A Review on the Technological Advances and Future Perspectives of Axon Guidance and Regeneration in Peripheral Nerve Repair
Abstract
1. Introduction
2. Strategies Adopted for Peripheral Nerve Regeneration
2.1. Surgical Approach
2.2. Biochemical Approach
2.3. Biomaterial Approach
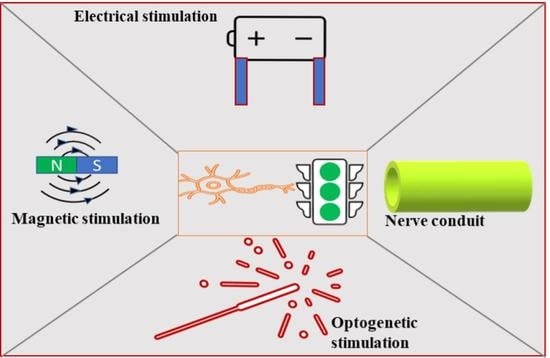
2.4. Electrical Stimulation Approach
2.5. Optogenetic Stimulation
2.6. Electromagnetic Stimulation
3. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Li, M.; Guan, Y.; Schreyer, D.; Chen, X. Effects of laminin blended with chitosan on axon guidance on patterned substrates. Biofabrication 2010, 2, 045002. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Brzezicki, G. Chapter 8 Current Techniques and Concepts in Peripheral Nerve Repair. Int. Rev. Neurobiol. 2009, 87, 141–172. [Google Scholar] [PubMed]
- Tang, S.; Zhu, J.; Xu, Y.; Xiang, A.P.; Jiang, M.H.; Quan, D. The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials 2013, 34, 7086–7096. [Google Scholar] [CrossRef]
- Meek, M.F. More than just sunshine with implantation of resorbable (p (DLLA-ε-CL)) biomaterials. Bio-Med. Mater. Eng. 2007, 17, 329–334. [Google Scholar]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77. [Google Scholar] [CrossRef]
- Sunderland, S. A classification of peripheral nerve injuries producing loss of function. Brain 1951, 74, 491–516. [Google Scholar] [CrossRef]
- Chhabra, A.; Andreisek, G. Magnetic Resonance Neurography; JP Medical Ltd.: London, UK, 2012. [Google Scholar]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef]
- Kline, D.G. Surgical repair of peripheral nerve injury. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1990, 13, 843–852. [Google Scholar] [CrossRef]
- Gordon, T. Nerve regeneration in the peripheral and central nervous systems. J. Physiol. 2016, 594, 3517–3520. [Google Scholar] [CrossRef]
- Hoben, G.M.; Ee, X.; Schellhardt, L.; Yan, Y.; Hunter, D.A.; Moore, A.M.; Snyder-Warwick, A.K.; Stewart, S.; Mackinnon, S.E.; Wood, M.D. Increasing nerve autograft length increases senescence and reduces regeneration. Plast. Reconstr. Surg. 2018, 142, 952. [Google Scholar] [CrossRef] [PubMed]
- Behar, O.; Golden, J.A.; Mashimo, H.; Schoen, F.J.; Fishman, M.C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 1996, 383, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Hancock, M.L.; Nowakowski, D.W.; Role, L.W.; Talmage, D.A.; Flanagan, J.G. Type III neuregulin 1 regulates pathfinding of sensory axons in the developing spinal cord and periphery. Development 2011, 138, 4887–4898. [Google Scholar] [CrossRef] [PubMed]
- Mokarram, N.; Dymanus, K.; Srinivasan, A.; Lyon, J.G.; Tipton, J.; Chu, J.; English, A.W.; Bellamkonda, R.V. Immunoengineering nerve repair. Proc. Natl. Acad. Sci. USA 2017, 114, E5077–E5084. [Google Scholar] [CrossRef]
- Schwieger, J.; Warnecke, A.; Lenarz, T.; Esser, K.-H.; Scheper, V. Neuronal survival, morphology and outgrowth of spiral ganglion neurons using a defined growth factor combination. PLoS ONE 2015, 10, e0133680. [Google Scholar]
- Emel, E.; Ergün, S.S.; Kotan, D.; Gürsoy, E.B.; Parman, Y.; Zengin, A.; Nurten, A. Effects of insulin-like growth factor–I and platelet-rich plasma on sciatic nerve crush injury in a rat model. J. Neurosurg. 2011, 114, 522–528. [Google Scholar] [CrossRef]
- Gordon, T.; Borschel, G.H. The use of the rat as a model for studying peripheral nerve regeneration and sprouting after complete and partial nerve injuries. Exp. Neurol. 2017, 287, 331–347. [Google Scholar] [CrossRef]
- Katori, S.; Noguchi-Katori, Y.; Itohara, S.; Iwasato, T. Spinal RacGAP α-chimaerin is required to establish the midline barrier for proper corticospinal axon guidance. J. Neurosci. 2017, 37, 7682–7699. [Google Scholar] [CrossRef]
- Magnaghi, V.; Conte, V.; Procacci, P.; Pivato, G.; Cortese, P.; Cavalli, E.; Pajardi, G.; Ranucci, E.; Fenili, F.; Manfredi, A. Biological performance of a novel biodegradable polyamidoamine hydrogel as guide for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2011, 98, 19–30. [Google Scholar] [CrossRef]
- Stanec, S.; Stanec, Z. Reconstruction of upper-extremity peripheral-nerve injuries with ePTFE conduits. J. Reconstr. Microsurg. 1998, 14, 227–232. [Google Scholar] [CrossRef]
- Hama, S.; Uemura, T.; Onode, E.; Yokoi, T.; Okada, M.; Takamatsu, K.; Nakamura, H. Nerve capping treatment using a bioabsorbable nerve conduit with open or closed end for rat sciatic neuroma. Clin. Neurol. Neurosurg. 2021, 209, 106920. [Google Scholar] [CrossRef] [PubMed]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Hirase, Y.; Isogai, N.; Sueyoshi, Y. A clinical multi-center registry study on digital nerve repair using a biodegradable nerve conduit of PGA with external and internal collagen scaffolding. Microsurgery 2019, 39, 395–399. [Google Scholar] [CrossRef]
- Siemionow, M.; Bozkurt, M.; Zor, F. Regeneration and repair of peripheral nerves with different biomaterials. Microsurgery 2010, 30, 574–588. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Chang, W.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Kohn, J. Engineering silk fibroin-based nerve conduit with neurotrophic factors for proximal protection after peripheral nerve injury. Adv. Healthc. Mater. 2021, 10, 2000753. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Ravnic, D.J.; Payne, R.; Rizk, E.; Koduru, S.V.; Hazard, S.W. Adipose-Derived Stem Cells in Peripheral Nerve Regeneration. Curr. Surg. Rep. 2017, 5, 5. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef]
- Bellamkonda, R.V. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials 2006, 27, 3515–3518. [Google Scholar] [CrossRef]
- Kim, Y.T.; Haftel, V.K.; Kumar, S.; Bellamkonda, R.V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials 2008, 29, 3117–3127. [Google Scholar] [CrossRef]
- Koh, H.; Yong, T.; Teo, W.; Chan, C.; Puhaindran, M.; Tan, T.; Lim, A.; Lim, B.; Ramakrishna, S. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J. Neural Eng. 2010, 7, 046003. [Google Scholar] [CrossRef]
- Ngo, T.T.B.; Waggoner, P.J.; Romero, A.A.; Nelson, K.D.; Eberhart, R.C.; Smith, G.M. Poly (l-lactide) microfilaments enhance peripheral nerve regeneration across extended nerve lesions. J. Neurosci. Res. 2003, 72, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Tiwari, A.P. Three dimensional polycaprolactone/cellulose scaffold containing calcium-based particles: A new platform for bone regeneration. Carbohydr. Polym. 2020, 250, 116880. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.-J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef]
- Luo, B.; Tiwari, A.P.; Chen, N.; Ramakrishna, S.; Yang, I.H. Development of an Axon-Guiding Aligned Nanofiber-Integrated Compartmentalized Microfluidic Neuron Culture System. ACS Appl. Bio Mater. 2021, 4, 8424–8432. [Google Scholar] [CrossRef]
- Thomson, S.E.; Charalambous, C.; Smith, C.-A.; Tsimbouri, P.M.; Déjardin, T.; Kingham, P.J.; Hart, A.M.; Riehle, M.O. Microtopographical cues promote peripheral nerve regeneration via transient mTORC2 activation. Acta Biomater. 2017, 60, 220–231. [Google Scholar] [CrossRef]
- Yang, X.; Huang, L.; Yi, X.; Huang, S.; Duan, B.; Yu, A. Multifunctional chitin-based hollow nerve conduit for peripheral nerve regeneration and neuroma inhibition. Carbohydr. Polym. 2022, 289, 119443. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Kim, J.I.; Won Ko, S.; Park, C.H.; Kim, C.S. Electrodeless coating polypyrrole on chitosan grafted polyurethane with functionalized multiwall carbon nanotubes electrospun scaffold for nerve tissue engineering. Carbon 2018, 136, 430–443. [Google Scholar] [CrossRef]
- Thibodeau, A.; Galbraith, T.; Fauvel, C.M.; Khuong, H.T.; Berthod, F. Repair of peripheral nerve injuries using a prevascularized cell-based tissue-engineered nerve conduit. Biomaterials 2022, 280, 121269. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Chan, S.-H.; Chiang, C.-M.; Chi-Chang Chen, C.; Jiang, C.-F. Peripheral nerve regeneration using a microporous polylactic acid asymmetric conduit in a rabbit long-gap sciatic nerve transection model. Biomaterials 2011, 32, 3764–3775. [Google Scholar] [CrossRef]
- Tao, J.; Liu, H.; Wu, W.; Zhang, J.; Liu, S.; Zhang, J.; Huang, Y.; Xu, X.; He, H.; Yang, S. 3D-Printed Nerve Conduits with Live Platelets for Effective Peripheral Nerve Repair. Adv. Funct. Mater. 2020, 30, 2004272. [Google Scholar] [CrossRef]
- Gordon, T.; Brushart, T.; Chan, K. Augmenting nerve regeneration with electrical stimulation. Neurol. Res. 2008, 30, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.; Willits, R.K. Short-duration, DC electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics 2006, 27, 328–331. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar] [PubMed]
- Geremia, N.M.; Gordon, T.; Brushart, T.M.; Al-Majed, A.A.; Verge, V.M. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp. Neurol. 2007, 205, 347–359. [Google Scholar] [CrossRef]
- Cobianchi, S.; Casals-Diaz, L.; Jaramillo, J.; Navarro, X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp. Neurol. 2013, 240, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Goganau, I.; Sandner, B.; Weidner, N.; Fouad, K.; Blesch, A. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp. Neurol. 2018, 300, 247–258. [Google Scholar] [CrossRef]
- Singh, B.; Xu, Q.-G.; Franz, C.K.; Zhang, R.; Dalton, C.; Gordon, T.; Verge, V.M.K.; Midha, R.; Zochodne, D.W. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm: Laboratory investigation. J. Neurosurg. JNS 2012, 116, 498–512. [Google Scholar] [CrossRef]
- Lu, M.C.; Ho, C.Y.; Hsu, S.F.; Lee, H.C.; Lin, J.H.; Yao, C.H.; Chen, Y.S. Effects of electrical stimulation at different frequencies on regeneration of transected peripheral nerve. Neurorehabilit. Neural Repair 2008, 22, 367–373. [Google Scholar] [CrossRef]
- Yang, I.H.; Gary, D.; Malone, M.; Dria, S.; Houdayer, T.; Belegu, V.; McDonald, J.W.; Thakor, N. Axon Myelination and Electrical Stimulation in a Microfluidic, Compartmentalized Cell Culture Platform. NeuroMolecular Med. 2012, 14, 112–118. [Google Scholar] [CrossRef]
- Nosaka, K.; Aldayel, A.; Jubeau, M.; Chen, T.C. Muscle damage induced by electrical stimulation. Eur. J. Appl. Physiol. 2011, 111, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Nag, S.; Blasiak, A.; Jin, Y.; Thakor, N.; Yang, I.H. Subcellular Optogenetic Stimulation for Activity-Dependent Myelination of Axons in a Novel Microfluidic Compartmentalized Platform. ACS Chem. Neurosci. 2016, 7, 1317–1324. [Google Scholar] [CrossRef]
- Maimon, B.E.; Sparks, K.; Srinivasan, S.; Zorzos, A.N.; Herr, H.M. Spectrally distinct channelrhodopsins for two-colour optogenetic peripheral nerve stimulation. Nat. Biomed. Eng. 2018, 2, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koppes, R.A.; Froriep, U.P.; Jia, X.; Achyuta, A.K.H.; McLaughlin, B.L.; Anikeeva, P. Optogenetic control of nerve growth. Sci. Rep. 2015, 5, 9669. [Google Scholar] [CrossRef] [PubMed]
- Antman-Passig, M.; Giron, J.; Karni, M.; Motiei, M.; Schori, H.; Shefi, O. Magnetic Assembly of a Multifunctional Guidance Conduit for Peripheral Nerve Repair. Adv. Funct. Mater. 2021, 31, 2010837. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Funnell, J.L.; Ziemba, A.M.; Nowak, J.F.; Awada, H.; Prokopiou, N.; Samuel, J.; Guari, Y.; Nottelet, B.; Gilbert, R.J. Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro. Acta Biomater. 2021, 131, 302–313. [Google Scholar] [CrossRef]
- Ben Yakir-Blumkin, M.; Loboda, Y.; Schächter, L.; Finberg, J.P.M. Neuroprotective effect of weak static magnetic fields in primary neuronal cultures. Neuroscience 2014, 278, 313–326. [Google Scholar] [CrossRef]
- Prasad, A.; Teh, D.B.L.; Blasiak, A.; Chai, C.; Wu, Y.; Gharibani, P.M.; Yang, I.H.; Phan, T.T.; Lim, K.L.; Yang, H.; et al. Static Magnetic Field Stimulation Enhances Oligodendrocyte Differentiation and Secretion of Neurotrophic Factors. Sci. Rep. 2017, 7, 6743. [Google Scholar] [CrossRef]
- Wassermann, E.M.; Zimmermann, T. Transcranial magnetic brain stimulation: Therapeutic promises and scientific gaps. Pharmacol. Ther. 2012, 133, 98–107. [Google Scholar] [CrossRef]
- Dey, S.; Bose, S.; Kumar, S.; Rathore, R.; Mathur, R.; Jain, S. Extremely low frequency magnetic field protects injured spinal cord from the microglia- and iron-induced tissue damage. Electromagn. Biol. Med. 2017, 36, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Sliow, A.; Ma, Z.; Gargiulo, G.; Mahns, D.; Mawad, D.; Breen, P.; Stoodley, M.; Houang, J.; Kuchel, R.; Tettamanzi, G.C.; et al. Stimulation and Repair of Peripheral Nerves Using Bioadhesive Graft-Antenna. Adv. Sci. 2019, 6, 1801212. [Google Scholar] [CrossRef] [PubMed]
- Lekhraj, R.; Cynamon, D.E.; DeLuca, S.E.; Taub, E.S.; Pilla, A.A.; Casper, D. Pulsed electromagnetic fields potentiate neurite outgrowth in the dopaminergic MN9D cell line. J. Neurosci. Res. 2014, 92, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Greenebaum, B.; Sutton, C.; Subramanian Vadula, M.; Battocletti, J.; Swiontek, T.; DeKeyser, J.; Sisken, B. Effects of pulsed magnetic fields on neurite outgrowth from chick embryo dorsal root ganglia. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1996, 17, 293–302. [Google Scholar] [CrossRef]
- Heo, D.N.; Kim, H.-J.; Lee, Y.J.; Heo, M.; Lee, S.J.; Lee, D.; Do, S.H.; Lee, S.H.; Kwon, I.K. Flexible and Highly Biocompatible Nanofiber-Based Electrodes for Neural Surface Interfacing. ACS Nano 2017, 11, 2961–2971. [Google Scholar] [CrossRef]


| Materials | Scaffold Type/Fabrication Technique | Key Results | Ref. |
|---|---|---|---|
| Polyacrylic acid (PAA) polyamidoamines | Hydrogel tubing/polymerization | Improved sciatic nerve regeneration, no inflammation | [20] |
| Polycaprolactone/Polydimethylsiloxane (PCL/PDMS) | Nanofibers-microfludic device/electrospinning-microfabrication | Improved axon guidance and myelination | [36] |
| PCL/PDMS | PCL coating on PDMS/Spin coating | Micro topographical cues improve nerve regeneration | [37] |
| Chitin/polydopamine | Hollow chitin hydrogel tube/freeze-thaw method | Inhibit neuroma formation | [38] |
| PCL-based | Hollow conduit (made by Neurolac) | Improved functional recovery | [5] |
| Polyurethane-carbon nanotube | Conductive Align nanofibers | Increased neuron cells aligned, differentiation and regeneration | [39] |
| Deendothelialized nerve conduit | Nerve tube/cellular manipulation | Motor recovery function compared with autograft, increased vasculaization | [40] |
| Polylactide | Microporous conduit/solvent-non-solvent phase conversion | nerve bundles formed and long-term support, achieving a functional recovery | [41] |
| Cell encapsulated-gelatin methacrylate (GelMA) and poly(ethyleneglycol)diacrylate (PEGDA) | 3D printing | Platelet encapsulation leading to the sustained release of multiple growth factors, platelets significantly promoted the hydrogel conduits in peripheral nerve repair in vivo | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.P.; Lokai, T.; Albin, B.; Yang, I.H. A Review on the Technological Advances and Future Perspectives of Axon Guidance and Regeneration in Peripheral Nerve Repair. Bioengineering 2022, 9, 562. https://doi.org/10.3390/bioengineering9100562
Tiwari AP, Lokai T, Albin B, Yang IH. A Review on the Technological Advances and Future Perspectives of Axon Guidance and Regeneration in Peripheral Nerve Repair. Bioengineering. 2022; 9(10):562. https://doi.org/10.3390/bioengineering9100562
Chicago/Turabian StyleTiwari, Arjun Prasad, Taylor Lokai, Bayne Albin, and In Hong Yang. 2022. "A Review on the Technological Advances and Future Perspectives of Axon Guidance and Regeneration in Peripheral Nerve Repair" Bioengineering 9, no. 10: 562. https://doi.org/10.3390/bioengineering9100562
APA StyleTiwari, A. P., Lokai, T., Albin, B., & Yang, I. H. (2022). A Review on the Technological Advances and Future Perspectives of Axon Guidance and Regeneration in Peripheral Nerve Repair. Bioengineering, 9(10), 562. https://doi.org/10.3390/bioengineering9100562