Biofabrication Strategies for Musculoskeletal Disorders: Evolution towards Clinical Applications
Abstract
1. Introduction
2. Biofabrication
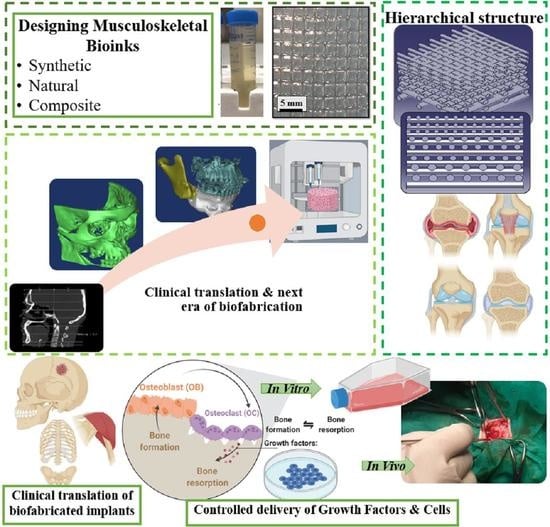
3. Designing Musculoskeletal Bioinks
3.1. Synthetic Materials
3.2. Natural Materials
3.3. Composite Materials
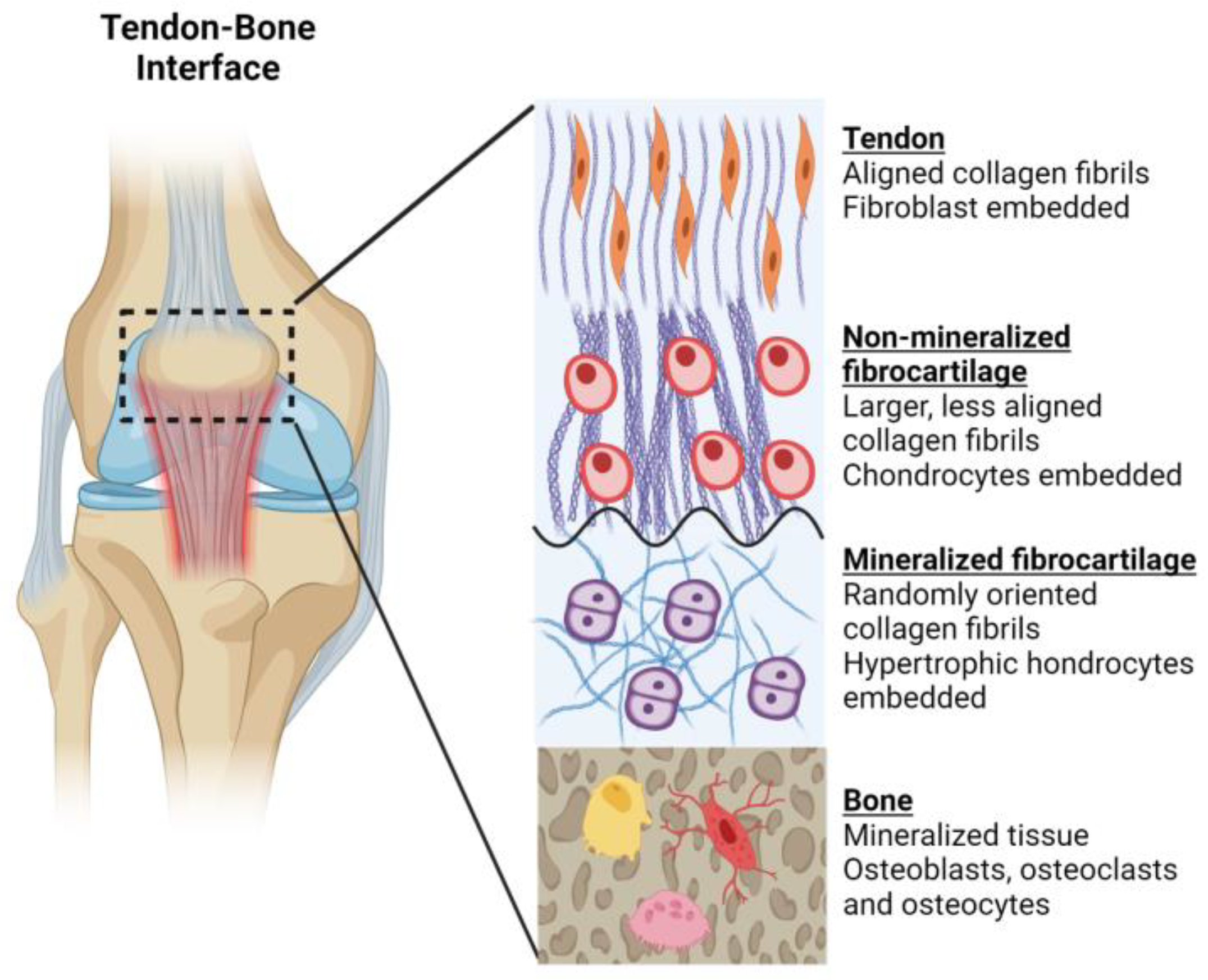
3.4. Functional Properties and Clinical Challenges of Musculoskeletal Bioinks
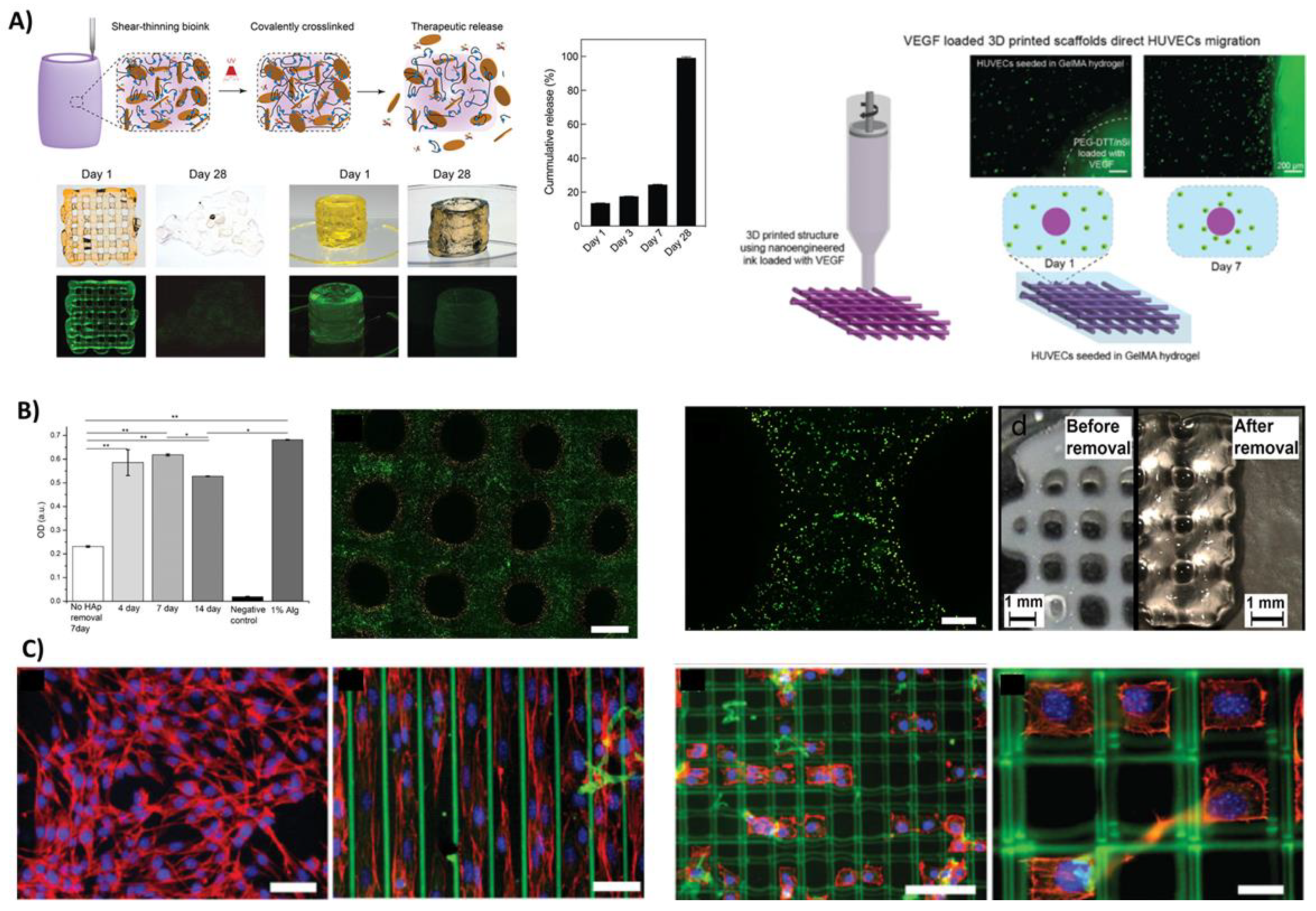
3.4.1. Low-Viscosity Bioinks
3.4.2. Controlled Delivery of Growth Factors and Cells
3.4.3. Hierarchical Structures
4. Challenges in Clinical Translation
4.1. Regulatory Classifications and Governing Bodies
4.2. Translational Pathways
4.2.1. Sterilisation
4.2.2. In Vitro Evaluation
4.2.3. In Vivo Evaluation
4.2.4. Premarket Evaluation
4.2.5. Clinical Trials
4.3. Other Considerations
5. Current Examples of Clinical Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Mus Culoskeletal Conditions. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 6 April 2021).
- Moraes, V.Y.; Lenza, M.; Tamaoki, M.J.; Faloppa, F.; Belloti, J.C. Platelet-Rich Therapies for Musculoskeletal Soft Tissue Injuries. Cochrane Database Syst. Rev. 2013, CD010071. [Google Scholar] [CrossRef]
- Collins, M.; Raleigh, S.M. Genetic Risk Factors for Musculoskeletal Soft Tissue Injuries. Med. Sport Sci. 2009, 54, 136–149. [Google Scholar] [CrossRef]
- Maffulli, N.; Del Buono, A.; Oliva, F.; Giai Via, A.; Frizziero, A.; Barazzuol, M.; Brancaccio, P.; Freschi, M.; Galletti, S.; Lisitano, G.; et al. Muscle Injuries: A Brief Guide to Classification and Management. Transl. Med. UniSa 2014, 12, 14–18. [Google Scholar] [PubMed]
- Chan, O.; Del Buono, A.; Best, T.M.; Maffulli, N. Acute Muscle Strain Injuries: A Proposed New Classification System. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Gilja, H.; Wang, L.; Oliveira, J.M.; Sun, X.; Tan, R.; Liu, C. Osteochondral Scaffolds for Early Treatment of Cartilage Defects in Osteoarthritic Joints: From Bench to Clinic. Biomater. Transl. 2020, 1, 3–17. [Google Scholar]
- Gomoll, A.H.; Filardo, G.; Almqvist, F.K.; Bugbee, W.D.; Jelic, M.; Monllau, J.C.; Puddu, G.; Rodkey, W.G.; Verdonk, P.; Verdonk, R.; et al. Surgical Treatment for Early Osteoarthritis. Part Ii: Allografts and Concurrent Procedures. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 468–486. [Google Scholar] [CrossRef]
- Melton, J.T.; Wilson, A.J.; Chapman-Sheath, P.; Cossey, A.J. Trufit Cb Bone Plug: Chondral Repair, Scaffold Design, Surgical Technique and Early Experiences. Expert Rev. Med. Devices 2010, 7, 333–341. [Google Scholar] [CrossRef]
- Angele, P.; Niemeyer, P.; Steinwachs, M.; Filardo, G.; Gomoll, A.H.; Kon, E.; Zellner, J.; Madry, H. Chondral and Osteochondral Operative Treatment in Early Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1743–1752. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Zhang, W.; Riches, P.; Li, B.; Shu, W. 3d Biofabrication for Soft Tissue and Cartilage Engineering. Med. Eng. Phys. 2020, 82, 13–39. [Google Scholar] [CrossRef]
- Dhammi, I.K.; Rehan Ul, H.; Kumar, S. Graft Choices for Anterior Cruciate Ligament Reconstruction. Indian J. Orthop. 2015, 49, 127–128. [Google Scholar] [CrossRef]
- Salzler, M.J.; Chang, J.; Richmond, J. Management of Anterior Cruciate Ligament Injuries in Adults Aged >40 Years. J. Am. Acad. Orthop. Surg. 2018, 26, 553–561. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for Neural Tissue Engineering Applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Mosher, C.Z.; Boushell, M.K.; Lu, H.H. Engineering Complex Orthopaedic Tissues Via Strategic Biomimicry. Ann. Biomed. Eng. 2015, 43, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.B. Ligament Structure, Physiology and Function. J. Musculoskelet. Neuronal Interact. 2004, 4, 199–201. [Google Scholar]
- Barajaa, M.A.; Nair, L.S.; Laurencin, C.T. Bioinspired Scaffold Designs for Regenerating Musculoskeletal Tissue Interfaces. Regen. Eng. Transl. Med. 2020, 6, 451–483. [Google Scholar] [CrossRef]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where Tendons and Ligaments Meet Bone: Attachment Sites (‘Entheses’) in Relation to Exercise and/or Mechanical Load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef]
- Rossetti, L.; Kuntz, L.A.; Kunold, E.; Schock, J.; Müller, K.W.; Grabmayr, H.; Stolberg-Stolberg, J.; Pfeiffer, F.; Sieber, S.A.; Burgkart, R.; et al. The Microstructure and Micromechanics of the Tendon–Bone Insertion. Nat. Mater. 2017, 16, 664–670. [Google Scholar] [CrossRef]
- Moffat, K.L.; Sun, W.-H.S.; Pena, P.E.; Chahine, N.O.; Doty, S.B.; Ateshian, G.A.; Hung, C.T.; Lu, H.H. Characterization of the Structure–Function Relationship at the Ligament-to-Bone Interface. Proc. Natl. Acad. Sci. USA 2008, 105, 7947–7952. [Google Scholar] [CrossRef] [PubMed]
- Pavlovich, M.J.; Hunsberger, J.; Atala, A. Biofabrication: A Secret Weapon to Advance Manufacturing, Economies, and Healthcare. Trends Biotechnol. 2016, 34, 679–680. [Google Scholar] [CrossRef]
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng. Part C Methods 2020, 26, 91–106. [Google Scholar] [CrossRef]
- Harley, W.S.; Li, C.C.; Toombs, J.; O’Connell, C.D.; Taylor, H.K.; Heath, D.E.; Collins, D.J. Advances in Biofabrication Techniques Towards Functional Bioprinted Heterogeneous Engineered Tissues: A Comprehensive Review. Bioprinting 2021, 23, e00147. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent Trends in Natural Polysaccharide Based Bioinks for Multiscale 3d Printing in Tissue Regeneration: A Review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.P.; Thorat, N.D.; Pricl, S.; Patil, R.M.; Rohiwal, S.; Townley, H. Bioink: A 3d-Bioprinting Tool for Anticancer Drug Discovery and Cancer Management. Drug Discov. Today 2021, 26, 1574–1590. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3d Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85. [Google Scholar] [CrossRef]
- Lindberg, G.C.J.; Lim, K.S.; Soliman, B.G.; Nguyen, A.; Hooper, G.J.; Narayan, R.J.; Woodfield, T.B.F. Biological Function Following Radical Photo-Polymerization of Biomedical Polymers and Surrounding Tissues: Design Considerations and Cellular Risk Factors. Appl. Phys. Rev. 2021, 8, 011301. [Google Scholar] [CrossRef]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photocrosslinking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef]
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, 1906423. [Google Scholar] [CrossRef]
- Woodfield, T.; Lim, K.; Morouço, P.; Levato, R.; Malda, J.; Melchels, F. Biofabrication in Tissue Engineering. In Comprehensive Biomaterials Ii; Elsevier: Oxford, UK, 2017; pp. 587–606. [Google Scholar]
- Popov, A.A.; Malferrari, S.; Kalaskar, D. 3d Bioprinting for Musculoskeletal Applications. J. 3D Print. Med. 2017, 1, 191–211. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3d Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Parr, W.C.H.; Burnard, J.L.; Wilson, P.J.; Mobbs, R.J. 3d Printed Anatomical (Bio)Models in Spine Surgery: Clinical Benefits and Value to Health Care Providers. J. Spine Surg. 2019, 5, 549–560. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.; Izadifar, M.; Chen, X. Dispensing-Based Bioprinting of Mechanically-Functional Hybrid Scaffolds with Vessel-Like Channels for Tissue Engineering Applications—A Brief Review. J. Mech. Behav. Biomed. Mater. 2018, 78, 298–314. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of Natural Hydrogels for Cardiac, Neural, and Bone Tissue Engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Soliman, B.G.; Lindberg, G.C.; Jungst, T.; Hooper, G.J.; Groll, J.; Woodfield, T.B.; Lim, K.S. Stepwise Control of Crosslinking in a One-Pot System for Bioprinting of Low-Density Bioinks. Adv. Healthc. Mater. 2020, 9, 1901544. [Google Scholar] [CrossRef]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-Thinning and Self-Healing Hydrogels as Injectable Therapeutics and for 3d-Printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3d Bioprinting of Hydrogels from Nonviscous Photo-Crosslinkable Inks. Adv. Mater. 2017, 29, 1604983. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Kumar, D.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Design and Characterisation of Multi-Functional Strontium-Gelatin Nanocomposite Bioinks with Improved Print Fidelity and Osteogenic Capacity. Bioprinting 2020, 18, e00073. [Google Scholar] [CrossRef]
- Leucht, A.; Volz, A.C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced Gelatin-Based Vascularization Bioinks for Extrusion-Based Bioprinting of Vascularized Bone Equivalents. Sci. Rep. 2020, 10, 5330. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed Microgel Inks for 3d Printing Applications. Adv. Sci. 2019, 6, 1801076. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Levato, R.; Costa, P.F.; Castilho, M.D.; Alcala-Orozco, C.R.; van Dorenmalen, K.M.A.; Melchels, F.P.W.; Gawlitta, D.; Hooper, G.J.; Malda, J.; et al. Bio-Resin for High Resolution Lithography-Based Biofabrication of Complex Cell-Laden Constructs. Biofabrication 2018, 10, 034101. [Google Scholar] [CrossRef]
- Petta, D.; Armiento, A.R.; Grijpma, D.; Alini, M.; Eglin, D.; D’Este, M. 3d Bioprinting of a Hyaluronan Bioink through Enzymatic-and Visible Light-Crosslinking. Biofabrication 2018, 10, 044104. [Google Scholar] [CrossRef]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3d Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3d Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404. [Google Scholar] [CrossRef]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3d Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Schon, B.S.; Hooper, G.J.; Woodfield, T.B. Modular Tissue Assembly Strategies for Biofabrication of Engineered Cartilage. Ann. Biomed. Eng. 2017, 45, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Duchi, S.; Onofrillo, C.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Quigley, A.F.; Kapsa, R.M.I.; Pivonka, P.; Wallace, G.; Di Bella, C.; et al. Handheld Co-Axial Bioprinting: Application to in Situ Surgical Cartilage Repair. Sci. Rep. 2017, 7, 5837. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a Clay Based Bioink for 3d Cell Printing for Skeletal Application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct Msc Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3d Bioprinting Is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the Printability of Hydrogels in 3d Bioprinting. Sci. Rep. 2016, 6, 29977. Available online: https://www.nature.com/articles/srep29977#supplementary-information (accessed on 15 August 2021). [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3d Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Kyburz, K.A.; Anseth, K.S. Synthetic Mimics of the Extracellular Matrix: How Simple Is Complex Enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3d Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3d Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Stichler, S.; Jungst, T.; Schamel, M.; Zilkowski, I.; Kuhlmann, M.; Böck, T.; Blunk, T.; Teßmar, J.; Groll, J. Thiol-Ene Clickable Poly(Glycidol) Hydrogels for Biofabrication. Ann. Biomed. Eng. 2017, 45, 273–285. [Google Scholar] [CrossRef]
- Peak, C.W.; Singh, K.A.; Adlouni, M.A.; Chen, J.; Gaharwar, A.K. Printing Therapeutic Proteins in 3d Using Nanoengineered Bioink to Control and Direct Cell Migration. Adv. Healthc. Mater. 2019, 8, 1801553. [Google Scholar] [CrossRef]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef]
- Hribar, K.C.; Soman, P.; Warner, J.; Chung, P.; Chen, S. Light-Assisted Direct-Write of 3d Functional Biomaterials. Lab A Chip 2014, 14, 268–275. [Google Scholar] [CrossRef]
- Wüst, S.; Müller, R.; Hofmann, S. 3d Bioprinting of Complex Channels—Effects of Material, Orientation, Geometry, and Cell Embedding. J. Biomed. Mater. Res. Part A 2015, 103, 2558–2570. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, G.; Gelinsky, M.; Huang, P.; Ruan, C. 3d Bioprinting Scaffold Using Alginate/Polyvinyl Alcohol Bioinks. Mater. Lett. 2017, 189, 295–298. [Google Scholar] [CrossRef]
- Atienza-Roca, P.; Kieser, D.C.; Cui, X.; Bathish, B.; Ramaswamy, Y.; Hooper, G.J.; Clarkson, A.N.; Rnjak-Kovacina, J.; Martens, P.J.; Wise, L.M.; et al. Visible Light Mediated Pva-Tyramine Hydrogels for Covalent Incorporation and Tailorable Release of Functional Growth Factors. Biomater. Sci. 2020, 8, 5005–5019. [Google Scholar] [CrossRef]
- Kim, H.; Hwangbo, H.; Koo, Y.; Kim, G. Fabrication of Mechanically Reinforced Gelatin/Hydroxyapatite Bio-Composite Scaffolds by Core/Shell Nozzle Printing for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 3401. [Google Scholar] [CrossRef]
- Kesti, M.; Müller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A Versatile Bioink for Three-Dimensional Printing of Cellular Scaffolds Based on Thermally and Photo-Triggered Tandem Gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, X.; Zhang, J.; Ju, J. Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A Photocurable Hybrid Chitosan/Acrylamide Bioink for Dlp Based 3d Bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Barry, R.A.; Shepherd, R.F.; Hanson, J.N.; Nuzzo, R.G.; Wiltzius, P.; Lewis, J.A. Direct-Write Assembly of 3d Hydrogel Scaffolds for Guided Cell Growth. Adv. Mater. 2009, 21, 2407–2410. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, X.; Rahman, S.E.; Su, S.; Wei, J.; Ning, F.; Hu, Z.; Martínez-Zaguilán, R.; Sennoune, S.R.; et al. 3d Printed Agar/ Calcium Alginate Hydrogels with High Shape Fidelity and Tailorable Mechanical Properties. Polymer 2021, 214, 123238. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Kang, H.W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S.; et al. A Hydrogel Bioink Toolkit for Mimicking Native Tissue Biochemical and Mechanical Properties in Bioprinted Tissue Constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3d Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multimaterial Bioink Method for 3d Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of Multi-Material Anatomically Shaped Tissue Constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture Healing: Mechanisms and Interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, O.; Guaita, M.; Trossarelli, L. Solution Properties of Poly(N-Isopropylacrylamide). Die Makromol. Chem. 1979, 180, 969–973. [Google Scholar] [CrossRef]
- Grimm, O.; Schacher, F.H. Dual Stimuli-Responsive P(Nipaam-Co-Spa) Copolymers: Synthesis and Response in Solution and in Films. Polymers 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Sanzari, I.; Buratti, E.; Huang, R.; Tusan, C.G.; Dinelli, F.; Evans, N.D.; Prodromakis, T.; Bertoldo, M. Poly(N-Isopropylacrylamide) Based Thin Microgel Films for Use in Cell Culture Applications. Sci. Rep. 2020, 10, 6126. [Google Scholar] [CrossRef]
- Schuurman, W.; Gawlitta, D.; Klein, T.J.; ten Hoope, W.; van Rijen, M.H.; Dhert, W.J.; van Weeren, P.R.; Malda, J. Zonal Chondrocyte Subpopulations Reacquire Zone-Specific Characteristics During in Vitro Redifferentiation. Am. J. Sports Med. 2009, 37 (Suppl. S1), 97–104. [Google Scholar] [CrossRef]
- Guilak, F.; Alexopoulos, L.G.; Upton, M.L.; Youn, I.; Choi, J.B.; Cao, L.; Setton, L.A.; Haider, M.A. The Pericellular Matrix as a Transducer of Biomechanical and Biochemical Signals in Articular Cartilage. Ann. N. Y. Acad. Sci. 2006, 1068, 498–512. [Google Scholar] [CrossRef]
- Yu, H.; Grynpas, M.; Kandel, R.A. Composition of Cartilagenous Tissue with Mineralized and Non-Mineralized Zones Formed in Vitro. Biomaterials 1997, 18, 1425–1431. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Shah, A.R.; Hernandez, R.J.; LeBaron, R.G. Basic Science of Articular Cartilage Repair. Clin. Sports Med. 2001, 20, 223–247. [Google Scholar] [CrossRef]
- Armitage, O.E.; Oyen, M.L. Hard-Soft Tissue Interface Engineering. Adv. Exp. Med. Biol. 2015, 881, 187–204. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Sathish, J.G.; Sethu, S.; Bielsky, M.C.; de Haan, L.; French, N.S.; Govindappa, K.; Green, J.; Griffiths, C.E.; Holgate, S.; Jones, D.; et al. Challenges and Approaches for the Development of Safer Immunomodulatory Biologics. Nat. Rev. Drug Discov. 2013, 12, 306–324. [Google Scholar] [CrossRef]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering Alginate as Bioink for Bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A Bioprintable Form of Chitosan Hydrogel for Bone Tissue Engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Oh, S.; Seok, J.M.; Park, S.A.; Lee, J.Y. Graphene Oxide/Alginate Composites as Novel Bioinks for Three-Dimensional Mesenchymal Stem Cell Printing and Bone Regeneration Applications. Nanoscale 2019, 11, 23275–23285. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pal, U. 3d Hydroxyapatite Scaffold for Bone Regeneration and Local Drug Delivery Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101131. [Google Scholar] [CrossRef]
- Rathan, S.; Dejob, L.; Schipani, R.; Haffner, B.; Möbius, M.E.; Kelly, D.J. Fiber Reinforced Cartilage Ecm Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1801501. [Google Scholar] [CrossRef]
- Aydin, L.; Kucuk, S.; Kenar, H. A Universal Self-Eroding Sacrificial Bioink That Enables Bioprinting at Room Temperature. Polym. Adv. Technol. 2020, 31, 1634–1647. [Google Scholar] [CrossRef]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet Bioprinting of 3d Silk Fibroin Cellular Constructs Using Sacrificial Alginate. ACS Biomater. Sci. Eng. 2017, 3, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3d Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.K.; Lin, Y.; Wojciechowski, J.P.; Lee-Reeves, C.; Hachim, D.; Zhou, K.; Burdick, J.A.; Stevens, M.M. Expanding and Optimizing 3d Bioprinting Capabilities Using Complementary Network Bioinks. Sci. Adv. 2020, 6, eabc5529. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Liu, J.; Yu, X.; Zhang, Y.; Wu, J.; Wan, Y. Sequential Delivery of Dual Growth Factors from Injectable Chitosan-Based Composite Hydrogels. Mar. Drugs 2019, 17, 365. [Google Scholar] [CrossRef]
- Comblain, F.; Rocasalbas, G.; Gauthier, S.; Henrotin, Y. Chitosan: A Promising Polymer for Cartilage Repair and Viscosupplementation. Bio-Med. Mater. Eng. 2017, 28, S209–S215. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Ahlfeld, T.; Lode, A.; Gelinsky, M. A Versatile Method for Combining Different Biopolymers in a Core/Shell Fashion by 3d Plotting to Achieve Mechanically Robust Constructs. Biofabrication 2016, 8, 045001. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Shin, S.R.; Park, Y.J.; Bae, H. Hydrogel Production Platform with Dynamic Movement Using Photo-Crosslinkable/Temperature Reversible Chitosan Polymer and Stereolithography 4d Printing Technology. Tissue Eng. Regen. Med. 2020, 17, 423–431. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Kwon, M.Y.; Burdick, J.A. 3d Bioprinting Via an In Situ Crosslinking Technique Towards Engineering Cartilage Tissue. Sci. Rep. 2019, 9, 19987. [Google Scholar] [CrossRef]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent Advances in Hydrogels for Cartilage Tissue Engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Shpichka, A.; Osipova, D.; Efremov, Y.; Bikmulina, P.; Kosheleva, N.; Lipina, M.; Bezrukov, E.A.; Sukhanov, R.B.; Solovieva, A.B.; Vosough, M.; et al. Fibrin-Based Bioinks: New Tricks from an Old Dog. Int. J. Bioprint. 2020, 6, 269. [Google Scholar] [CrossRef]
- Barthes, J.; Lagarrigue, P.; Riabov, V.; Lutzweiler, G.; Kirsch, J.; Muller, C.; Courtial, E.J.; Marquette, C.; Projetti, F.; Kzhyskowska, J.; et al. Biofunctionalization of 3d-Printed Silicone Implants with Immunomodulatory Hydrogels for Controlling the Innate Immune Response: An in Vivo Model of Tracheal Defect Repair. Biomaterials 2021, 268, 120549. [Google Scholar] [CrossRef]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk Fibroin/Gelatin Microcarriers as Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Klotz, B.J.; Lindberg, G.C.J.; Melchels, F.P.W.; Hooper, G.J.; Malda, J.; Gawlitta, D.; Woodfield, T.B.F. Visible Light Cross-Linking of Gelatin Hydrogels Offers an Enhanced Cell Microenvironment with Improved Light Penetration Depth. Macromol. Biosci. 2019, 19, 1900098. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent Polysaccharide Alginate-Based Bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, J.; Bergman, J.; Kerins, C.; Hermanson, O. Optimization of 3d Bioprinting of Human Neuroblastoma Cells Using Sodium Alginate Hydrogel. Bioprinting 2019, 16, e00053. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Feinberg, A.W. Emergence of Fresh 3d Printing as a Platform for Advanced Tissue Biofabrication. APL Bioeng. 2021, 5, 010904. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4d Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
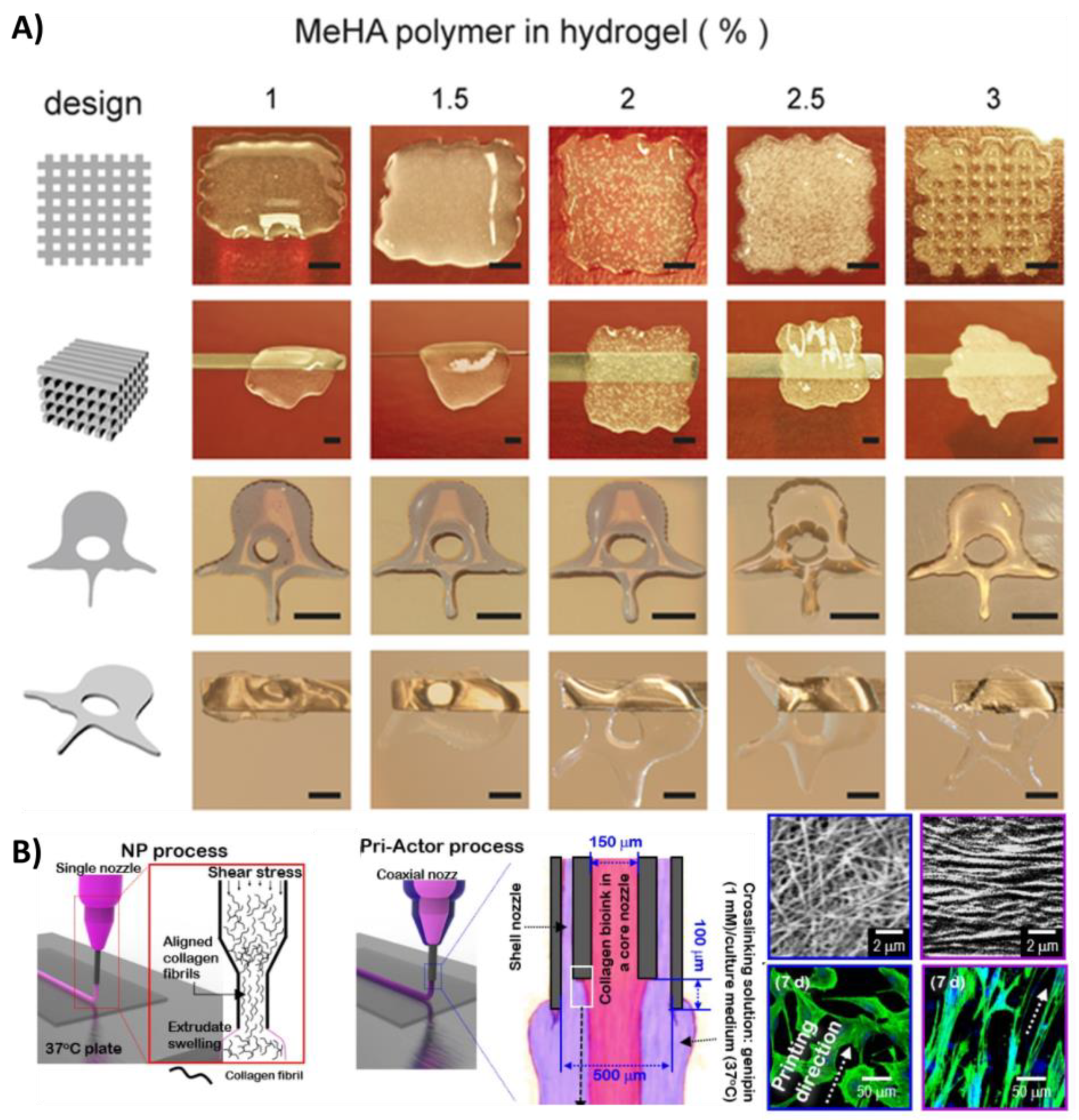
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D Bioprinting of Methacrylated Hyaluronic Acid (Meha) Hydrogel with Intrinsic Osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef]
- Xing, F.; Zhou, C.; Hui, D.; Du, C.; Wu, L.; Wang, L.; Wang, W.; Pu, X.; Gu, L.; Liu, L.; et al. Hyaluronic Acid as a Bioactive Component for Bone Tissue Regeneration: Fabrication, Modification, Properties, and Biological Functions. Nanotechnol. Rev. 2020, 9, 1059–1079. [Google Scholar] [CrossRef]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3d Printable Hyaluronic Acid-Based Hydrogel for Its Potential Application as a Bioink in Tissue Engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.H.; Kim, B.S.; Cho, Y.S.; Park, Y. Development and Evaluation of Hyaluronic Acid-Based Hybrid Bio-Ink for Tissue Regeneration. Tissue Eng. Regen. Med. 2018, 15, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Highley, C.B.; Yeh, Y.C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-Dimensional Extrusion Bioprinting of Single- and Double-Network Hydrogels Containing Dynamic Covalent Crosslinks. J. Biomed. Mater. Res. Part A 2018, 106, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-Inspired Hydrogel Composed of Hyaluronic Acid and Alginate as a Potential Bioink for 3d Bioprinting of Articular Cartilage Engineering Constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Fang, D.; Long, Z.; Hou, J. Clinical Application of Concentrated Growth Factor Fibrin Combined with Bone Repair Materials in Jaw Defects. J. Oral Maxillofac. Surg. 2020, 78, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Al-Maawi, S.; Sader, R.; Ghanaati, S. Individualized Titanium Mesh Combined with Platelet-Rich Fibrin and Deproteinized Bovine Bone: A New Approach for Challenging Augmentation. J. Oral Implantol. 2018, 44, 345–351. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Amler, A.-K.; Thomas, A.; Tüzüner, S.; Lam, T.; Geiger, M.-A.; Kreuder, A.-E.; Palmer, C.; Nahles, S.; Lauster, R.; Kloke, L. 3d Bioprinting of Tissue-Specific Osteoblasts and Endothelial Cells to Model the Human Jawbone. Sci. Rep. 2021, 11, 4876. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3d Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel Bioprinted Microchannel Networks for Vascularization of Tissue Engineering Constructs. Lab A Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3d Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Kakiuchi, M.; Hosoya, T.; Takaoka, K.; Amitani, K.; Ono, K. Human Bone Matrix Gelatin as a Clinical Alloimplant. Int. Orthop. 1985, 9, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Gudas, R.; Mačiulaitis, J.; Staškūnas, M.; Smailys, A. Clinical Outcome after Treatment of Single and Multiple Cartilage Defects by Autologous Matrix-Induced Chondrogenesis. J. Orthop. Surg. 2019, 27, 2309499019851011. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, W.; Skinner, J.A.; Gooding, C.R.; Carrington, R.W.J.; Flanagan, A.M.; Briggs, T.W.R.; Bentley, G. Autologous Chondrocyte Implantation Versus Matrix-Induced Autologous Chondrocyte Implantation for Osteochondral Defects of the Knee. J. Bone Jt. Surg. 2005, 87, 640–645. [Google Scholar] [CrossRef]
- Nawaz, S.Z.; Bentley, G.; Briggs, T.W.; Carrington, R.W.; Skinner, J.A.; Gallagher, K.R.; Dhinsa, B.S. Autologous Chondrocyte Implantation in the Knee: Mid-Term to Long-Term Results. J Bone Jt. Surg. 2014, 96, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.L.; Remmers, A.E.; Flanigan, D.C. Use of Maci (Autologous Cultured Chondrocytes on Porcine Collagen Membrane) in the United States: Preliminary Experience. Orthop. J. Sports Med. 2020, 8, 2325967120941816. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.K.; Ashton, T.R.; Cunningham, J.D.; Maini, R.; Pollock, J.G. Experimental and Clinical Experience with a Gelatin Impregnated Dacron Prosthesis. Ann. Vasc. Surg. 1987, 1, 542–547. [Google Scholar] [CrossRef]
- Khan, I.M.; Gilbert, S.J.; Singhrao, S.K.; Duance, V.C.; Archer, C.W. Cartilage Integration: Evaluation of the Reasons for Failure of Integration During Cartilage Repair. A Review. Eur. Cells Mater. 2008, 16, 26–39. [Google Scholar] [CrossRef]
- Martín, A.R.; Patel, J.M.; Zlotnick, H.M.; Carey, J.L.; Mauck, R.L. Emerging Therapies for Cartilage Regeneration in Currently Excluded ‘Red Knee’ Populations. NPJ Regen. Med. 2019, 4, 12. [Google Scholar] [CrossRef]
- Kim, W.; Jang, C.H.; Kim, G. Bioprinted Hasc-Laden Structures with Cell-Differentiation Niches for Muscle Regeneration. Chem. Eng. J. 2021, 419, 129570. [Google Scholar] [CrossRef]
- Groen, W.M.; Utomo, L.; Castilho, M.; Gawlitta, D.; Malda, J.; van Weeren, P.R.; Levato, R.; Korthagen, N.M. Impact of Endotoxins in Gelatine Hydrogels on Chondrogenic Differentiation and Inflammatory Cytokine Secretion in Vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Munarin, F.; Kabelac, C.; Coulombe, K.L.K. Heparin-Modified Alginate Microspheres Enhance Neovessel Formation in Hipsc-Derived Endothelial Cells and Heterocellular in Vitro Models by Controlled Release of Vegf. bioRxiv 2021. [Google Scholar] [CrossRef]
- Freeman, F.E.; Pitacco, P.; van Dommelen, L.H.A.; Nulty, J.; Browe, D.C.; Shin, J.-Y.; Alsberg, E.; Kelly, D.J. 3d Bioprinting Spatiotemporally Defined Patterns of Growth Factors to Tightly Control Tissue Regeneration. Sci. Adv. 2020, 6, eabb5093. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3d Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3d Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Li, H.; Tay, R.Y.; Sun, B.; Tsang, S.H.; Cometto, O.; Lin, J.; Teo, E.H.; Tok, A.I. Biocompatible Hydroxylated Boron Nitride Nanosheets/Poly(Vinyl Alcohol) Interpenetrating Hydrogels with Enhanced Mechanical and Thermal Responses. ACS Nano 2017, 11, 3742–3751. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xu, J.; Lu, X.; Gan, D.; Wang, Z.; Wang, K.; Zhang, H.; Yuan, H.; Weng, J. Biohybrid Methacrylated Gelatin/Polyacrylamide Hydrogels for Cartilage Repair. J. Mater. Chem. B 2017, 5, 731–741. [Google Scholar] [CrossRef]
- Boere, K.W.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; van Steenbergen, M.J.; Dhert, W.J.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent Attachment of a Three-Dimensionally Printed Thermoplast to a Gelatin Hydrogel for Mechanically Enhanced Cartilage Constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cao, C.; Wang, Q.; Gonzalez, M.; Dolbow, J.E.; Zhao, X. Design of Stiff, Tough and Stretchy Hydrogel Composites Via Nanoscale Hybrid Crosslinking and Macroscale Fiber Reinforcement. Soft Matter 2014, 10, 7519–7527. [Google Scholar] [CrossRef]
- Bas, O.; De-Juan-Pardo, E.M.; Chhaya, M.P.; Wunner, F.M.; Jeon, J.E.; Klein, T.J.; Hutmacher, D.W. Enhancing Structural Integrity of Hydrogels by Using Highly Organised Melt Electrospun Fibre Constructs. Eur. Polym. J. 2015, 72, 451–463. [Google Scholar] [CrossRef]
- Iviglia, G.; Cassinelli, C.; Torre, E.; Baino, F.; Morra, M.; Vitale-Brovarone, C. Novel Bioceramic-Reinforced Hydrogel for Alveolar Bone Regeneration. Acta Biomater. 2016, 44, 97. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of Hydrogels Using Three-Dimensionally Printed Microfibres. Nat. Commun. 2015, 6, 6933. Available online: http://www.nature.com/articles/ncomms7933#supplementary-information (accessed on 15 August 2021). [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3d Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. Available online: http://www.nature.com/nbt/journal/v34/n3/abs/nbt.3413.html#supplementary-information (accessed on 15 August 2021). [CrossRef] [PubMed]
- de Ruijter, M.; Hrynevich, A.; Haigh, J.N.; Hochleitner, G.; Castilho, M.; Groll, J.; Malda, J.; Dalton, P.D. Out-of-Plane 3d-Printed Microfibers Improve the Shear Properties of Hydrogel Composites. Small 2018, 14, 1702773. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.; Melchels, F.P.; Visser, J.; Dhert, W.J.; Gawlitta, D.; Malda, J. Yield Stress Determines Bioprintability of Hydrogels Based on Gelatin-Methacryloyl and Gellan Gum for Cartilage Bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3d Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in Extrusion Bioprinting. Biofabrication 2021, 13, 033001. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Tamjid, E.; Simchi, A. Fabrication of a Highly Ordered Hierarchically Designed Porous Nanocomposite Via Indirect 3d Printing: Mechanical Properties and in Vitro Cell Responses. Mater. Des. 2015, 88, 924–931. [Google Scholar] [CrossRef]
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3d Printed Microchannel Networks to Direct Vascularisation During Endochondral Bone Repair. Biomaterials 2018, 162, 34–46. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. A 3d Bioprinted Pseudo-Bone Drug Delivery Scaffold for Bone Tissue Engineering. Pharmaceutics 2020, 12, 166. [Google Scholar] [CrossRef]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Procedia Cirp 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Tan, E.Y.S.; Suntornnond, R.; Yeong, W.Y. High-Resolution Novel Indirect Bioprinting of Low-Viscosity Cell-Laden Hydrogels Via Model-Support Bioink Interaction. 3D Print. Addit. Manuf. 2021, 8, 69–78. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-Free Vascular Tissue Engineering Using Bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Negro, A.; Cherbuin, T.; Lutolf, M.P. 3d Inkjet Printing of Complex, Cell-Laden Hydrogel Structures. Sci. Rep. 2018, 8, 17099. [Google Scholar] [CrossRef]
- Gurkan, U.A.; El Assal, R.; Yildiz, S.E.; Sung, Y.; Trachtenberg, A.J.; Kuo, W.P.; Demirci, U. Engineering Anisotropic Biomimetic Fibrocartilage Microenvironment by Bioprinting Mesenchymal Stem Cells in Nanoliter Gel Droplets. Mol. Pharm. 2014, 11, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shim, J.-H.; Choi, S.-A.; Jang, J.; Kim, M.; Lee, S.H.; Cho, D.-W. 3d Printing Technology to Control Bmp-2 and Vegf Delivery Spatially and Temporally to Promote Large-Volume Bone Regeneration. J. Mater. Chem. B 2015, 3, 5415–5425. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth Factor Delivery-Based Tissue Engineering: General Approaches and a Review of Recent Developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef]
- Said, S.S.; Pickering, J.G.; Mequanint, K. Advances in Growth Factor Delivery for Therapeutic Angiogenesis. J. Vasc. Res. 2013, 50, 35–51. [Google Scholar] [CrossRef]
- Rajesh, V.; Dhirendra, K.S. Growth Factor-Delivery Systems for Tissue Engineering: A Materials Perspective. Expert Rev. Med Devices 2006, 3, 29–47. [Google Scholar] [CrossRef]
- Silva, A.K.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Growth Factor Delivery Approaches in Hydrogels. Biomacromolecules 2009, 10, 9–18. [Google Scholar] [CrossRef]
- Aguilar, L.M.C.; Silva, S.M.; Moulton, S.E. Growth Factor Delivery: Defining the Next Generation Platforms for Tissue Engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Longoni, A.; Li, J.; Lindberg, G.C.; Rnjak-Kovacina, J.; Wise, L.M.; Hooper, G.J.; Woodfield, T.B.; Kieser, D.C.; Lim, K.S. Strategies for Inclusion of Growth Factors into 3d Printed Bone Grafts. Essays Biochem. 2021, 65, 585. [Google Scholar] [CrossRef]
- Aimetti, A.A.; Machen, A.J.; Anseth, K.S. Poly(Ethylene Glycol) Hydrogels Formed by Thiol-Ene Photopolymerization for Enzyme-Responsive Protein Delivery. Biomaterials 2009, 30, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.J.; Lim, K.S.; Farrugia, B.L.; Hooper, G.J.; Woodfield, T.B.F. Covalent Incorporation of Heparin Improves Chondrogenesis in Photocurable Gelatin-Methacryloyl Hydrogels. Macromol. Biosci. 2017, 17, 1700158. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Sahu, A.; Vilos, C.; Kamaly, N.; Jo, S.-M.; Lee, J.H.; Tae, G. Bioinspired Heparin Nanosponge Prepared by Photo-Crosslinking for Controlled Release of Growth Factors. Sci. Rep. 2017, 7, 14351. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.D.; Anseth, K.S. Thiol-Ene Photopolymerizations Provide a Facile Method to Encapsulate Proteins and Maintain Their Bioactivity. Biomacromolecules 2012, 13, 2410–2417. [Google Scholar] [CrossRef]
- Masters, K.S. Covalent Growth Factor Immobilization Strategies for Tissue Repair and Regeneration. Macromol. Biosci. 2011, 11, 1149–1163. [Google Scholar] [CrossRef]
- Grim, J.C.; Marozas, I.A.; Anseth, K.S. Thiol-Ene and Photo-Cleavage Chemistry for Controlled Presentation of Biomolecules in Hydrogels. J. Control. Release 2015, 219, 95–106. [Google Scholar] [CrossRef]
- Enriquez-Ochoa, D.; Robles-Ovalle, P.; Mayolo-Deloisa, K.; Brunck, M.E.G. Immobilization of Growth Factors for Cell Therapy Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 620. [Google Scholar] [CrossRef]
- Kempen, D.H.R.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J.A. Effect of Local Sequential Vegf and Bmp-2 Delivery on Ectopic and Orthotopic Bone Regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef]
- Geuze, R.E.; Theyse, L.F.; Kempen, D.H.; Hazewinkel, H.A.; Kraak, H.Y.; Oner, F.C.; Dhert, W.J.; Alblas, J. A Differential Effect of Bone Morphogenetic Protein-2 and Vascular Endothelial Growth Factor Release Timing on Osteogenesis at Ectopic and Orthotopic Sites in a Large-Animal Model. Tissue Eng. Part A 2012, 18, 2052–2062. [Google Scholar] [CrossRef]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An Alginate-Based Hybrid System for Growth Factor Delivery in the Functional Repair of Large Bone Defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef]
- Henrionnet, C.; Pourchet, L.; Neybecker, P.; Messaoudi, O.; Gillet, P.; Loeuille, D.; Mainard, D.; Marquette, C.; Pinzano, A. Combining Innovative Bioink and Low Cell Density for the Production of 3d-Bioprinted Cartilage Substitutes: A Pilot Study. Stem Cells Int. 2020, 2020, 2487072. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and Structure of Articular Cartilage: A Template for Tissue Repair. Clin. Orthop. 2001, 391, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage: Tissue Design and Chondrocyte-Matrix Interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar]
- Amer, M.H.; Rose, F.R.A.J.; Shakesheff, K.M.; Modo, M.; White, L.J. Translational Considerations in Injectable Cell-Based Therapeutics for Neurological Applications: Concepts, Progress and Challenges. NPJ Regen. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Ker, E.D.; Nain, A.S.; Weiss, L.E.; Wang, J.; Suhan, J.; Amon, C.H.; Campbell, P.G. Bioprinting of Growth Factors onto Aligned Sub-Micron Fibrous Scaffolds for Simultaneous Control of Cell Differentiation and Alignment. Biomaterials 2011, 32, 8097–8107. [Google Scholar] [CrossRef] [PubMed]
- Albritton, J.L.; Miller, J.S. 3d Bioprinting: Improving in Vitro Models of Metastasis with Heterogeneous Tumor Microenvironments. Dis. Models Mech. 2017, 10, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sardelli, L.; Pacheco, D.P.; Zorzetto, L.; Rinoldi, C.; Święszkowski, W.; Petrini, P. Engineering Biological Gradients. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019829023. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Xia, Y. Multiple Facets for Extracellular Matrix Mimicking in Regenerative Medicine. Nanomedicine 2015, 10, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, X.; Lipner, J.; Manning, C.N.; Schwartz, A.G.; Thomopoulos, S.; Xia, Y. “Aligned-to-Random” Nanofiber Scaffolds for Mimicking the Structure of the Tendon-to-Bone Insertion Site. Nanoscale 2010, 2, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Cheng, M.-H.; Engel, H.; Kao, S.-W.; Larson, J.C.; Gupta, S.; Brey, E.M. The Role of Pore Size on Vascularization and Tissue Remodeling in Peg Hydrogels. Biomaterials 2011, 32, 6045–6051. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3d Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-Dimensional Plotted Scaffolds with Controlled Pore Size Gradients: Effect of Scaffold Geometry on Mechanical Performance and Cell Seeding Efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef]
- Wendt, D.; Marsano, A.; Jakob, M.; Heberer, M.; Martin, I. Oscillating Perfusion of Cell Suspensions through Three-Dimensional Scaffolds Enhances Cell Seeding Efficiency and Uniformity. Biotechnol. Bioeng. 2003, 84, 205–214. [Google Scholar] [CrossRef]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and Mechanical Characterization of 3d Printed Vertical Uniform and Gradient Scaffolds for Bone and Osteochondral Tissue Engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, K.J.; Park, S.Y.; Huh, J.E.; Kim, H.J.; Yu, W.-R. 3d Braid Scaffolds for Regeneration of Articular Cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Diloksumpan, P.; Bolaños, R.V.; Cokelaere, S.; Pouran, B.; de Grauw, J.; van Rijen, M.; van Weeren, R.; Levato, R.; Malda, J. Orthotopic Bone Regeneration within 3d Printed Bioceramic Scaffolds with Region-Dependent Porosity Gradients in an Equine Model. Adv. Healthc. Mater. 2020, 9, 1901807. [Google Scholar] [CrossRef] [PubMed]
- Ober, T.J.; Foresti, D.; Lewis, J.A. Active Mixing of Complex Fluids at the Microscale. Proc. Natl. Acad. Sci. USA 2015, 112, 12293–12298. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Kudva, A.K.; Saxena, N.S.; Roy, K. Engineering Articular Cartilage with Spatially-Varying Matrix Composition and Mechanical Properties from a Single Stem Cell Population Using a Multi-Layered Hydrogel. Biomaterials 2011, 32, 6946–6952. [Google Scholar] [CrossRef]
- Dobos, A.; Van Hoorick, J.; Steiger, W.; Gruber, P.; Markovic, M.; Andriotis, O.G.; Rohatschek, A.; Dubruel, P.; Thurner, P.J.; Van Vlierberghe, S.; et al. Thiol–Gelatin–Norbornene Bioink for Laser-Based High-Definition Bioprinting. Adv. Healthc. Mater. 2020, 9, 1900752. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Guiding Cell Migration in 3d: A Collagen Matrix with Graded Directional Stiffness. Cell Motil. Cytoskelet. 2009, 66, 121–128. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Fleischhammer, T.; Enders, A.; Pirmahboub, H.; Bahnemann, J.; Pepelanova, I. Fabrication of Stiffness Gradients of Gelma Hydrogels Using a 3d Printed Micromixer. Macromol. Biosci. 2020, 20, 2000107. [Google Scholar] [CrossRef]
- Idaszek, J.; Costantini, M.; Karlsen, T.A.; Jaroszewicz, J.; Colosi, C.; Testa, S.; Fornetti, E.; Bernardini, S.; Seta, M.; Kasarełło, K.; et al. 3d Bioprinting of Hydrogel Constructs with Cell and Material Gradients for the Regeneration of Full-Thickness Chondral Defect Using a Microfluidic Printing Head. Biofabrication 2019, 11, 044101. [Google Scholar] [CrossRef]
- Chae, S.; Sun, Y.; Choi, Y.-J.; Ha, D.-H.; Jeon, I.; Cho, D.-W. 3d Cell-Printing of Tendon-Bone Interface Using Tissue-Derived Extracellular Matrix Bioinks for Chronic Rotator Cuff Repair. Biofabrication 2021, 13, 035005. [Google Scholar] [CrossRef]
- de Ruijter, M.; Ribeiro, A.; Dokter, I.; Castilho, M.; Malda, J. Simultaneous Micropatterning of Fibrous Meshes and Bioinks for the Fabrication of Living Tissue Constructs. Adv. Healthc. Mater. 2019, 8, e1800418. [Google Scholar] [CrossRef]
- Guvendiren, M. 3D Bioprinting in Medicine: Technologies, Bioinks, and Applications; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Lu, L.; Arbit, H.M.; Herrick, J.L.; Segovis, S.G.; Maran, A.; Yaszemski, M.J. Tissue Engineered Constructs: Perspectives on Clinical Translation. Ann. Biomed. Eng. 2015, 43, 796–804. [Google Scholar] [CrossRef] [PubMed]
- US Food & Drug Administration. Technical Considerations for Additive Manufactured Medical Devices; US Food & Drug Administration: Silver Spring, MD, USA, 2016.
- Gilbert, F.; O’Connell, C.D.; Mladenovska, T.; Dodds, S. Print Me an Organ? Ethical and Regulatory Issues Emerging from 3d Bioprinting in Medicine. Sci. Eng. Ethics 2018, 24, 73–91. [Google Scholar] [CrossRef]
- Lee, M.H.; Arcidiacono, J.A.; Bilek, A.M.; Wille, J.J.; Hamill, C.A.; Wonnacott, K.M.; Wells, M.A.; Oh, S.S. Considerations for Tissue-Engineered and Regenerative Medicine Product Development Prior to Clinical Trials in the United States. Tissue Eng. Part B Rev. 2010, 16, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Bertram, T.; Hellman, K.B.; Bayon, Y.; Ellison, S.; Wilburn, S. The Regulatory Imperative: International Perspective. Tissue Eng. Part B Rev. 2013, 19, 191–193. [Google Scholar] [CrossRef]
- Vinck, I.; Vijverman, A.; Vollebregt, E.; Broeckx, N.; Wouters, K.; Piët, M.; Bacic, N.; Vlayen, J.; Thiry, N.; Neyt, M. Responsible Use of High-Risk Medical Devices: The Example of 3D Printed Medical Devices; Belgian Health Care Knowledge Centre (KCE): Brussels, Belgium, 2018.
- IMDRF Personalized Medical Devices. Definitions for Personalized Medical Devices. International Medical Device Regulators Forum: 2018. Available online: http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-181018-pmd-definitions-n49.pdf (accessed on 15 August 2021).
- Bayon, Y.; Vertès, A.A.; Ronfard, V.; Egloff, M.; Snykers, S.; Salinas, G.F.; Thomas, R.; Girling, A.; Lilford, R.; Clermont, G.; et al. Translating Cell-Based Regenerative Medicines from Research to Successful Products: Challenges and Solutions. Tissue Eng. Part B Rev. 2014, 20, 246–256. [Google Scholar] [CrossRef]
- Mendes, G.C.; Brandão, T.R.; Silva, C.L. Ethylene Oxide Sterilization of Medical Devices: A Review. Am. J. Infect. Control. 2007, 35, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Palmer, I.; Clarke, S.A.; Nelson, J.; Schatton, W.; Dunne, N.J.; Buchanan, F. Identification of a Suitable Sterilisation Method for Collagen Derived from a Marine Demosponge. Int. J. Nano Biomater. 2012, 4, 148–163. [Google Scholar] [CrossRef]
- Hodder, E.; Duin, S.; Kilian, D.; Ahlfeld, T.; Seidel, J.; Nachtigall, C.; Bush, P.; Covill, D.; Gelinsky, M.; Lode, A. Investigating the Effect of Sterilisation Methods on the Physical Properties and Cytocompatibility of Methyl Cellulose Used in Combination with Alginate for 3d-Bioplotting of Chondrocytes. J. Mater. Sci. Mater. Med. 2019, 30, 10. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Yoshii, F.; Nagasawa, N.; Wach, R.A.; Mitomo, H. Degradation of Chitosan and Sodium Alginate by Gamma Radiation, Sonochemical and Ultraviolet Methods. Radiat. Phys. Chem. 2005, 73, 287–295. [Google Scholar] [CrossRef]
- Nagai, N.; Matsunobe, T.; Imai, T. Infrared Analysis of Depth Profiles in Uv-Photochemical Degradation of Polymers. Polym. Degrad. Stab. 2005, 88, 224–233. [Google Scholar] [CrossRef]
- Arnosti, C.; Repeta, D.J.; Blough, N.V. Rapid Bacterial Degradation of Polysaccharides in Anoxic Marine Systems. Geochim. Cosmochim. Acta 1994, 58, 2639–2652. [Google Scholar] [CrossRef]
- Stoppel, W.L.; White, J.C.; Horava, S.D.; Henry, A.C.; Roberts, S.C.; Bhatia, S.R. Terminal Sterilization of Alginate Hydrogels: Efficacy and Impact on Mechanical Properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 877–884. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Use of International Standard Iso 10993-1, Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2020.
- Food and Drug Administration. Guidance on Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products; Food and Drug Administration: Rockville, MD, USA, 1999; Volume 64, pp. 44928–44935.
- US Food and Drug Administration. Guidance for the Preparation of a Premarket Notification Application for a Surgical Mesh; U.S. Department of Health and Human Services: Rockville, MD, USA, 1999.
- Flanagan, T.C.; Sachweh, J.S.; Frese, J.; Schnöring, H.; Gronloh, N.; Koch, S.; Tolba, R.H.; Schmitz-Rode, T.; Jockenhoevel, S. In Vivo Remodeling and Structural Characterization of Fibrin-Based Tissue-Engineered Heart Valves in the Adult Sheep Model. Tissue Eng. Part A 2009, 15, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. General Considerations for Animal Studies for Cardiovascular Devices—Guidance for Industry and Fda Staff; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2010.
- Zhang, B.L.; Bianco, R.W.; Schoen, F.J. Preclinical Assessment of Cardiac Valve Substitutes: Current Status and Considerations for Engineered Tissue Heart Valves. Front. Cardiovasc. Med. 2019, 6, 72. [Google Scholar] [CrossRef]
- Taylor, D.A.; Caplan, A.L.; Macchiarini, P. Ethics of Bioengineering Organs and Tissues. Expert Opin. Biol. Ther. 2014, 14, 879–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oerlemans, A.J.; van den Berg, P.P.; van Leeuwen, E.; Dekkers, W.J. Ethical Issues Regarding the Donation and Source of Cells for Tissue Engineering: A European Focus Group Study. Tissue Eng. Part B Rev. 2011, 17, 229–234. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.B.; Oerlemans, A.; Trommelmans, L.; Dierickx, K.; Gordijn, B. Ethical Aspects of Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2008, 14, 367–375. [Google Scholar] [CrossRef]
- Shapiro, J.K.; Wesoloski, B.J. Fda’s Regulatory Scheme for Human Tissue. Hum. Tissue Regul. 2007, 11, 9–12. [Google Scholar]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef]
- Jorgensen, C.; Simon, M. In Vitro Human Joint Models Combining Advanced 3d Cell Culture and Cutting-Edge 3d Bioprinting Technologies. Cells 2021, 10, 596. [Google Scholar] [CrossRef]
- Nichols, D.A.; Sondh, I.S.; Little, S.R.; Zunino, P.; Gottardi, R. Design and Validation of an Osteochondral Bioreactor for the Screening of Treatments for Osteoarthritis. Biomed. Microdevices 2018, 20, 18. [Google Scholar] [CrossRef]
- Paggi, C.; Venzac, B.; Leijten, J.; Teixeira Leijten, L.M.; Le Gac, S.; Karperien, M. Cartilage-on-Chip: A Multi-Modal Platform to Study Human Chondrocyte’s Response to Mechanical Stimuli. Osteoarthr. Cartil. 2020, 28, S176–S177. [Google Scholar] [CrossRef]
- Occhetta, P.; Mainardi, A.; Votta, E.; Vallmajo-Martin, Q.; Ehrbar, M.; Martin, I.; Barbero, A.; Rasponi, M. Hyperphysiological Compression of Articular Cartilage Induces an Osteoarthritic Phenotype in a Cartilage-on-a-Chip Model. Nat. Biomed. Eng. 2019, 3, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Verma, T.; Vaish, A.; Vaish, R.; Vaishya, R.; Maini, L. Virtual Preoperative Planning and 3d Printing Are Valuable for the Management of Complex Orthopaedic Trauma. Chin. J. Traumatol. 2019, 22, 350–355. [Google Scholar] [CrossRef]
- Segaran, N.; Saini, G.; Mayer, J.L.; Naidu, S.; Patel, I.; Alzubaidi, S.; Oklu, R. Application of 3d Printing in Preoperative Planning. J. Clin. Med. 2021, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, G.; Coles-Black, J.; Chuen, J.; Hardidge, A. Three-Dimensional Printing in Orthopaedic Preoperative Planning Improves Intraoperative Metrics: A Systematic Review. ANZ J. Surg. 2020, 90, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ratinam, R.; Quayle, M.; Crock, J.; Lazarus, M.; Fogg, Q.; McMenamin, P. Challenges in Creating Dissectible Anatomical 3d Prints for Surgical Teaching. J. Anat. 2019, 234, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Cardea, S.; Maffulli, N.; Reverchon, E. Regeneration Techniques for Bone-to-Tendon and Muscle-to-Tendon Interfaces Reconstruction. Br. Med. Bull. 2016, 117, 25–37. [Google Scholar] [CrossRef]
- Schwab, A.; Helary, C.; Richards, G.; Alini, M.; Eglin, D.; D’Este, M. Tissue Mimetic Hyaluronan Bioink Containing Collagen Fibers with Controlled Orientation Modulating Cell Morphology and Alignment. bioRxiv 2020. [Google Scholar] [CrossRef]
- Full, S.M.; Delman, C.; Gluck, J.M.; Abdmaulen, R.; Shemin, R.J.; Heydarkhan-Hagvall, S. Effect of Fiber Orientation of Collagen-Based Electrospun Meshes on Human Fibroblasts for Ligament Tissue Engineering Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.S.; Verbridge, S.S.; Dahlgren, L.A.; Kakar, S.; Guelcher, S.A.; Goldstein, A.S. Fiber/Collagen Composites for Ligament Tissue Engineering: Influence of Elastic Moduli of Sparse Aligned Fibers on Mesenchymal Stem Cells. J. Biomed. Mater. Res. Part A 2016, 104, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Girão, A.F.; Semitela, Â.; Ramalho, G.; Completo, A.; Marques, P.A.A.P. Mimicking Nature: Fabrication of 3d Anisotropic Electrospun Polycaprolactone Scaffolds for Cartilage Tissue Engineering Applications. Compos. Part B Eng. 2018, 154, 99–107. [Google Scholar] [CrossRef]
- Di Luca, A.; Longoni, A.; Criscenti, G.; Lorenzo-Moldero, I.; Klein-Gunnewiek, M.; Vancso, J.; van Blitterswijk, C.; Mota, C.; Moroni, L. Surface Energy and Stiffness Discrete Gradients in Additive Manufactured Scaffolds for Osteochondral Regeneration. Biofabrication 2016, 8, 015014. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naghieh, S.; Lindberg, G.; Tamaddon, M.; Liu, C. Biofabrication Strategies for Musculoskeletal Disorders: Evolution towards Clinical Applications. Bioengineering 2021, 8, 123. https://doi.org/10.3390/bioengineering8090123
Naghieh S, Lindberg G, Tamaddon M, Liu C. Biofabrication Strategies for Musculoskeletal Disorders: Evolution towards Clinical Applications. Bioengineering. 2021; 8(9):123. https://doi.org/10.3390/bioengineering8090123
Chicago/Turabian StyleNaghieh, Saman, Gabriella Lindberg, Maryam Tamaddon, and Chaozong Liu. 2021. "Biofabrication Strategies for Musculoskeletal Disorders: Evolution towards Clinical Applications" Bioengineering 8, no. 9: 123. https://doi.org/10.3390/bioengineering8090123
APA StyleNaghieh, S., Lindberg, G., Tamaddon, M., & Liu, C. (2021). Biofabrication Strategies for Musculoskeletal Disorders: Evolution towards Clinical Applications. Bioengineering, 8(9), 123. https://doi.org/10.3390/bioengineering8090123