Apical Medium Flow Influences the Morphology and Physiology of Human Proximal Tubular Cells in a Microphysiological System
Abstract
:1. Introduction
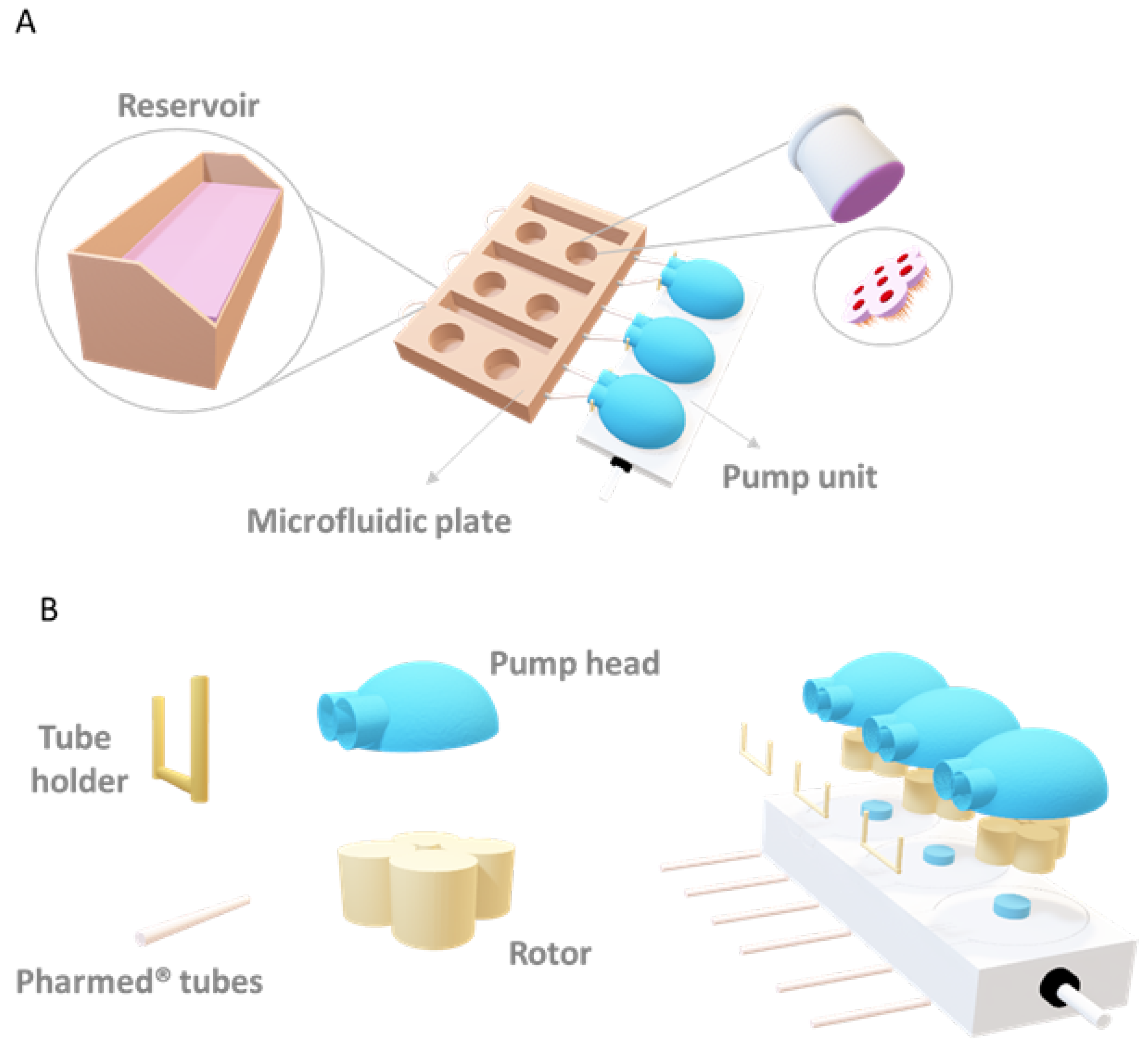
2. Materials and Methods
2.1. Cell Culture
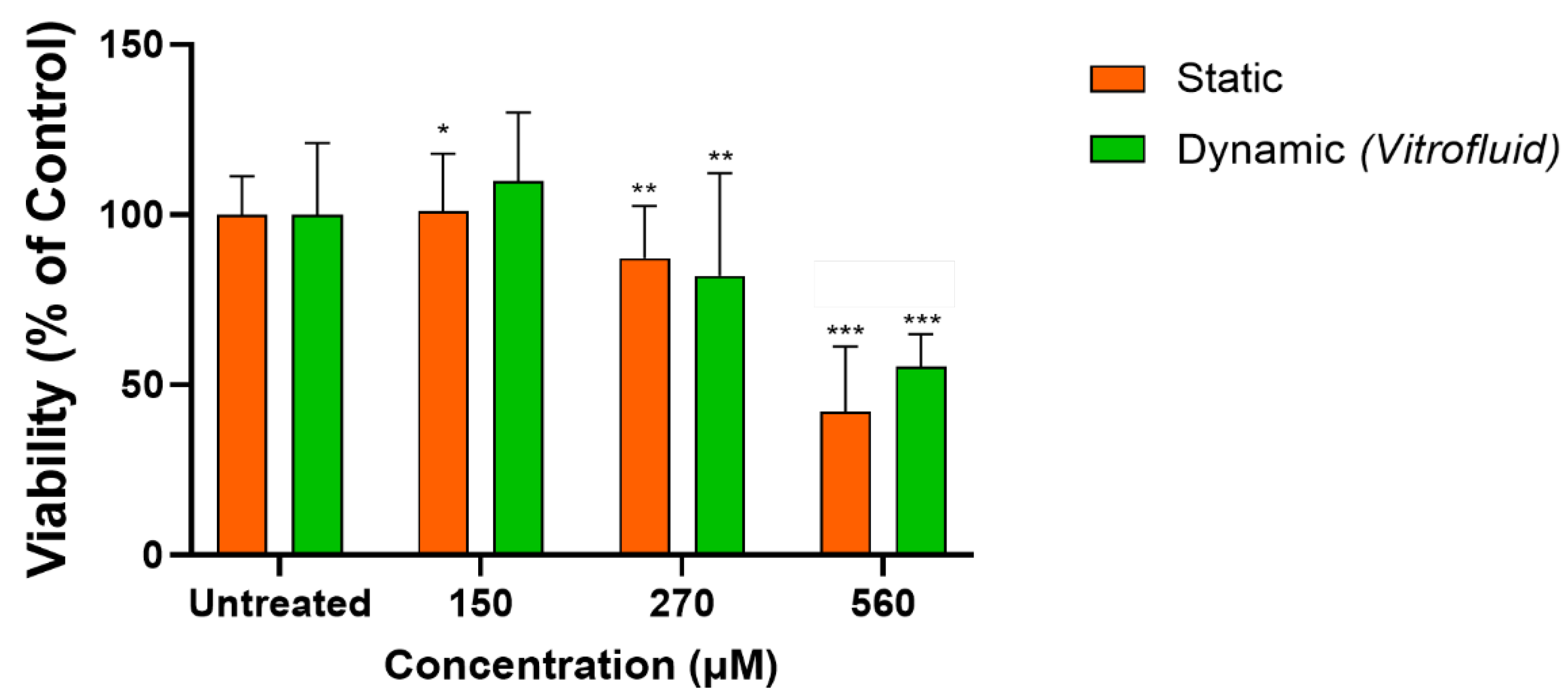
2.2. Cell Viability Assay
2.3. Membrane Permeance Assay
2.4. Immunostaining
2.5. Detection of Reactive Oxygen Species (ROS)
2.6. Determination of Cilia Morphology and Orientation
2.7. Albumin Uptake
2.8. Transferrin Uptake
2.9. Determination of Apparent Permeability
2.10. Gene Expression Analysis
2.11. Cytokine Detection
2.12. Finite Element Simulation
3. Results
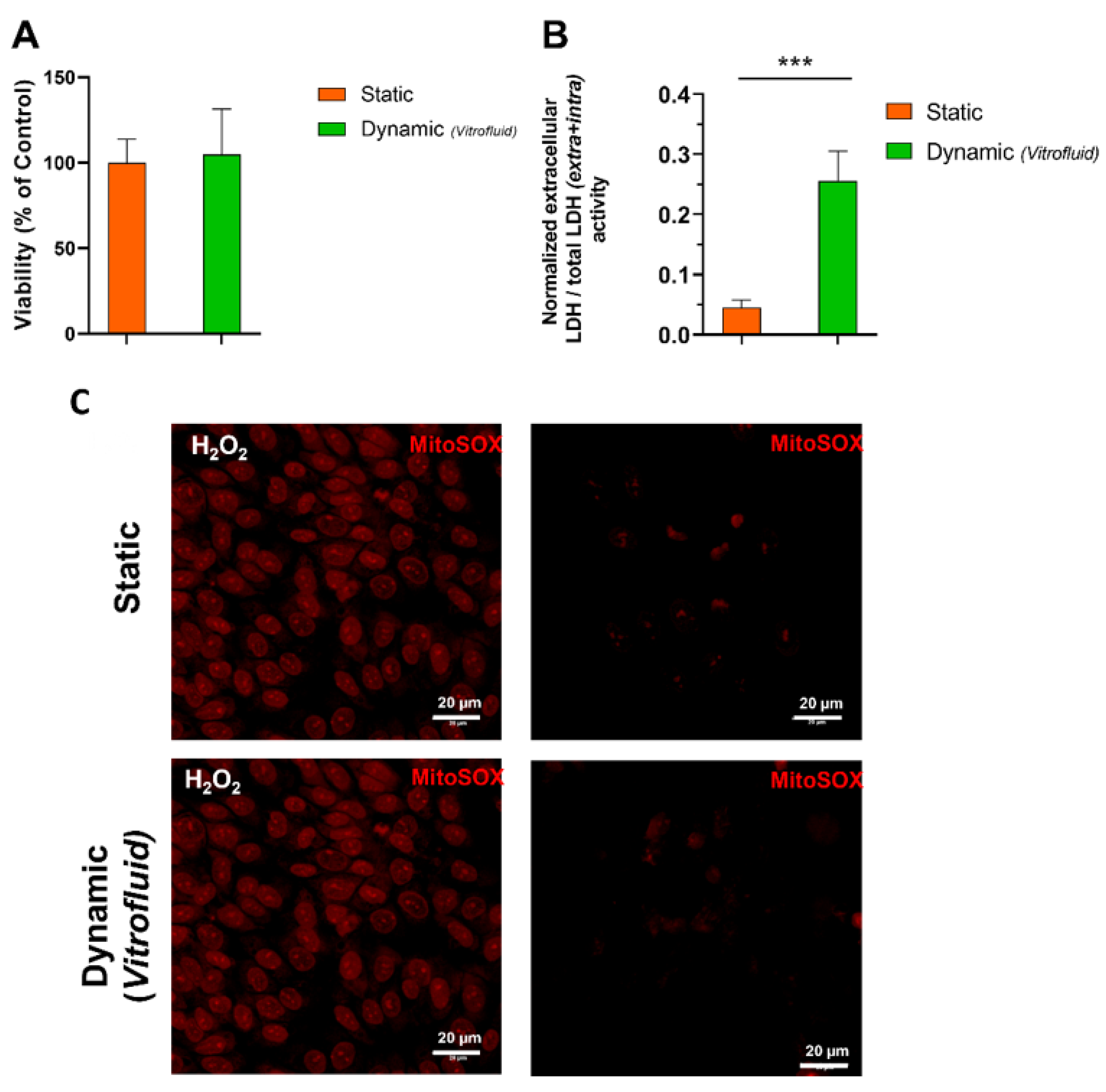
3.1. Medium Flow Does Not Cause Cytotoxicity but Affects the RPTEC Plasma Membrane
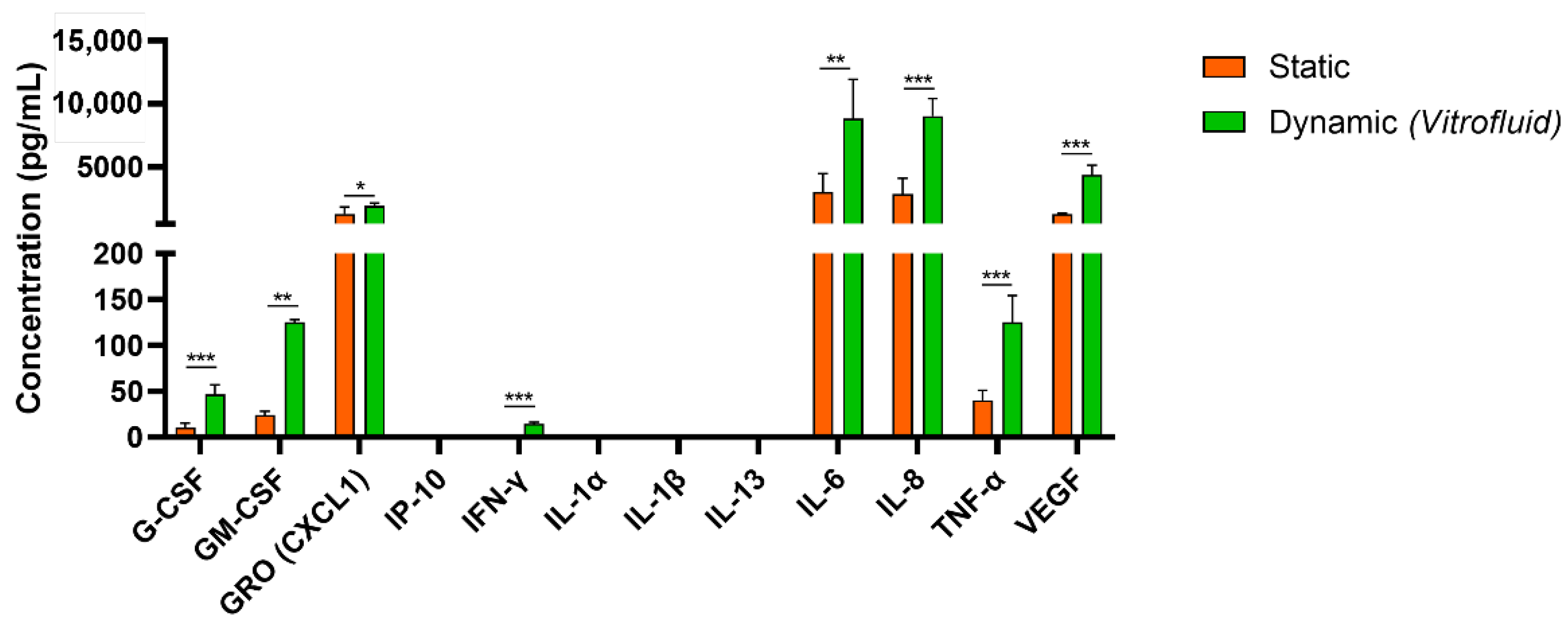
3.2. Dynamic Culture Conditions Induce RPTEC Cytokine/Chemokine Secretion
3.3. RPTECs Maintain a Tight Barrier under Static and Dynamic Conditions
3.4. Effect of FSS on Specific Transporters in RPTECs
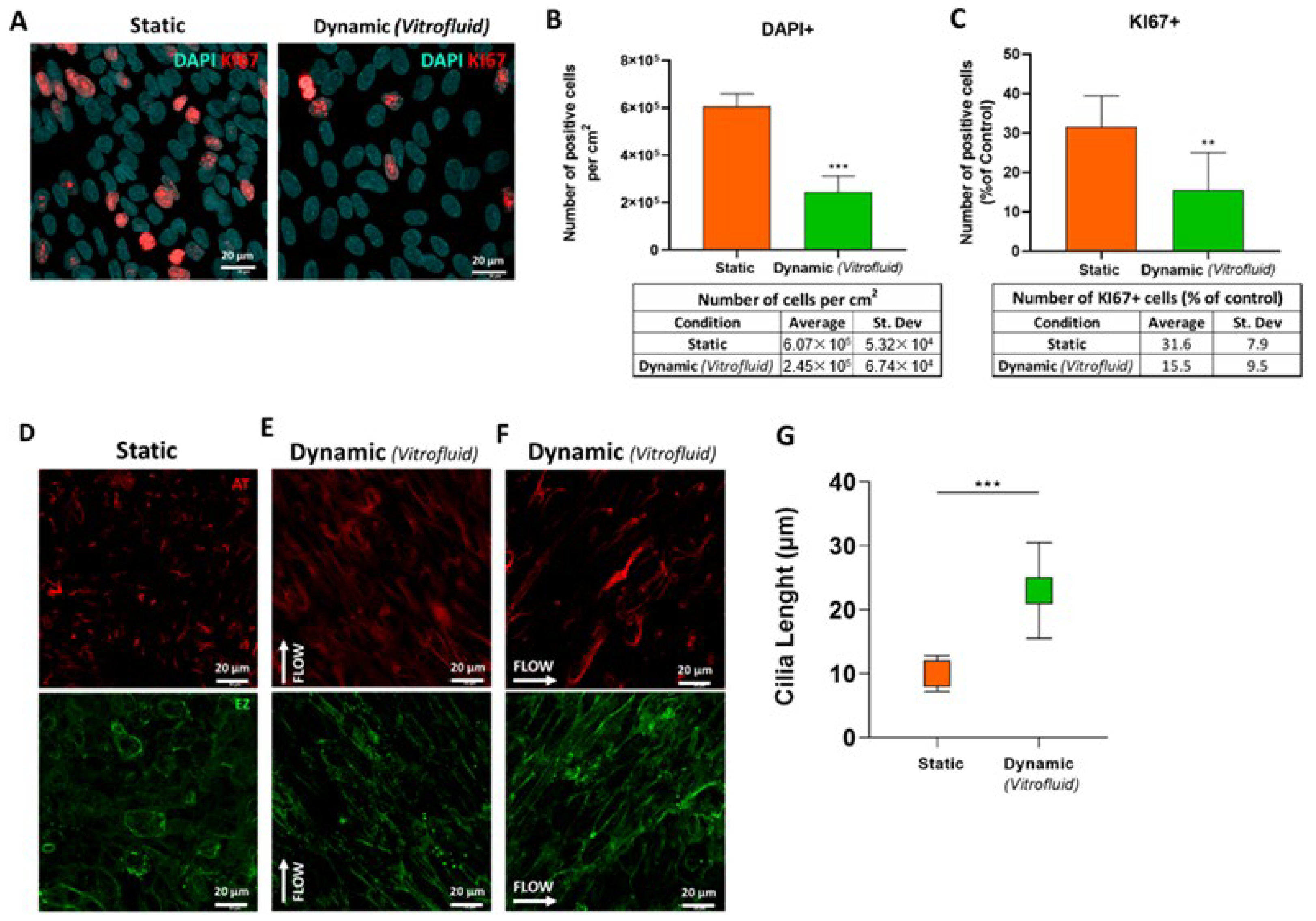
3.5. FSS Influences RPTEC Proliferation and Differentiation
3.6. RPTECs under Flow Remain Susceptible to Colistin-Induced Toxicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, M.A. Anatomy and physiology of the kidney. Aorn J. 1998, 68, 799–800, 803–804, 806, 808, 810–811, 813–816, 819–820. [Google Scholar] [CrossRef]
- Morrissey, K.M.; Stocker, S.L.; Wittwer, M.B.; Xu, L.; Giacomini, K.M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Nadim, M.K. An overview of drug-induced acute kidney injury. Crit. Care Med. 2008, 36, S216–S223. [Google Scholar] [CrossRef] [PubMed]
- George, B.; You, D.; Joy, M.S.; Aleksunes, L.M. Xenobiotic transporters and kidney injury. Adv. Drug Deliv. Rev. 2017, 116, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Koslowski, S.; Latapy, C.; Auvray, P.; Blondel, M.; Meijer, L. An overview of in vivo and in vitro models for autosomal dominant polycystic kidney disease: A journey from 3D-cysts to mini-pigs. Int. J. Mol. Sci. 2020, 21, 4537. [Google Scholar] [CrossRef]
- Bajaj, P.; Chowdhury, S.K.; Yucha, R.; Kelly, E.J.; Xiao, G. Emerging kidney models to investigate metabolism, transport, and toxicity of drugs and xenobiotics. Drug Metab. Dispos. 2018, 46, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Weisz, O.A. Endocytic adaptation to functional demand by the kidney proximal tubule. J. Physiol. 2021, 599, 3437–3446. [Google Scholar] [CrossRef]
- Narayanan, K.; Schumacher, K.M.; Tasnim, F.; Kandasamy, K.; Schumacher, A.; Ni, M.; Gao, S.; Gopalan, B.; Zink, D.; Ying, J.Y. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013, 83, 593–603. [Google Scholar] [CrossRef]
- Pfaller, W.; Gstraunthaler, G. Nephrotoxicity testing in vitro--what we know and what we need to know. Environ. Health Perspect. 1998, 106 (Suppl. S2), 559–569. [Google Scholar] [CrossRef]
- Jang, K.J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Minuth, W.W.; Denk, L. Bridging the gap between traditional cell cultures and bioreactors applied in regenerative medicine: Practical experiences with the MINUSHEET perfusion culture system. Cytotechnology 2016, 68, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, N.; Desai, R.R.; Fleischman, A.J.; Roy, S.; Humes, H.D.; Fissell, W.H. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnol. Bioeng. 2010, 107, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Brakeman, P.; Miao, S.; Cheng, J.; Lee, C.Z.; Roy, S.; Fissell, W.H.; Ferrell, N. A modular microfluidic bioreactor with improved throughput for evaluation of polarized renal epithelial cells. Biomicrofluidics 2016, 10, 064106. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gotoh, N.; Yan, Q.; Du, Z.; Weinstein, A.M.; Wang, T.; Weinbaum, S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 11418–11423. [Google Scholar] [CrossRef]
- Duan, Y.; Weinstein, A.M.; Weinbaum, S.; Wang, T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21860–21865. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Nieskens, T.T.G.; Vormann, M.K.; van den Berge, B.T.; van den Heuvel, A.; Russel, F.G.M.; Suter-Dick, L.; Lanz, H.L.; Vulto, P.; Masereeuw, R.; et al. Screening of drug-transporter interactions in a 3D microfluidic renal proximal tubule on a chip. AAPS J. 2018, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.A.; Spring, K.R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 2001, 184, 71–79. [Google Scholar] [CrossRef]
- Ran, J.; Yang, Y.; Li, D.; Liu, M.; Zhou, J. Deacetylation of α-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci. Rep. 2015, 5, 12917. [Google Scholar] [CrossRef]
- Maggiorani, D.; Dissard, R.; Belloy, M.; Saulnier-Blache, J.S.; Casemayou, A.; Ducasse, L.; Grès, S.; Bellière, J.; Caubet, C.; Bascands, J.L.; et al. Shear stress-induced alteration of epithelial organization in human renal tubular cells. PLoS ONE 2015, 10, e0131416. [Google Scholar] [CrossRef]
- Marx, U.; Andersson, T.B.; Bahinski, A.; Beilmann, M.; Beken, S.; Cassee, F.R.; Cirit, M.; Daneshian, M.; Fitzpatrick, S.; Frey, O.; et al. Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. Altex 2016, 33, 272–321. [Google Scholar] [CrossRef]
- Renggli, K.; Rousset, N.; Lohasz, C.; Nguyen, O.T.P.; Hierlemann, A. Integrated microphysiological systems: Transferable organ models and recirculating flow. Adv. Biosyst. 2019, 3, e1900018. [Google Scholar] [CrossRef] [PubMed]
- Beilmann, M.; Boonen, H.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Newham, P.; Oinonen, T.; Pognan, F.; Roth, A.; et al. Optimizing drug discovery by investigative toxicology: Current and future trends. Altex 2019, 36, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Lohasz, C.; Loretan, J.; Sterker, D.; Görlach, E.; Renggli, K.; Argast, P.; Frey, O.; Wiesmann, M.; Wartmann, M.; Rausch, M.; et al. A Microphysiological cell-culturing system for pharmacokinetic drug exposure and high-resolution imaging of arrays of 3D microtissues. Front. Pharmacol. 2021, 12, 785851. [Google Scholar] [CrossRef] [PubMed]
- Lohasz, C.; Bonanini, F.; Hoelting, L.; Renggli, K.; Frey, O.; Hierlemann, A. Predicting metabolism-related drug-drug interactions using a microphysiological multitissue system. Adv. Biosyst. 2020, 4, e2000079. [Google Scholar] [CrossRef] [PubMed]
- Marx, U.; Akabane, T.; Andersson, T.B.; Baker, E.; Beilmann, M.; Beken, S.; Brendler-Schwaab, S.; Cirit, M.; David, R.; Dehne, E.M.; et al. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. Altex 2020, 37, 365–394. [Google Scholar] [CrossRef]
- Wilmer, M.J.; Ng, C.P.; Lanz, H.L.; Vulto, P.; Suter-Dick, L.; Masereeuw, R. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016, 34, 156–170. [Google Scholar] [CrossRef]
- Nieskens, T.T.G.; Sjögren, A.K. Emerging in vitro systems to screen and predict drug-induced kidney toxicity. Semin. Nephrol. 2019, 39, 215–226. [Google Scholar] [CrossRef]
- Zanetti, F. Chapter 7—Kidney-on-a-chip. In Organ-on-a-Chip; Hoeng, J., Bovard, D., Peitsch, M.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 233–253. [Google Scholar]
- Brown, C.D.; Sayer, R.; Windass, A.S.; Haslam, I.S.; De Broe, M.E.; D’Haese, P.C.; Verhulst, A. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol. Appl. Pharmacol. 2008, 233, 428–438. [Google Scholar] [CrossRef]
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, 3814–3829. [Google Scholar] [CrossRef]
- Javan, A.O.; Shokouhi, S.; Sahraei, Z. A review on colistin nephrotoxicity. Eur. J. Clin. Pharmacol. 2015, 71, 801–810. [Google Scholar] [CrossRef]
- Soo, J.Y.; Jansen, J.; Masereeuw, R.; Little, M.H. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat. Rev. Nephrol. 2018, 14, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.; Piontek, J.; Rosenthal, R.; Günzel, D.; Krug, S.M. Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. Eur. J. Physiol. 2017, 469, 877–887. [Google Scholar] [CrossRef]
- Frost, T.S.; Jiang, L.; Lynch, R.M.; Zohar, Y. Permeability of epithelial/endothelial barriers in transwells and microfluidic bilayer devices. Micromachines 2019, 10, 533. [Google Scholar] [CrossRef]
- Gudi, S.R.; Clark, C.B.; Frangos, J.A. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ. Res. 1996, 79, 834–839. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Frangos, J.A. The shear stress of it all: The cell membrane and mechanochemical transduction. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1459–1467. [Google Scholar] [CrossRef]
- Jenq, W.; Cooper, D.R.; Bittle, P.; Ramirez, G. Aquaporin-1 expression in proximal tubule epithelial cells of human kidney is regulated by hyperosmolarity and contrast agents. Biochem. Biophys. Res. Commun. 1999, 256, 240–248. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J. Am. Soc. Nephrol. 2006, 17, 39–45. [Google Scholar] [CrossRef]
- Fukuda, Y.; Kaishima, M.; Ohnishi, T.; Tohyama, K.; Chisaki, I.; Nakayama, Y.; Ogasawara-Shimizu, M.; Kawamata, Y. Fluid shear stress stimulates MATE2-K expression via Nrf2 pathway activation. Biochem. Biophys. Res. Commun. 2017, 484, 358–364. [Google Scholar] [CrossRef]
- Uwai, Y.; Ida, H.; Tsuji, Y.; Katsura, T.; Inui, K. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm. Res. 2007, 24, 811–815. [Google Scholar] [CrossRef]
- Filipski, K.K.; Mathijssen, R.H.; Mikkelsen, T.S.; Schinkel, A.H.; Sparreboom, A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009, 86, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Aoki, N.; Kuwahara, S.; Hosojima, M.; Kaseda, R.; Goto, S.; Iida, T.; De, S.; Kabasawa, H.; Kaneko, R.; et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J. Am. Soc. Nephrol. 2017, 28, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Amsellem, S.; Gburek, J.; Hamard, G.; Nielsen, R.; Willnow, T.E.; Devuyst, O.; Nexo, E.; Verroust, P.J.; Christensen, E.I.; Kozyraki, R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J. Am. Soc. Nephrol. 2010, 21, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Nislow, C.; Christov, I.C.; Batuman, V.; Nagrani, P.P.; Barazandeh, M.; Upadhyay, R.; Giaever, G.; Allen, P.L.; Armbruster, M.; et al. Cell spinpods are a simple inexpensive suspension culture device to deliver fluid shear stress to renal proximal tubular cells. Sci. Rep. 2021, 11, 21296. [Google Scholar] [CrossRef]
- Park, K.M. Can tissue cilia lengths and urine cilia proteins be markers of kidney diseases? Chonnam Med. J. 2018, 54, 83–89. [Google Scholar] [CrossRef]
- Kim, S.; Tsiokas, L. Cilia and cell cycle re-entry: More than a coincidence. Cell Cycle 2011, 10, 2683–2690. [Google Scholar] [CrossRef]
- Yu, F.; Ran, J.; Zhou, J. Ciliopathies: Does HDAC6 represent a new therapeutic target? Trends Pharmacol. Sci. 2016, 37, 114–119. [Google Scholar] [CrossRef]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent aurora a activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The predictive role of the biomarker kidney molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef]
- Song, J.; Yu, J.; Prayogo, G.W.; Cao, W.; Wu, Y.; Jia, Z.; Zhang, A. Understanding kidney injury molecule 1: A novel immune factor in kidney pathophysiology. Am. J. Transl. Res. 2019, 11, 1219–1229. [Google Scholar]
- Pazour, G.J.; Dickert, B.L.; Vucica, Y.; Seeley, E.S.; Rosenbaum, J.L.; Witman, G.B.; Cole, D.G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000, 151, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; El-Jouni, W.; Kong, F.; Ramesh, J.; Kumar, R.S.; Shen, X.; Ren, J.; Devendra, S.; Dorschel, A.; Wu, M.; et al. Genetic reduction of cilium length by targeting intraflagellar transport 88 protein impedes kidney and liver cyst formation in mouse models of autosomal polycystic kidney disease. Kidney Int. 2020, 98, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, N.; Sandoval, R.M.; Molitoris, B.A.; Brakeman, P.; Roy, S.; Fissell, W.H. Application of physiological shear stress to renal tubular epithelial cells. Methods Cell Biol. 2019, 153, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Velkov, T.; Nation, R.L.; Forrest, A.; Tsuji, B.T.; Bergen, P.J.; Li, J. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: Are we there yet? Int. J. Antimicrob. Agents 2016, 48, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.D.; Azad, M.A.; Wang, J.; Horne, A.S.; Thompson, P.E.; Nation, R.L.; Velkov, T.; Li, J. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: Last-line antibiotics against multidrug-resistant gram-negative bacteria. ACS Infect. Dis. 2015, 1, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Vormann, M.K.; Vriend, J.; Lanz, H.L.; Gijzen, L.; van den Heuvel, A.; Hutter, S.; Joore, J.; Trietsch, S.J.; Stuut, C.; Nieskens, T.T.G.; et al. Implementation of a human renal proximal tubule on a chip for nephrotoxicity and drug interaction studies. J. Pharm. Sci. 2021, 110, 1601–1614. [Google Scholar] [CrossRef]



| Gene | Gene Name | Protein | Assay Number |
|---|---|---|---|
| Solute Carrier Family 22 Member 6 | SLC22A6 | Organic Anion Transporter 1 (OAT-1) | Hs00537914_m1 |
| Solute Carrier Family 22 Member 2 | SLC22A2 | Organic Cation Transporter (OCT-2) | Hs00533907_m1 |
| LDL Receptor Related Protein 2 | LRP2 | Megalin | Hs00189742_m1 |
| ATP Binding Cassette Subfamily C Member 2 | ABCC2 | Multidrug Resistance- Associated Protein 2 (MRP2) | Hs00166123_m1 |
| Aquaporin-1 | AQP1 | Aquaporin-1 | Hs01028916_m1 |
| Histone Deacetylase 6 | HDAC6 | Histone Deacetylase 6 | Hs00997427_m1 |
| Hepatitis A Virus Cellular Receptor 1 | HAVCR1 | Kidney Injury Molecule 1 (KIM-1) | Hs00273334_m1 |
| Solute Carrier Family 47 Member 1 | SLC47A1 | Multidrug And Toxin Extrusion Protein 1 (MATE 1) | Hs00217320_m1 |
| ATP Binding Cassette Subfamily B Member 1 | ABCB1 | P-Glycoprotein 1 (P-Gp) | Hs00184500_m1 |
| Cubilin | CUBN | Cubilin | Hs00153607_m1 |
| Glyceraldehyde-3-Phosphate Dehydrogenase | GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | Hs02786624_g1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Specioso, G.; Bovard, D.; Zanetti, F.; Maranzano, F.; Merg, C.; Sandoz, A.; Titz, B.; Dalcanale, F.; Hoeng, J.; Renggli, K.; et al. Apical Medium Flow Influences the Morphology and Physiology of Human Proximal Tubular Cells in a Microphysiological System. Bioengineering 2022, 9, 516. https://doi.org/10.3390/bioengineering9100516
Specioso G, Bovard D, Zanetti F, Maranzano F, Merg C, Sandoz A, Titz B, Dalcanale F, Hoeng J, Renggli K, et al. Apical Medium Flow Influences the Morphology and Physiology of Human Proximal Tubular Cells in a Microphysiological System. Bioengineering. 2022; 9(10):516. https://doi.org/10.3390/bioengineering9100516
Chicago/Turabian StyleSpecioso, Gabriele, David Bovard, Filippo Zanetti, Fabio Maranzano, Céline Merg, Antonin Sandoz, Bjoern Titz, Federico Dalcanale, Julia Hoeng, Kasper Renggli, and et al. 2022. "Apical Medium Flow Influences the Morphology and Physiology of Human Proximal Tubular Cells in a Microphysiological System" Bioengineering 9, no. 10: 516. https://doi.org/10.3390/bioengineering9100516
APA StyleSpecioso, G., Bovard, D., Zanetti, F., Maranzano, F., Merg, C., Sandoz, A., Titz, B., Dalcanale, F., Hoeng, J., Renggli, K., & Suter-Dick, L. (2022). Apical Medium Flow Influences the Morphology and Physiology of Human Proximal Tubular Cells in a Microphysiological System. Bioengineering, 9(10), 516. https://doi.org/10.3390/bioengineering9100516







