Unraveling the Mechanism of Platelet Aggregation Suppression by Monoterpenoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Synthesis of 2-((((1S,5R)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)thio)ethan-1-ol (4)
2.1.2. Synthesis of 2-((((1S,5R)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)amino)ethan-1-ol (5)
2.2. Platelet Aggregation Assays
2.3. Molecular Docking
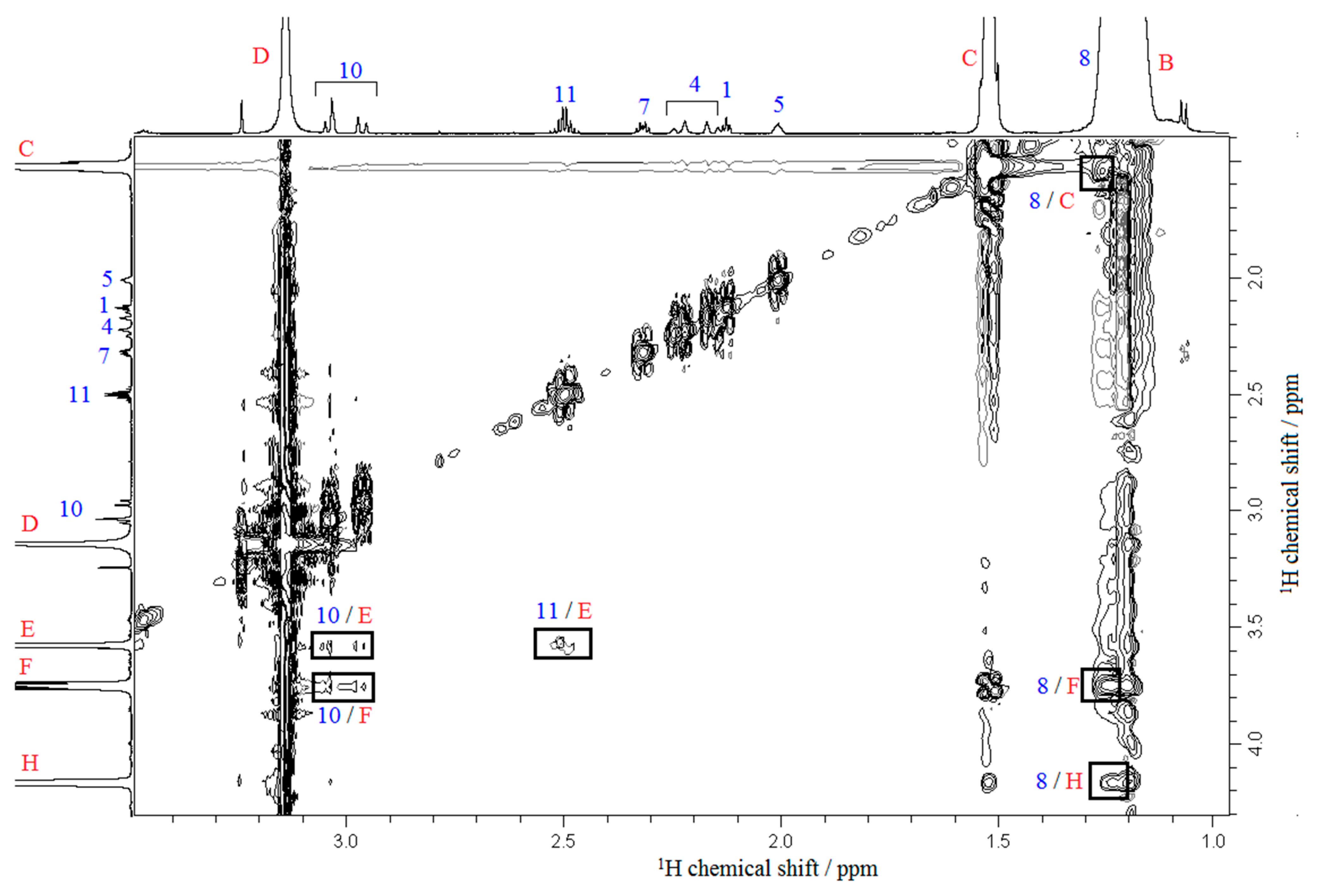
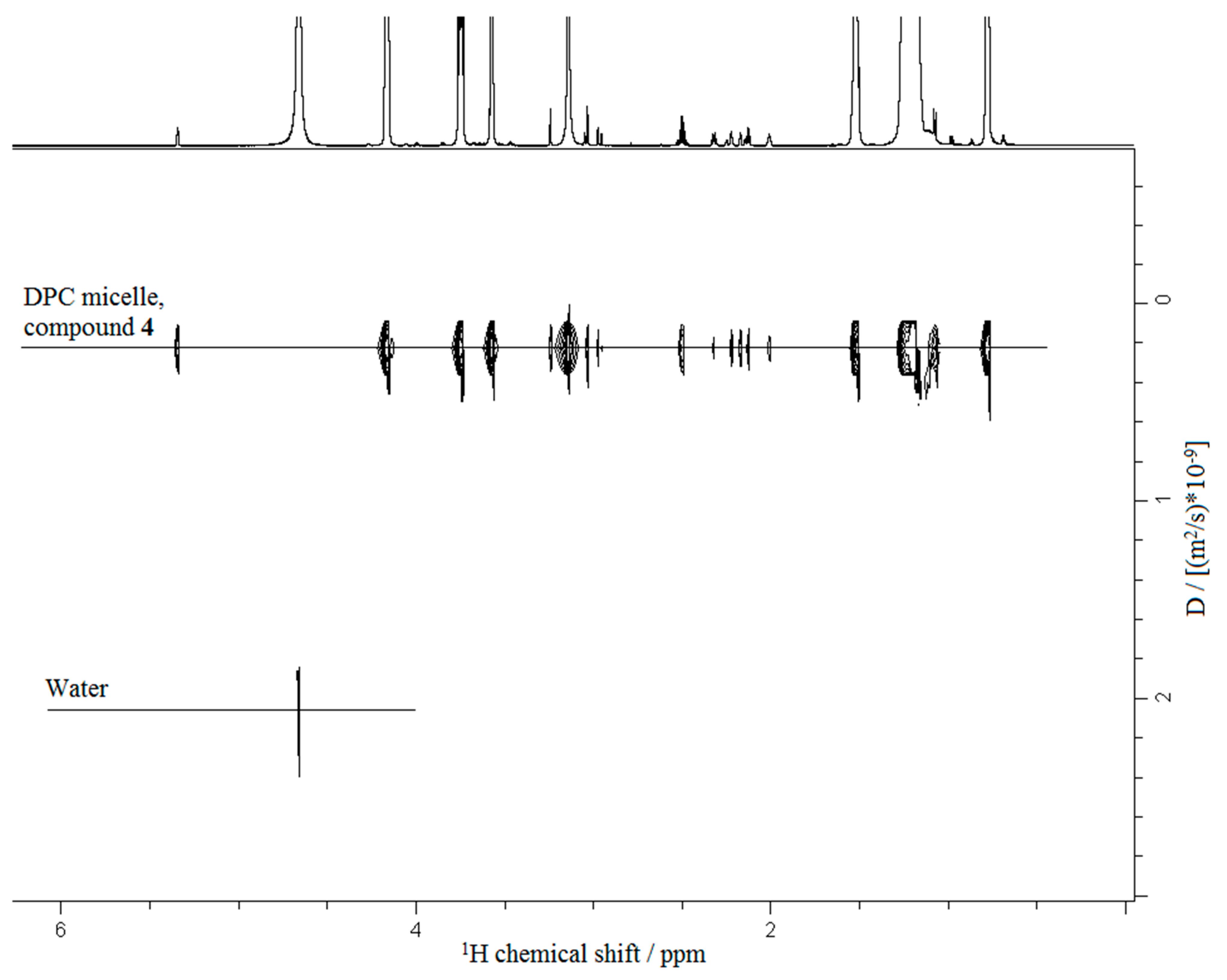
2.4. NMR Experiments
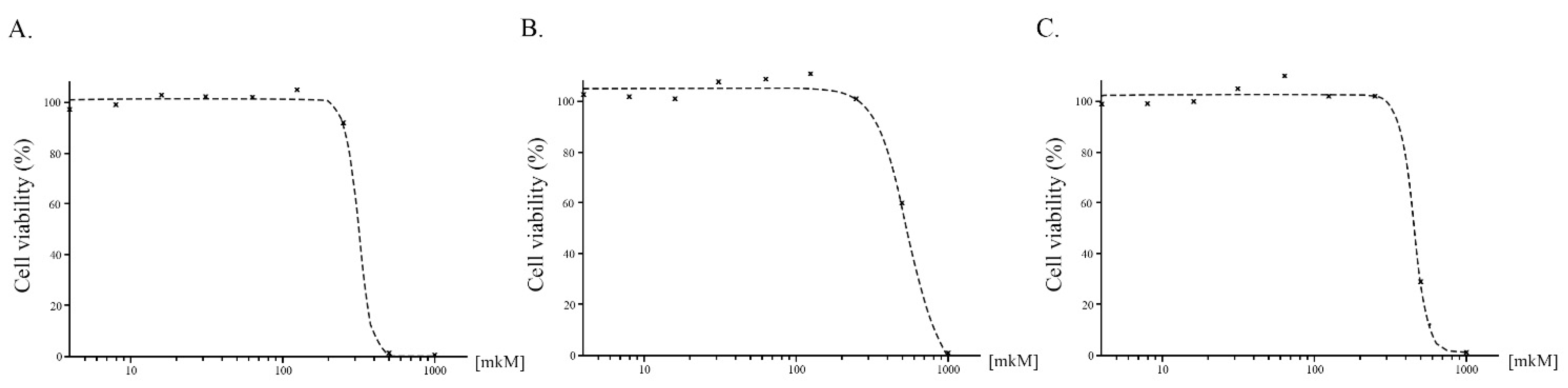
2.5. Cells Viability MTS Assay
2.6. Statistic Analysis
3. Results
3.1. Synthesis of Monoterpenoids and Their Effect on Platelet aggregation
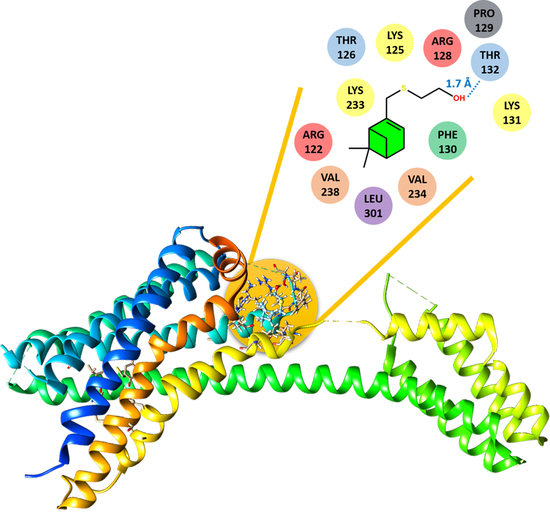
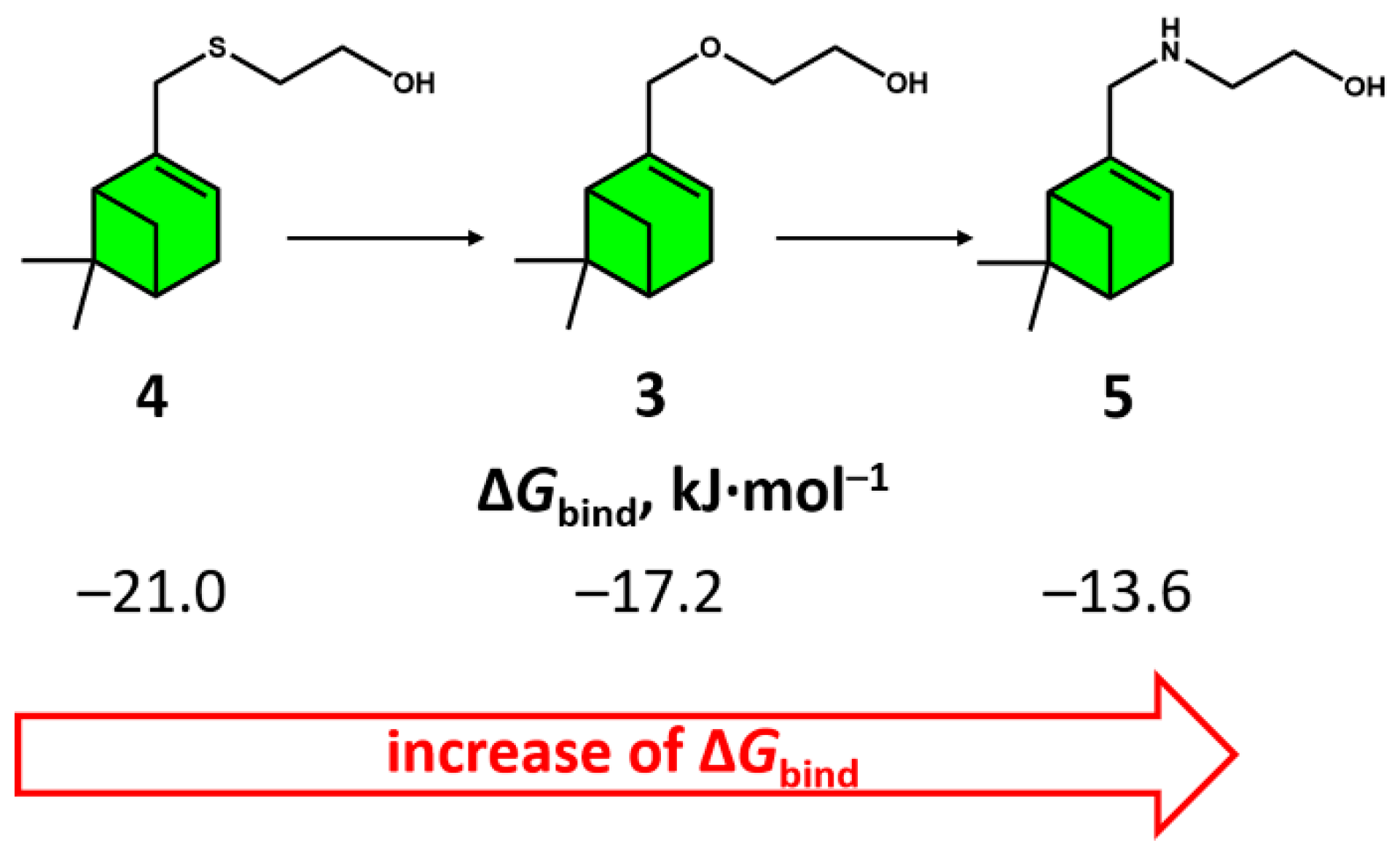
3.2. Molecular Docking
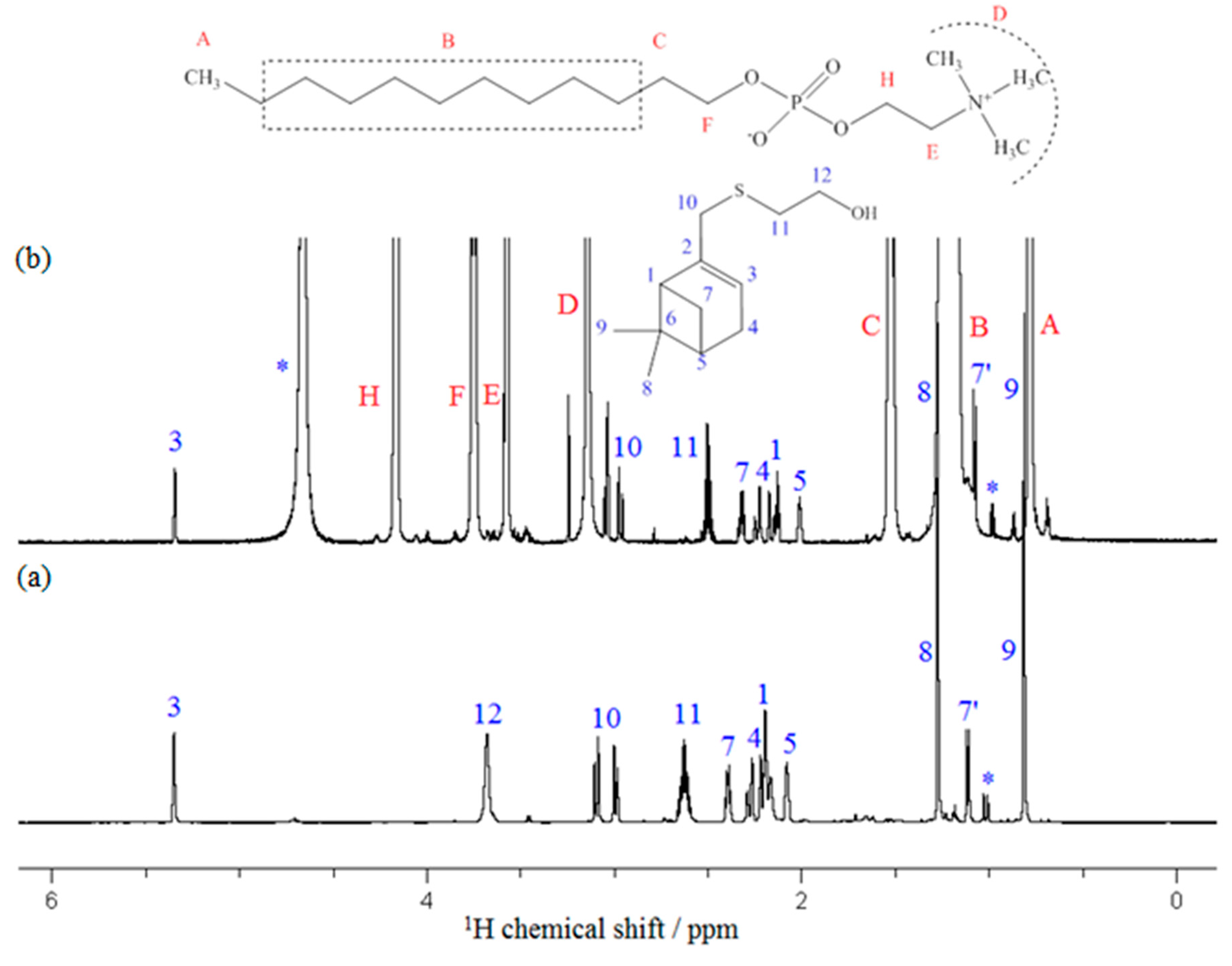
3.3. NMR Experiments
3.4. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders—A systematic review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiasakul, T.; De Jesus, E.; Tong, J.; Chen, Y.; Crowther, M.; Garcia, D.; Chai-Adisaksopha, C.; Messé, S.R.; Cuker, A. Inherited thrombophilia and the risk of arterial ischemic stroke: A systematic review and meta-analysis. J. Am. Heart Assoc. 2019, 8, e012877. [Google Scholar] [CrossRef]
- Jakobsen, C.; Larsen, J.B.; Fuglsang, J.; Hvas, A.-M. Platelet function in preeclampsia–A systematic review and meta-analysis. Platelets 2019, 30, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.A.; Anand, S.S.; Hankey, G.J.; Eikelboom, J.W. Aspirin resistance. Thromb. Res. 2007, 120, 337–346. [Google Scholar] [CrossRef]
- Sugunaraj, J.P.; Palaniswamy, C.; Selvaraj, D.R.; Arudra, S.K.C.; Sukhija, R. Clopidogrel resistance. Am. J. Ther. 2010, 17, 210–215. [Google Scholar] [CrossRef]
- Tomimatsu, T.; Mimura, K.; Matsuzaki, S.; Endo, M.; Kumasawa, K.; Kimura, T. Preeclampsia: Maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int. J. Mol. Sci. 2019, 20, 4246. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, C.; Palomo, I.; Fuentes, E. Mechanisms of endothelial dysfunction during aging: Predisposition to thrombosis. Mech. Ageing Dev. 2017, 164, 91–99. [Google Scholar] [CrossRef]
- Farzaneh, M.; Ahmadzadeh, M.; Hadian, J.; Tehrani, A.S. Chemical composition and antifungal activity of the essential oils of three species of Artemisia on some soil-borne phytopathogens. Commun. Agric. Appl. Biol. Sci. 2006, 71, 1327–1333. [Google Scholar]
- Nikitina, L.E.; Startseva, V.A.; Dorofeeva, L.Y.; Artemova, N.P.; Kuznetsov, I.V.; Lisovskaya, S.A.; Glushko, N.P. Antifungal activity of bicyclic monoterpenoids and terpenesulfides. Chem. Nat. Compd. 2010, 46, 28–32. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Startseva, V.A.; Artemova, N.P.; Dorofeeva, L.Y.; Kuznetsov, I.V.; Lisovskaya, S.A.; Glushko, N.P.; Kutyreva, M.P. Synthesis and antifungal activity of monoterpenoids of the carane series. Pharm. Chem. J. 2012, 45, 664–667. [Google Scholar] [CrossRef]
- Ishmuratov, G.Y.; Yakovleva, M.P.; Tukhvatshin, V.S.; Talipov, R.F.; Nikitina, L.E.; Artemova, N.P.; Startseva, V.A.; Tolstikov, A.G. Sulfur-containing derivatives of mono- and bicyclic natural monoterpenoids. Chem. Nat. Compd. 2014, 50, 22–47. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Pavelyev, R.S.; Startseva, V.A.; Kiselev, S.V.; Galiullina, L.F.; Aganova, O.V.; Timerova, A.F.; Boichuk, S.V.; Azizova, Z.R.; Klochkov, V. V Structural details on the interaction of biologically active sulfur-containing monoterpenoids with lipid membranes. J. Mol. Liq. 2020, 301, 112366. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Bannwarth, C.; Grimme, S. A simplified time-dependent density functional theory approach for electronic ultraviolet and circular dichroism spectra of very large molecules. Comput. Theor. Chem. 2014, 1040, 45–53. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrescu, I.; Lamotte-Brasseur, J.; Chessa, J.-P.; Ntarima, P.; Claeyssens, M.; Devreese, B.; Marino, G.; Gerday, C. Xylanase from the psychrophilic yeast Cryptococcus adeliae. Extremophiles 2000, 4, 137–144. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhang, J.; Gao, Z.-G.; Zhang, D.; Zhu, L.; Han, G.W.; Moss, S.M.; Paoletta, S.; Kiselev, E.; Lu, W. Structure of the human P2Y 12 receptor in complex with an antithrombotic drug. Nature 2014, 509, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, K.; Gao, Z.-G.; Paoletta, S.; Zhang, D.; Han, G.W.; Li, T.; Ma, L.; Zhang, W.; Müller, C.E. Agonist-bound structure of the human P2Y 12 receptor. Nature 2014, 509, 119–122. [Google Scholar] [CrossRef]
- de Richter, R.K.; Bonato, M.; Follet, M.; Kamenka, J.M. The (+)-and (−)-[2-(1, 3-dithianyl)] myrtanylborane. Solid and stable monoalkylboranes for asymmetric hydroboration. J. Org. Chem. 1990, 55, 2855–2860. [Google Scholar] [CrossRef]
- Liu, H.-X.; Tan, H.-B.; He, M.-T.; Li, L.; Wang, Y.-H.; Long, C.-L. Isolation and synthesis of two hydroxychavicol heterodimers from Piper nudibaccatum. Tetrahedron 2015, 71, 2369–2375. [Google Scholar] [CrossRef]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1) combining 7-hydroxycoumarin and monoterpenoid moieties. Bioorg. Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Baguley, P.; Walton, J. Reductive free-radical alkylations and cyclisations mediated by 1-alkylcyclohexa-2, 5-diene-1-carboxylic acids. J. Chem. Soc. Perkin Trans. 1 1998, 2073–2082. [Google Scholar] [CrossRef]
- Cattaneo, M. P2Y12 receptors: Structure and function. J. Thromb. Haemost. 2015, 13, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, S.V.; Nikitina, L.E.; Startseva, V.A.; Artemova, N.P.; Bodrov, A.V.; Boichuk, S.V.; Vorontsova, M.M.; Rakhmatullina, A.A.; Turaev, R.G.; Klochkov, V.V. Hemocoagulation Activity of Sulfur-Containing Pinane-Type Terpenoids. Pharm. Chem. J. 2017, 51, 343–347. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Kiselev, S.V.; Bodrov, A.V.; Startseva, V.A.; Artemova, N.P.; Klochkov, V.V.; Galiullina, L.F.; Aganova, O.V.; Khaliullina, A.V.; Lodochnikova, O.A.; et al. Development of Approaches to the Study of the Interaction of Biologically Active Thioterpenoids with Model Membranes. Bionanoscience 2017, 7, 600–607. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Kiselev, S.V.; Startseva, V.A.; Bodrov, A.V.; Azizova, Z.R.; Shipina, O.T.; Fedyunina, I.V.; Boichuk, S.V.; Lodochnikova, O.A.; Klochkov, V.V.; et al. Sulfur-containing monoterpenoids as potential antithrombotic drugs: Research in the molecular mechanism of coagulation activity using pinanyl sulfoxide as an example. Front. Pharmacol. 2018, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, A.S.; Rakhmatullin, I.Z.; Shurshalova, G.S.; Klochkov, A.V.; Il’yasov, K.A.; Klochkov, V.V. The Affect of Gadolinium Ion on Micelles and Reverse Micelles by NMR Spectroscopy. Bionanoscience 2021, 11, 136–141. [Google Scholar] [CrossRef]
- Irazazabal, L.N.; Porto, W.F.; Ribeiro, S.M.; Casale, S.; Humblot, V.; Ladram, A.; Franco, O.L. Selective amino acid substitution reduces cytotoxicity of the antimicrobial peptide mastoparan. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G.; Carboni, M.; Rinaldi, A.C.; Casu, M.; Scorciapino, M.A. Characterization of sodium dodecylsulphate and dodecylphosphocholine mixed micelles through NMR and dynamic light scattering. Magn. Reson. Chem. 2013, 51, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Mäler, L.; Gräslund, A. Artificial membrane models for the study of macromolecular delivery. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 480, pp. 129–139. [Google Scholar]
- Usachev, K.S.; Kolosova, O.A.; Klochkova, E.A.; Yulmetov, A.R.; Aganov, A.V.; Klochkov, V.V. Oligomerization of the antimicrobial peptide Protegrin-5 in a membrane-mimicking environment. Structural studies by high-resolution NMR spectroscopy. Eur. Biophys. J. 2017, 46, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Usachev, K.S.; Efimov, S.V.; Kolosova, O.A.; Klochkova, E.A.; Aganov, A.V.; Klochkov, V.V. Antimicrobial peptide protegrin-3 adopt an antiparallel dimer in the presence of DPC micelles: A high-resolution NMR study. J. Biomol. NMR 2015, 62, 71–79. [Google Scholar] [CrossRef] [PubMed]

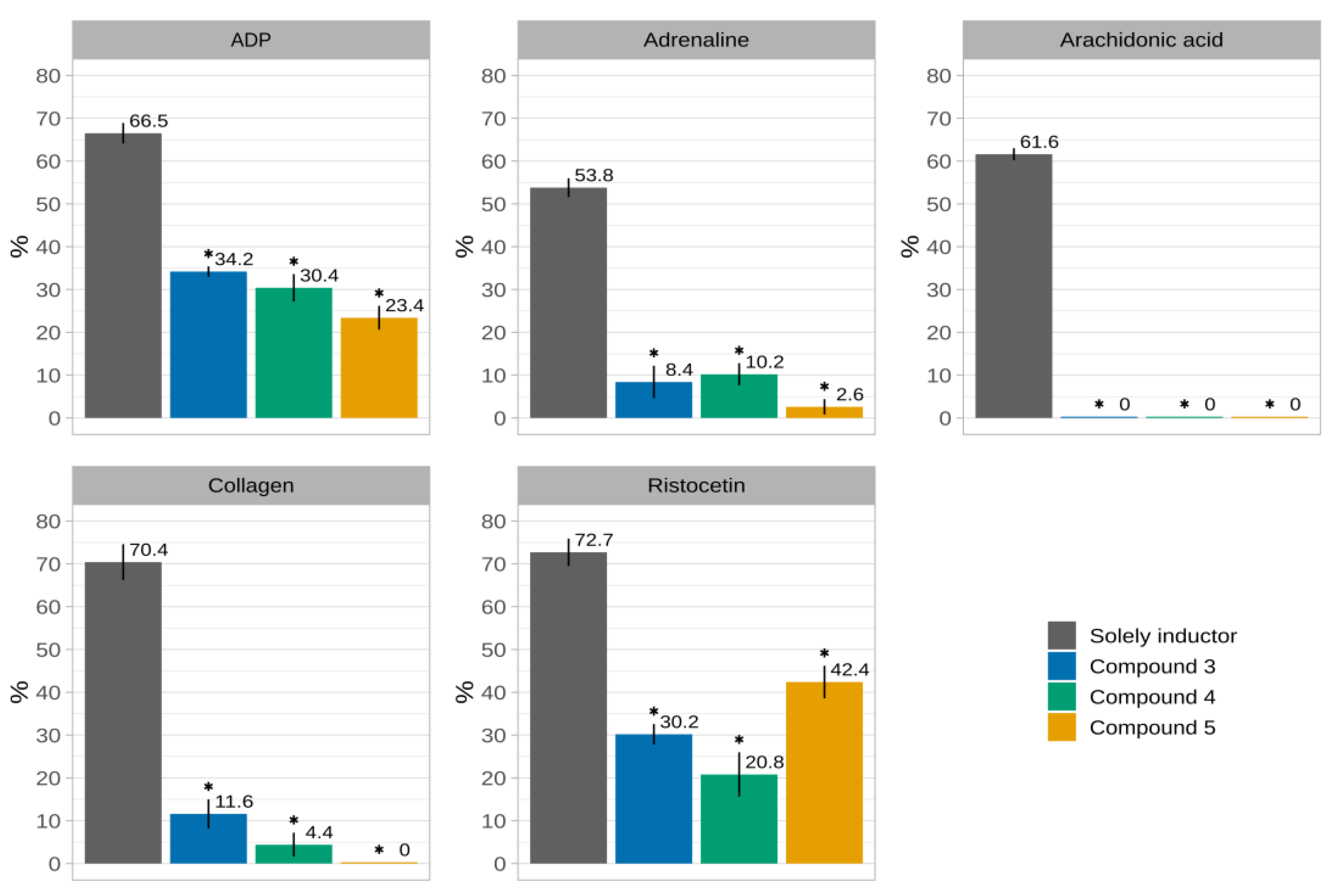



| Inductor | Solely Inductor | 3 | 4 | 5 |
|---|---|---|---|---|
| ADP | 66.5 ± 2.4 | 34.2 ± 1.2 * | 30.4 ± 3.2 * | 23.4 ± 2.8 * |
| adrenaline | 53.8 ± 2.2 | 8.4 ± 3.8 * | 10.2 ± 2.6 * | 2.6 ± 1.8 * |
| arachidonic acid | 61.6 ± 1.4 | 0 * | 0 * | 0 * |
| collagen | 70.4 ± 4.2 | 11.6 ± 3.4 * | 4.4 ± 2.8 * | 0 * |
| ristocetin | 72.7 ± 3.2 | 30.2 ± 2.4 * | 20.8 ± 5.2 * | 42.4 ± 3.8 * |
| Compound | Amino Acid Residues |
|---|---|
| 3 | ARG122, LYS125, THR126, ARG128, PRO129, PHE130, LYS131, THR132, LYS232, LYS233, VAL238 |
| 4 | ARG122, LYS125, THR126, ARG128, PRO129, PHE130, LYS131, THR132, LYS233, VAL234, VAL238, LEU301 |
| 5 | ASP1005, GLU1008, THR1009, ASP1012, ASN1013, VAL1016, LYS1032 |
| Compound | IC50 Value, µM |
|---|---|
| 3 | 548 ± 52.7 |
| 4 | 314 ± 46.5 |
| 5 | 450 ± 71.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitina, L.E.; Pavelyev, R.S.; Gilfanov, I.R.; Kiselev, S.V.; Azizova, Z.R.; Ksenofontov, A.A.; Bocharov, P.S.; Antina, E.V.; Klochkov, V.V.; Timerova, A.F.; et al. Unraveling the Mechanism of Platelet Aggregation Suppression by Monoterpenoids. Bioengineering 2022, 9, 24. https://doi.org/10.3390/bioengineering9010024
Nikitina LE, Pavelyev RS, Gilfanov IR, Kiselev SV, Azizova ZR, Ksenofontov AA, Bocharov PS, Antina EV, Klochkov VV, Timerova AF, et al. Unraveling the Mechanism of Platelet Aggregation Suppression by Monoterpenoids. Bioengineering. 2022; 9(1):24. https://doi.org/10.3390/bioengineering9010024
Chicago/Turabian StyleNikitina, Liliya E., Roman S. Pavelyev, Ilmir R. Gilfanov, Sergei V. Kiselev, Zulfiya R. Azizova, Alexander A. Ksenofontov, Pavel S. Bocharov, Elena V. Antina, Vladimir V. Klochkov, Ayzira F. Timerova, and et al. 2022. "Unraveling the Mechanism of Platelet Aggregation Suppression by Monoterpenoids" Bioengineering 9, no. 1: 24. https://doi.org/10.3390/bioengineering9010024
APA StyleNikitina, L. E., Pavelyev, R. S., Gilfanov, I. R., Kiselev, S. V., Azizova, Z. R., Ksenofontov, A. A., Bocharov, P. S., Antina, E. V., Klochkov, V. V., Timerova, A. F., Rakhmatullin, I. Z., Ostolopovskaya, O. V., Khelkhal, M. A., Boichuk, S. V., Galembikova, A. R., Andriutsa, N. S., Frolova, L. L., Kutchin, A. V., & Kayumov, A. R. (2022). Unraveling the Mechanism of Platelet Aggregation Suppression by Monoterpenoids. Bioengineering, 9(1), 24. https://doi.org/10.3390/bioengineering9010024