Functionalizing Fibrin Hydrogels with Thermally Responsive Oligonucleotide Tethers for On-Demand Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymatic Incorporation of Peptides into Fibrin Hydrogels
2.1.1. Fabrication of Fibrin Hydrogels
2.1.2. Comparing FXIII Substrate Peptide Incorporation
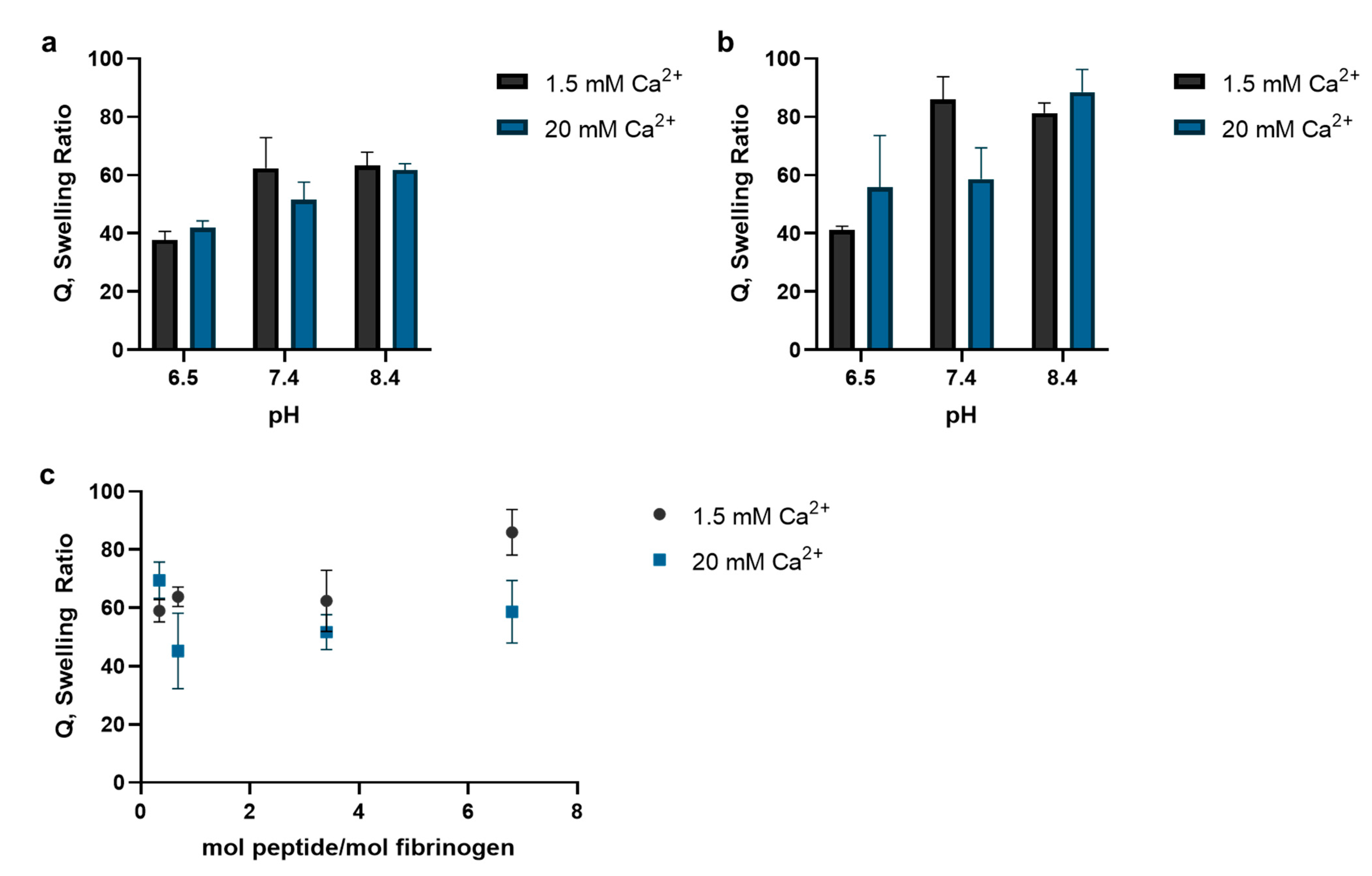
2.1.3. Polymerization Reaction Conditions’ Effect on Peptide Incorporation
2.2. Thermally Triggered Release of Complement Oligonucleotide
2.2.1. Annealing Oligonucleotides
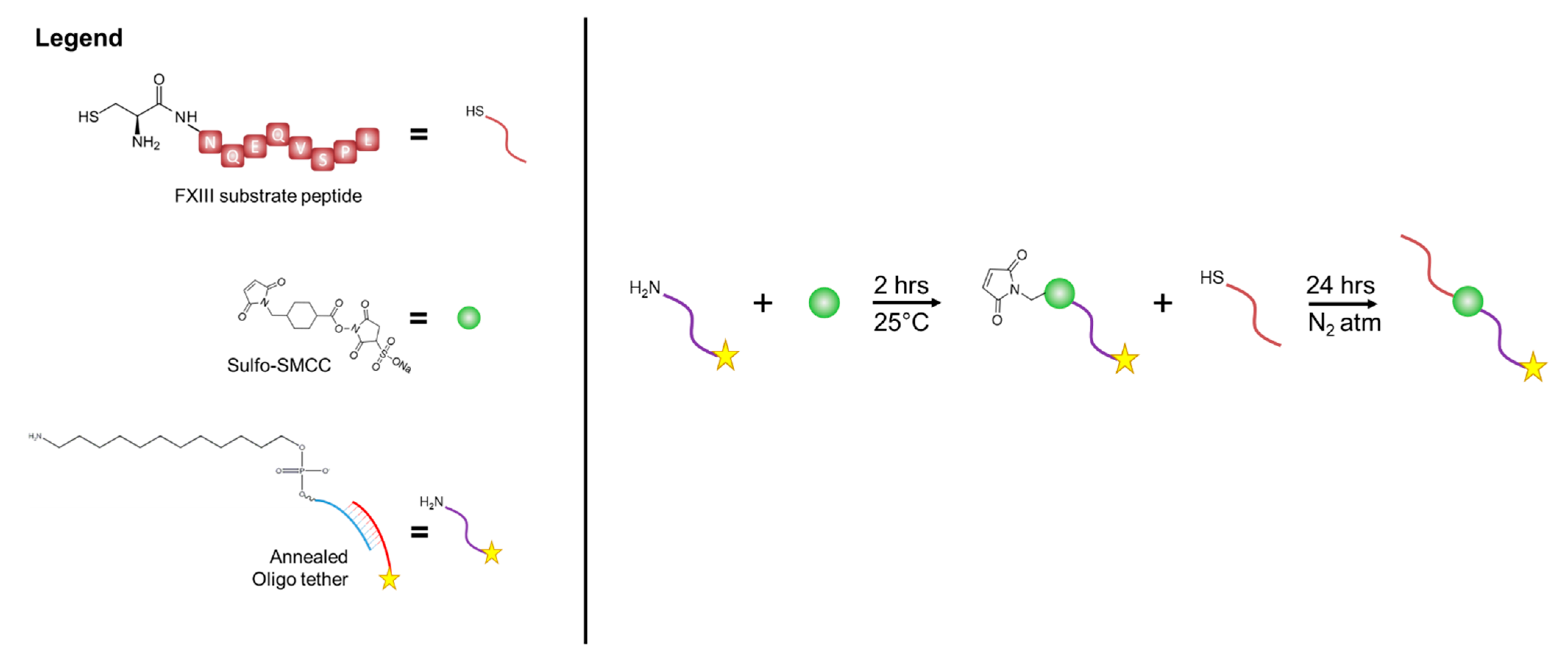
2.2.2. Functionalization of Substrate
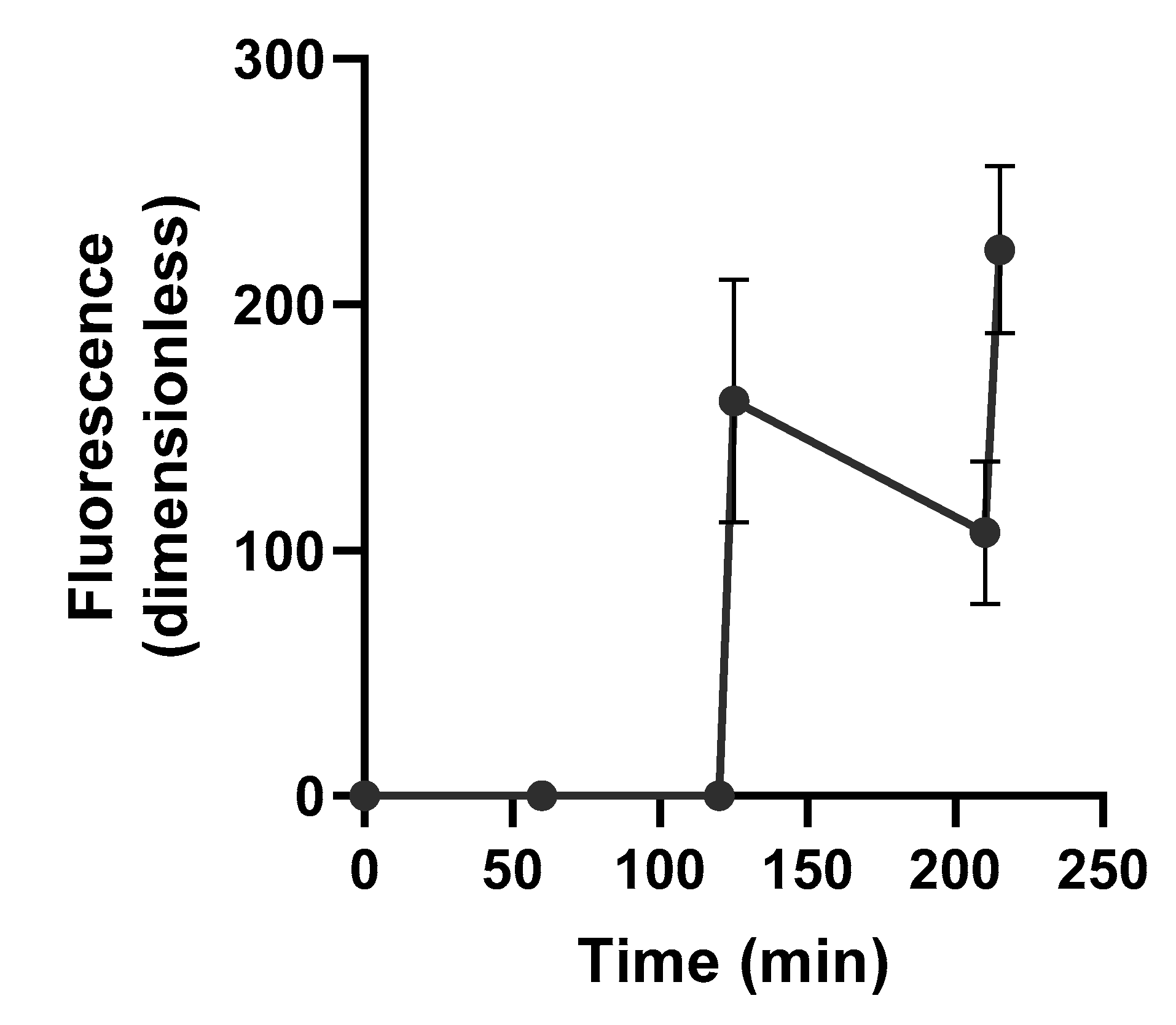
2.2.3. Triggered Release of Fluorescently Labeled Complement Oligonucleotide
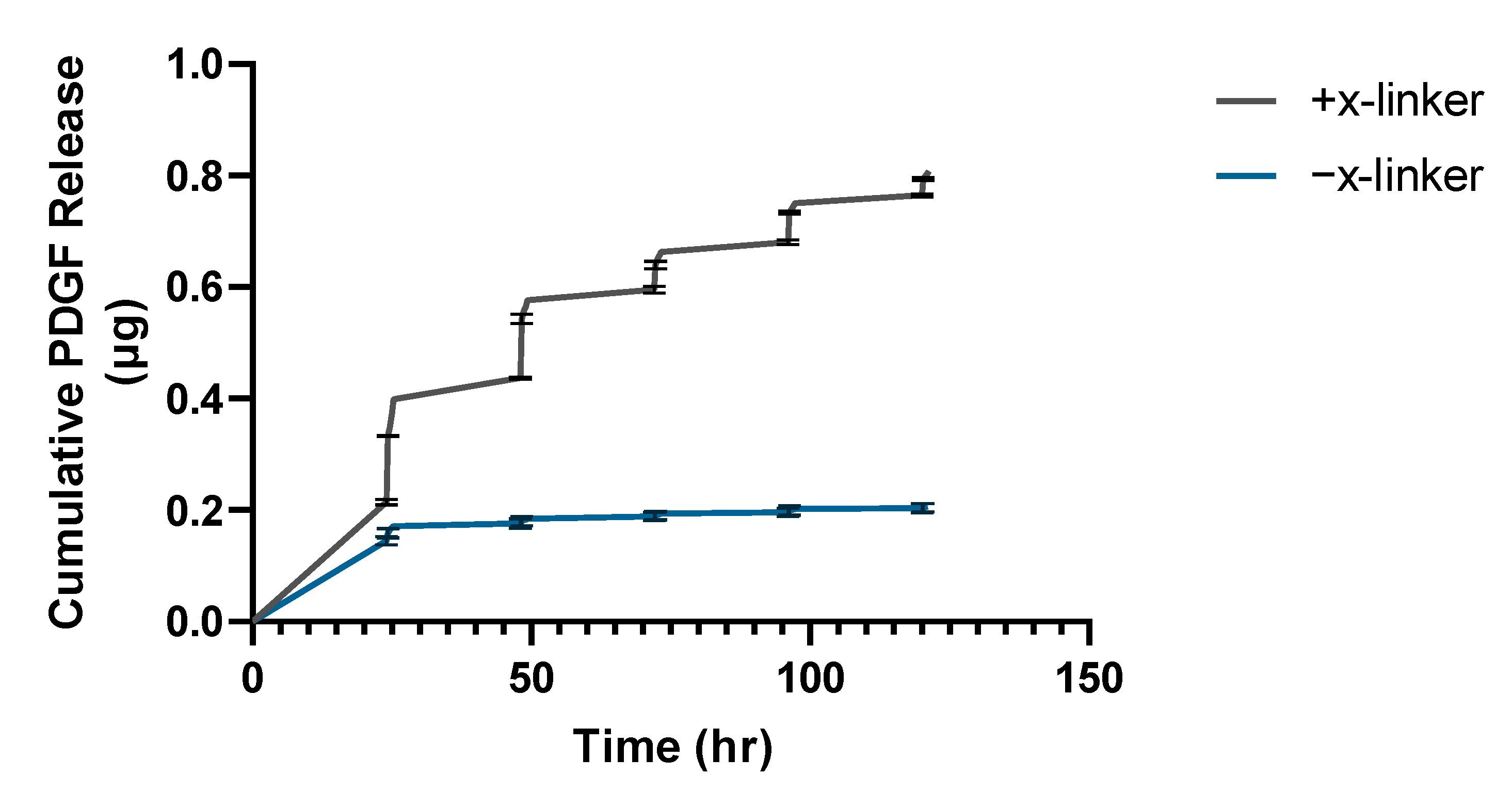
2.2.4. Triggered Release of Growth Factor-Oligonucleotide Conjugate
2.3. Thermally Triggered Release of Complement Oligonucleotide
2.3.1. Oligonucleotide-Peptide Conjugate Synthesis
2.3.2. Enzymatic Incorporation of Oligonucleotide-Peptide Conjugate into Fibrin Hydrogels
2.3.3. Thermally Triggered Release of Complement Oligonucleotide Strands from Fibrin Hydrogels
2.4. Statistical Analysis
3. Results
3.1. FXIII Substrate Peptide Incorporation into Fibrin Hydrogels
3.2. Thermally Triggered Release of Complement Oligonucleotide
3.3. Thermally Triggered Release from Fibrin Hydrogels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, I.S.; Ginsburg, G.S. Personalized medicine: Progress and promise. Annu. Rev. Genomics Hum. Genet. 2011, 12, 217–244. [Google Scholar] [CrossRef]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018, 10, eaam8645. [Google Scholar] [CrossRef] [PubMed]
- Goversen, B.; van der Heyden, M.A.G.; van Veen, T.A.B.; de Boer, T.P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on IK1. Pharmacol. Ther. 2018, 183, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Azimi, T.; Loizidou, M.; Dwek, M.V. Cancer cells grown in 3D under fluid flow exhibit an aggressive phenotype and reduced responsiveness to the anti-cancer treatment doxorubicin. Sci. Rep. 2020, 10, 12020. [Google Scholar] [CrossRef]
- Garnett, M.J.; Edelman, E.J.; Heidorn, S.J.; Greenman, C.D.; Dastur, A.; Lau, K.W.; Greninger, P.; Thompson, I.R.; Luo, X.; Soares, J.; et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012, 483, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesti, F.; Abbott, G.W.; Wei, J.; Murray, K.T.; Saksena, S.; Schwartz, P.J.; Priori, S.G.; Roden, D.M.; George, A.L., Jr.; Goldstein, S.A. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc. Natl. Acad. Sci. USA 2000, 97, 10613–10618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petralia, F.; Tignor, N.; Reva, B.; Koptyra, M.; Chowdhury, S.; Rykunov, D.; Krek, A.; Ma, W.; Zhu, Y.; Ji, J.; et al. Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell 2020, 183, 1962–1985.e31. [Google Scholar] [CrossRef] [PubMed]
- Crystal, A.S.; Shaw, A.T.; Sequist, L.V.; Friboulet, L.; Niederst, M.J.; Lockerman, E.L.; Frias, R.L.; Gainor, J.F.; Amzallag, A.; Greninger, P.; et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014, 346, 1480–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrinpar, A.; Lee, D.K.; Silva, A.; Datta, N.; Kee, T.; Eriksen, C.; Weigle, K.; Agopian, V.; Kaldas, F.; Farmer, D.; et al. Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Sci. Transl. Med. 2016, 8, 333ra49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, G.J.; Saul, J.M.; Furth, M.E.; Andersson, K.E. The pharmacology of regenerative medicine. Pharmacol. Rev. 2013, 65, 1091–1133. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef] [PubMed]
- Mirvakili, S.M.; Langer, R. Wireless on-demand drug delivery. Nat. Electron. 2021, 4, 464–477. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Tavakol, D.N.; Fleischer, S.; Falcucci, T.; Graney, P.L.; Halligan, S.P.; Kaplan, D.L.; Vunjak-Novakovic, G. Emerging Trajectories for Next Generation Tissue Engineers. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1064, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Mogford, J.E.; Tawil, B.; Jia, S.; Mustoe, T.A. Fibrin sealant combined with fibroblasts and platelet-derived growth factor enhance wound healing in excisional wounds. Wound Repair Regen. 2009, 17, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Schense, J.C.; Bloch, J.; Aebischer, P.; Hubbell, J.A. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat. Biotechnol. 2000, 18, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, M.; Metters, A.; Zammaretti, P.; Hubbell, J.A.; Zisch, A.H. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J. Control. Release 2005, 101, 93–109. [Google Scholar] [CrossRef]
- Zisch, A.H.; Schenk, U.; Schense, J.C.; Sakiyama-Elbert, S.E.; Hubbell, J.A. Covalently conjugated VEGF-fibrin matrices for endothelialization. J. Control. Release 2001, 72, 101–113. [Google Scholar] [CrossRef]
- Ehrbar, M.; Djonov, V.G.; Schnell, C.; Tschanz, S.A.; Martiny-Baron, G.; Schenk, U.; Wood, J.; Burri, P.H.; Hubbell, J.A.; Zisch, A.H. Cell-demanded liberation of VEGF(121) from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 2004, 94, 1124–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrbar, M.; Rizzi, S.C.; Hlushchuk, R.; Djonov, V.; Zisch, A.H.; Hubbell, J.A.; Weber, F.E.; Lutolf, M.P. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials 2007, 28, 3856–3866. [Google Scholar] [CrossRef]
- Vallmajo-Martin, Q.; Broguiere, N.; Millan, C.; Zenobi-Wong, M.; Ehrbar, M. PEG/HA Hybrid Hydrogels for Biologically and Mechanically Tailorable Bone Marrow Organoids. Adv. Funct. Mater. 2020, 30, 1910282. [Google Scholar] [CrossRef]
- Broguiere, N.; Isenmann, L.; Zenobi-Wong, M. Novel enzymatically cross-linked hyaluronan hydrogels support the formation of 3D neuronal networks. Biomaterials 2016, 99, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef]
- Jin, R.; Wu, G.; Li, Z.; Mirkin, C.A.; Schatz, G.C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2003, 125, 1643–1654. [Google Scholar] [CrossRef]
- Derfus, A.M.; von Maltzahn, G.; Harris, T.J.; Duza, T.; Vecchio, K.S.; Ruoslahti, E.; Bhatia, S.N. Remotely triggered release from magnetic nanoparticles. Adv. Mater. 2007, 19, 3932–3936. [Google Scholar] [CrossRef]
- Ruiz-Hernandez, E.; Baeza, A.; Vallet-Regi, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef]
- Lee, K.; Kim, T.; Kim, Y.M.; Yang, K.; Choi, I.; Roh, Y.H. Multifunctional DNA Nanogels for Aptamer-Based Targeted Delivery and Stimuli-Triggered Release of Cancer Therapeutics. Macromol. Rapid Comm. 2021, 42, e2000457. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Nam, K.; Kim, Y.M.; Yang, K.; Roh, Y.H. DNA-Assisted Smart Nanocarriers: Progress, Challenges, and Opportunities. ACS Nano 2021, 15, 1942–1951. [Google Scholar] [CrossRef]
- Yamashita, S.; Fukushima, H.; Akiyama, Y.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. Controlled-release system of single-stranded DNA triggered by the photothermal effect of gold nanorods and its in vivo application. Bioorg. Med. Chem. 2011, 19, 2130–2135. [Google Scholar] [CrossRef]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mandieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Inter. 2016, 8, 21107–21133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolajsen, C.L.; Dyrlund, T.F.; Poulsen, E.T.; Enghild, J.J.; Scavenius, C. Coagulation factor XIIIa substrates in human plasma: Identification and incorporation into the clot. J. Biol. Chem. 2014, 289, 6526–6534. [Google Scholar] [CrossRef] [Green Version]
- Buchta, C.; Hedrich, H.C.; Macher, M.; Hocker, P.; Redl, H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal (R) and Vivostat (R) in comparison to the homologous fibrin sealant product Tissucol/Tisseel (R). Biomaterials 2005, 26, 6233–6241. [Google Scholar] [CrossRef]
- Corbett, S.A.; Lee, L.; Wilson, C.L.; Schwarzbauer, J.E. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J. Biol. Chem. 1997, 272, 24999–25005. [Google Scholar] [CrossRef] [Green Version]
- Podor, T.J.; Campbell, S.; Chindemi, P.; Foulon, D.M.; Farrell, D.H.; Walton, P.D.; Weitz, J.I.; Peterson, C.B. Incorporation of vitronectin into fibrin clots. Evidence for a binding interaction between vitronectin and gamma A/gamma’ fibrinogen. J. Biol. Chem. 2002, 277, 7520–7528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, J.; Aoyama, T.; Ueki, S.; Ohba, H.; Saito, Y.; Lorand, L. Identification of factor-XIIIa-reactive glutaminyl residues in the propolypeptide of bovine von Willebrand factor. Eur. J. Biochem. 1995, 232, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, K.F.; Rodriguez, A.L.; Parish, C.L.; Williams, R.J.; Nisbet, D.R. Temporally controlled release of multiple growth factors from a self-assembling peptide hydrogel. Nanotechnology 2016, 27, 385102. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, K.H.; Lee, J.Y.; Ku, Y.; Lee, S.J.; Min, B.M.; Chung, C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnol. Appl. Biochem. 2006, 43, 17–24. [Google Scholar] [CrossRef]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Lee, C.S.; Chun, J.G.; Mckee, P.A. Why alpha(2)-antiplasmin must be converted to a derivative form for optimal function. J. Thromb. Haemost. 2007, 5, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Penzes, K.; Kover, K.E.; Fazakas, F.; Haramura, G.; Muszbek, L. Molecular mechanism of the interaction between activated factor XIII and its glutamine donor peptide substrate. J. Thromb. Haemost. 2009, 7, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komaromi, I.; Katona, E. Factor XIII: A coagulation factor with multiple plasmatic and cellular functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamboa-Martinez, T.C.; Luque-Guillen, V.; Gonzalez-Garcia, C.; Ribelles, J.L.G.; Ferrer, G.G. Crosslinked fibrin gels for tissue engineering: Two approaches to improve their properties. J. Biomed. Mater. Res. Part A 2015, 103, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci Rep. 2019, 9, 11324. [Google Scholar] [CrossRef] [PubMed]
- Sanstead, P.J.; Stevenson, P.; Tokmakoff, A. Sequence-Dependent Mechanism of DNA Oligonucleotide Dehybridization Resolved through Infrared Spectroscopy. J. Am. Chem. Soc. 2016, 138, 11792–11801. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Fiche, J.B.; Buhot, A.; Calemczuk, R.; Livache, T. Salt concentration effects on equilibrium melting curves from DNA microarrays. Biophys. J. 2010, 99, 1886–1895. [Google Scholar] [CrossRef] [Green Version]
- Kibbe, W.A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35, W43–W46. [Google Scholar] [CrossRef]
- Andersen, T.; Strand, B.L.; Formo, K.; Alsberg, E.; Christensen, B.E. Alginates as biomaterials in tissue engineering. Carbohydr. Chem. Chem. Biol. Approaches 2012, 37, 227–258. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]


| Peptide 3 | CaCl2 Conc. (mM) | Incorporation (%) 1 | |
|---|---|---|---|
| +FXIII 2 | −FXIII | ||
| NQEQ | 1.5 | 26.3% (±1.1%) | 0.3% (±0%) |
| 20 | 36.9% (±1.8%) | 0.4% (±0%) | |
| RQAQQ | 1.5 | 0.5% (±0.1%) | 0.1% (±0%) |
| 20 | 0.8% (±0.1%) | 0.1% (±0%) | |
| NPEQ | 1.5 | 1.2% (±0%) | 0.1% (±0%) |
| 20 | 1.6% (±0.2%) | 0.1% (±0%) | |
| TCQS | 1.5 | 4.0% (±0.1%) | 3.5% (±0.1%) |
| 20 | 4.6% (±0.1%) | 4.3% (±0.1%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linsley, C.S.; Sung, K.; White, C.; Abecunas, C.A.; Tawil, B.J.; Wu, B.M. Functionalizing Fibrin Hydrogels with Thermally Responsive Oligonucleotide Tethers for On-Demand Delivery. Bioengineering 2022, 9, 25. https://doi.org/10.3390/bioengineering9010025
Linsley CS, Sung K, White C, Abecunas CA, Tawil BJ, Wu BM. Functionalizing Fibrin Hydrogels with Thermally Responsive Oligonucleotide Tethers for On-Demand Delivery. Bioengineering. 2022; 9(1):25. https://doi.org/10.3390/bioengineering9010025
Chicago/Turabian StyleLinsley, Chase S., Kevin Sung, Cameron White, Cara A. Abecunas, Bill J. Tawil, and Benjamin M. Wu. 2022. "Functionalizing Fibrin Hydrogels with Thermally Responsive Oligonucleotide Tethers for On-Demand Delivery" Bioengineering 9, no. 1: 25. https://doi.org/10.3390/bioengineering9010025
APA StyleLinsley, C. S., Sung, K., White, C., Abecunas, C. A., Tawil, B. J., & Wu, B. M. (2022). Functionalizing Fibrin Hydrogels with Thermally Responsive Oligonucleotide Tethers for On-Demand Delivery. Bioengineering, 9(1), 25. https://doi.org/10.3390/bioengineering9010025






