Hewett, T.; Bogert, A.J.V.D. Evaluation of a two dimensional analysis method as a screening and evaluation tool for anterior cruciate ligament injury. Br. J. Sport. Med. 2005, 39, 355–362. [Google Scholar] [CrossRef]
- Pearsall, D.; Costigan, P. The effect of segment parameter error on gait analysis results. Gait Posture 1999, 9, 173–183. [Google Scholar] [CrossRef]
- Ganley, K.J.; Powers, C.M. Determination of lower extremity anthropometric parameters using dual energy X-ray absorptiometry: The influence on net joint moments during gait. Clin. Biomech. 2004, 19, 50–56. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Rozumalski, A. A new method for estimating joint parameters from motion data. J. Biomech. 2005, 38, 107–116. [Google Scholar] [CrossRef]
- Richards, J.G. The measurement of human motion: A comparison of commercially available systems. Hum. Mov. Sci. 1999, 18, 589–602. [Google Scholar] [CrossRef]
- Cappozzo, A.; Catani, F.; Della Croce, U.; Leardini, A. Position and orientation in space of bones during movement: Anatomical frame definition and determination. Clin. Biomech. 1995, 10, 171–178. [Google Scholar] [CrossRef]
- Stagni, R.; Fantozzi, S.; Cappello, A.; Leardini, A. Quantification of soft tissue artefact in motion analysis by combining 3D fluoroscopy and stereophotogrammetry: A study on two subjects. Clin. Biomech. 2005, 20, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.R. Quantification of human motion: Gait analysis—Benefits and limitations to its application to clinical problems. J. Biomech. 2004, 37, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Gorton, G.E., III; Hebert, D.A.; Gannotti, M.E. Assessment of the kinematic variability among 12 motion analysis laboratories. Gait Posture 2009, 29, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Alcock, L.; McArdle, R.; Rehman, R.; Del Din, S.; Mazzà, C.; Yarnall, A.; Rochester, L. The Role of Movement Analysis in Diagnosing and Monitoring Neurodegenerative Conditions: Insights from Gait and Postural Control. Brain Sci. 2019, 9, 34. [Google Scholar] [CrossRef]
- Whittle, M.W. Clinical gait analysis: A review. Hum. Mov. Sci. 1996, 15, 369–387. [Google Scholar] [CrossRef]
- Kanko, R.M.; Laende, E.K.; Strutzenberger, G.; Brown, M.; Selbie, W.S.; DePaul, V.; Scott, S.H.; Deluzio, K.J. Assessment of spatiotemporal gait parameters using a deep learning algorithm-based markerless motion capture system. J. Biomech. 2021, 122, 110414. [Google Scholar] [CrossRef]
- Kanko, R.M.; Laende, E.; Selbie, W.S.; Deluzio, K.J. Inter-session repeatability of markerless motion capture gait kinematics. J. Biomech. 2021, 121, 110422. [Google Scholar] [CrossRef]
- Kanko, R.M.; Laende, E.K.; Davis, E.M.; Selbie, W.S.; Deluzio, K.J. Concurrent assessment of gait kinematics using marker-based and markerless motion capture. J. Biomech. 2021, 127, 110665. [Google Scholar] [CrossRef]
- Derrick, T.R.; Bogert, A.J.V.D.; Cereatti, A.; Dumas, R.; Fantozzi, S.; Leardini, A. ISB recommendations on the reporting of intersegmental forces and moments during human motion analysis. J. Biomech. 2019, 99, 109533. [Google Scholar] [CrossRef]
- Woltring, H.J. Representation and calculation of 3-D joint movement. Hum. Mov. Sci. 1991, 10, 603–616. [Google Scholar] [CrossRef]
- Gruber, A.H.; Zhang, S.; Pan, J.; Li, L. Leg and Joint Stiffness Adaptations to Minimalist and Maximalist Running Shoes. J. Appl. Biomech. 2021, 37, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Documentation, C.-M.W. Tutorial: Foot and Ankle Angles. 2020. Available online: https://www.c-motion.com/v3dwiki/index.php?title=Visual3D_Documentation (accessed on 12 October 2022).
- Dempster, W.T. Space Requirements of the Seated Operator, Geometrical, Kinematic, and Mechanical Aspects of the Body with Special Reference to the Limbs; Michigan State University: East Lansing, MI, USA, 1955. [Google Scholar]
- Hanavan, E.P. A Mathematical Model of the Human Body; AMRL Technical Report; AMRL: Tamil Nadu, India, 1964; pp. 64–102. [Google Scholar] [CrossRef]
- Bell, A.L.; Brand, R.A.; Pedersen, D.R. Prediction of hip joint centre location from external landmarks. Hum. Mov. Sci. 1989, 8, 3–16. [Google Scholar] [CrossRef]
- Kristianslund, E.; Krosshaug, T.; Bogert, A.J.V.D. Effect of low pass filtering on joint moments from inverse dynamics: Implications for injury prevention. J. Biomech. 2012, 45, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.G.E.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S.N. Research Methods in Biomechanics, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2014. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, J.; Li, L. Non-linear changes of lower extremity kinetics prior to gait transition. J. Biomech. 2018, 77, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Dugan, S.A.; Bhat, K.P. Biomechanics and Analysis of Running Gait. Phys. Med. Rehabil. Clin. 2005, 16, 603–621. [Google Scholar] [CrossRef]
- Novacheck, T.F. The biomechanics of running. Gait Posture 1998, 7, 77–95. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Trost, J.P.; Wervey, R.A. Measurement and management of errors in quantitative gait data. Gait Posture 2004, 20, 196–203. [Google Scholar] [CrossRef]
- Fukuchi, R.K.; Fukuchi, C.A.; Duarte, M. A public dataset of running biomechanics and the effects of running speed on lower extremity kinematics and kinetics. PeerJ 2017, 5, e3298. [Google Scholar] [CrossRef]
- Schache, A.G.; Blanch, P.D.; Dorn, T.W.; Brown, N.; Rosemond, D.; Pandy, M. Effect of Running Speed on Lower Limb Joint Kinetics. Med. Sci. Sport. Exerc. 2011, 43, 1260–1271. [Google Scholar] [CrossRef]
- Stagni, R.; Leardini, A.; Cappozzo, A.; Benedetti, M.G.; Cappello, A. Effects of hip joint centre mislocation on gait analysis results. J. Biomech. 2000, 33, 1479–1487. [Google Scholar] [CrossRef]
- Holden, J.P.; Stanhope, S.J. The effect of variation in knee center location estimates on net knee joint moments. Gait Posture 1998, 7, 1–6. [Google Scholar] [CrossRef]
- Wade, L.; Needham, L.; McGuigan, P.; Bilzon, J. Applications and limitations of current markerless motion capture methods for clinical gait biomechanics. PeerJ 2022, 10, e12995. [Google Scholar] [CrossRef] [PubMed]
- Reinschmidt, C.; Bogert, A.V.D.; Nigg, B.; Lundberg, A.; Murphy, N. Effect of skin movement on the analysis of skeletal knee joint motion during running. J. Biomech. 1997, 30, 729–732. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, R.; Ong, J.N.; Lee, C.Y.; Jeon, S.H.; Park, H.; Kim, H.-J.; Lee, J.; Jeon, B. Quantitative Gait Analysis Using a Pose-Estimation Algorithm with a Single 2D-Video of Parkinson’s Disease Patients. J. Park. Dis. 2021, 11, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Kidziński, Ł.; Yang, B.; Hicks, J.L.; Rajagopal, A.; Delp, S.L.; Schwartz, M.H. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cronin, N.J.; Rantalainen, T.; Ahtiainen, J.P.; Hynynen, E.; Waller, B. Markerless 2D kinematic analysis of underwater running: A deep learning approach. J. Biomech. 2019, 87, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Colyer, S.; Cosker, D.; Salo, A. Foot Contact Timings and Step Length for Sprint Training. In Proceedings of the 2018 IEEE Winter Conference on Applications of Computer Vision (WACV), Lake Tahoe, NV, USA, 12–15 March 2018. [Google Scholar]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; Jácome, M.A.; García-Sancho, C.; Cudeiro, J.; Arias, P. Spatiotemporal Gait Patterns During Overt and Covert Evaluation in Patients With Parkinson’s Disease and Healthy Subjects: Is There a Hawthorne Effect? J. Appl. Biomech. 2015, 31, 189–194. [Google Scholar] [CrossRef]
- Liu, H.; Garrett, W.E.; Moorman, C.T.; Yu, B. Injury rate, mechanism, and risk factors of hamstring strain injuries in sports: A review of the literature. J. Sport Health Sci. 2012, 1, 92–101. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, S.; Zhong, Y.; Fu, W.; Li, L.; Liu, Y. How Joint Torques Affect Hamstring Injury Risk in Sprinting Swing–Stance Transition. Med. Sci. Sport. Exerc. 2015, 47, 373–380. [Google Scholar] [CrossRef]
- Lenhart, R.L.; Thelen, D.G.; Wille, C.M.; Chumanov, E.S.; Heiderscheit, B.C. Increasing Running Step Rate Reduces Patellofemoral Joint Forces. Med. Sci. Sport. Exerc. 2014, 46, 557–564. [Google Scholar] [CrossRef]
- Zago, M.; Luzzago, M.; Marangoni, T.; De Cecco, M.; Tarabini, M.; Galli, M. 3D Tracking of Human Motion Using Visual Skeletonization and Stereoscopic Vision. Front. Bioeng. Biotechnol. 2020, 8, 181. [Google Scholar] [CrossRef] [PubMed]

| Parameters | MB | ML | T & p-Value | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Moment (Nm/kg) | Hip first peak | 2.09 | 0.66 | 2.05 | 0.73 | t159 = −1.42, p < 0.2832 | 0.1 |
| Hip second peak | −1.38 | 0.23 | −1.73 | 0.27 | t159 = −22.99, p < 0.0001 * | 1.8 | |
| Hip third peak | 1.42 | 0.29 | 2.27 | 0.45 | t159 = 39.612, p < 0.0001 | 3.1 | |
| Knee first peak | 1.40 | 0.42 | 1.28 | 0.32 | t159 = 5.907, p < 0.0001 * | 0.5 | |
| Knee second peak | −0.74 | 0.13 | −1.17 | 0.24 | t159 = 40.804, p < 0.0001 * | 3.2 | |
| Ankle first peak | 3.14 | 0.51 | 3.32 | 0.55 | t159 = 10.450, p < 0.0001 * | 0.8 | |
| Power (W/kg) | Hip first peak | −5.02 | 2.60 | −4.80 | 2.37 | t159 = −1.570, p = 0.118 | 0.1 |
| Hip second peak | 4.29 | 1.14 | 8.07 | 2.11 | t159 = −27.082, p < 0.0001 * | 2.1 | |
| Hip third peak | 3.99 | 2.13 | 5.68 | 2.71 | t159 = −13.049, p < 0.0001 * | 1.0 | |
| Knee first peak | −5.00 | 2.77 | −4.08 | 1.79 | t159 = −6.138, p = 0.0229 | 0.5 | |
| Knee second peak | 3.15 | 1.41 | 2.64 | 1.09 | t159 = 6.628, p < 0.0001 * | 0.5 | |
| Knee third peak | −3.45 | 1.29 | −5.42 | 1.61 | t159 = 21.188, p < 0.0001 * | 1.7 | |
| Knee forth peak | −7.15 | 1.83 | −9.65 | 2.10 | t159 = 31.515, p < 0.0001 * | 2.5 | |
| Ankle first peak | −8.38 | 2.48 | −9.44 | 1.81 | t159 = 7.295, p = 0.6656 | 0.6 | |
| Ankle second peak | 16.07 | 3.60 | 18.40 | 4.91 | t159 = −10.726, p = 0.2426 | 0.9 | |
| Parameters | MB | ML | T & p-Value | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Moment (%stride Cycle) | Hip first peak | 5.16 | 1.27 | 6.74 | 3.40 | t159 = −5.946, p< 0.0001 * | 0.5 |
| Hip second peak | 40.26 | 6.90 | 43.59 | 7.00 | t159 = −7.179, p < 0.0001 * | 0.6 | |
| Hip third peak | 90.58 | 3.39 | 92.73 | 3.00 | t159 = −8.637, p < 0.0001 * | 0.7 | |
| Knee first peak | 12.98 | 2.18 | 13.53 | 3.89 | t159 = −2.008, p = 0.0015 * | 0.2 | |
| Knee second peak | 90.93 | 2.21 | 92.19 | 2.51 | t159 = −10.031, p < 0.0001 * | 0.8 | |
| Ankle first peak | 18.29 | 1.90 | 18.33 | 1.82 | t159 = −0.569, p = 0.57 | 0.0 | |
| Power (%Stride Cycle) | Hip first peak | 26.61 | 4.45 | 28.30 | 3.68 | t159 = −6.983, p < 0.0001 * | 0.6 |
| Hip second peak | 51.89 | 3.90 | 52.36 | 4.37 | t159 = −1.243, p = 0.216 | 0.1 | |
| Hip third peak | 91.23 | 3.47 | 89.63 | 4.15 | t159 = 4.458, p < 0.0001 * | 0.4 | |
| Knee first peak | 8.19 | 2.21 | 8.73 | 3.01 | t159 = −2.380, p = 0.0006 * | 0.2 | |
| Knee second peak | 19.45 | 3.09 | 21.17 | 3.51 | t159 = −6.606, p < 0.0001 * | 0.5 | |
| Knee third peak | 49.31 | 2.77 | 49.06 | 3.30 | t159 = 0.860, p = 0.391 | 0.1 | |
| Knee forth peak | 86.59 | 2.18 | 86.83 | 2.31 | t159 = −1.740, p = 0.084 | 0.1 | |
| Ankle first peak | 10.58 | 2.20 | 10.82 | 2.22 | t159 = −1.794, p = 0.075 | 0.6 | |
| Ankle second peak | 24.10 | 2.32 | 24.21 | 2.08 | t159 = −0.943, p = 0.347 | 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Pan, J.; Munkasy, B.; Duffy, K.; Li, L. Comparison of Lower Extremity Joint Moment and Power Estimated by Markerless and Marker-Based Systems during Treadmill Running. Bioengineering 2022, 9, 574. https://doi.org/10.3390/bioengineering9100574
Tang H, Pan J, Munkasy B, Duffy K, Li L. Comparison of Lower Extremity Joint Moment and Power Estimated by Markerless and Marker-Based Systems during Treadmill Running. Bioengineering. 2022; 9(10):574. https://doi.org/10.3390/bioengineering9100574
Chicago/Turabian StyleTang, Hui, Jiahao Pan, Barry Munkasy, Kim Duffy, and Li Li. 2022. "Comparison of Lower Extremity Joint Moment and Power Estimated by Markerless and Marker-Based Systems during Treadmill Running" Bioengineering 9, no. 10: 574. https://doi.org/10.3390/bioengineering9100574
APA StyleTang, H., Pan, J., Munkasy, B., Duffy, K., & Li, L. (2022). Comparison of Lower Extremity Joint Moment and Power Estimated by Markerless and Marker-Based Systems during Treadmill Running. Bioengineering, 9(10), 574. https://doi.org/10.3390/bioengineering9100574








