On the Left Ventricular Remodeling of Patients with Stenotic Aortic Valve: A Statistical Shape Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Segmentation and Anatomical Measurements
2.3. Statistical Shape Analysis Method
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef]
- Allison, M.A.; Cheung, P.; Criqui, M.H.; Langer, R.D.; Wright, C.M. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation 2006, 113, 861–866. [Google Scholar] [CrossRef]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Keshavarz-Motamed, Z.; García, J.; Gaillard, E.; Capoulade, R.; Le Ven, F.; Cloutier, G.; Kadem, L.; Pibarot, P. Non-invasive determination of left ventricular workload in patients with aortic stenosis using magnetic resonance imaging and Doppler echocardiography. PLoS ONE 2014, 9, e86793. [Google Scholar]
- Pasta, S.; Agnese, V.; Di Giuseppe, M.; Gentile, G.; Raffa, G.M.; Bellavia, D.; Pilato, T. In Vivo Strain Analysis of Dilated Ascending Thoracic Aorta by ECG-Gated CT Angiographic Imaging. Ann. Biomed. Eng. 2017, 45, 2911–2920. [Google Scholar] [CrossRef]
- Rinaudo, A.; Raffa, G.M.; Scardulla, F.; Pilato, M.; Scardulla, C.; Pasta, S. Biomechanical implications of excessive endograft protrusion into the aortic arch after thoracic endovascular repair. Comput. Biol. Med. 2015, 66, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.; Teixeira, R.; Oliveira-Santos, M.; Barbosa, A.; Martins, R.; Castro, G.; Gonçalves, L.; Pego, M. Aortic Valve Disease and Vascular Mechanics: Two-Dimensional Speckle Tracking Echocardiographic Analysis. Echocardiography 2016, 33, 1121–1130. [Google Scholar] [CrossRef]
- Mendez, V.; Di Giuseppe, M.; Pasta, S. Comparison of hemodynamic and structural indices of ascending thoracic aortic aneurysm as predicted by 2-way FSI, CFD rigid wall simulation and patient-specific displacement-based FEA. Comput. Biol. Med. 2018, 100, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Gentile, G.; Raffa, G.M.; Scardulla, F.; Bellavia, D.; Luca, A.; Pilato, M.; Scardulla, C. Three-dimensional parametric modeling of bicuspid aortopathy and comparison with computational flow predictions. Artif. Organs 2017, 41, E92–E102. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Cannata, S.; Gentile, G.; Ingrassia, T.; Nigrelli, V.; Gandolfo, C. CAD Modeling for Evaluating LVOT Obstruction in Transcatheter Mitral Valve Replacement. In Design Tools and Methods in Industrial Engineering. ADM 2019; Lecture Notes in Mechanical Engineering; Spinger: Berlin, Germany, 2020; pp. 776–787. [Google Scholar]
- Gjesdal, O.; Bluemke, D.A.; Lima, J.A. Cardiac remodeling at the population level–risk factors, screening, and outcomes. Nat. Rev. Cardiol. 2011, 8, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Raffa, G.M.; Gentile, G.; Agnese, V.; Bellavia, D.; Pilato, M.; Pasta, S. Statistical Shape Analysis of Ascending Thoracic Aortic Aneurysm: Correlation between Shape and Biomechanical Descriptors. J. Pers. Med. 2020, 10, 28. [Google Scholar] [CrossRef]
- Roohi, S.F.; Zoroofi, R.A. 4D statistical shape modeling of the left ventricle in cardiac MR images. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 335–341. [Google Scholar] [CrossRef]
- Suinesiaputra, A.; D’Hooge, J.; Duchateau, N.; Ehrhardt, J.; Frangi, A.F.; Gooya, A.; Grau, V.; Lekadir, K.; Lu, A.; Mukhopadhyay, A.; et al. Statistical shape modeling of the left ventricle: Myocardial infarct classification challenge. IEEE J. Biomed. Health Inform. 2018, 22, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Agnese, V.; Gallo, A.; Cosentino, F.; Di Giuseppe, M.; Gentile, G.; Raffa, G.M.; Maalouf, J.F.; Michelena, H.I.; Bellavia, D.; et al. Stress and Aortic Strain Associations with Biomarkers of Ascending Thoracic Aortic Aneurysm. Ann. Thorac. Surg. 2020, 110, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Suinesiaputra, A.; Medrano-Gracia, P.; Cowan, B.R.; Young, A.A. Big heart data: Advancing health informatics through data sharing in cardiovascular imaging. IEEE J. Biomed. Health Inform. 2015, 19, 1283–1290. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Mirulla, A.; Bragonzoni, L.; Zaffagnini, S.; Bontempi, M.; Nigrelli, V.; Ingrassia, T. Virtual simulation of an osseointegrated trans-humeral prosthesis: A falling scenario. Injury 2018, 49, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, T.; Nigrelli, V.; Ricotta, V.; Tartamella, C. Process parameters influence in additive manufacturing. In Advances on Mechanics, Design Engineering and Manufacturing; Lecture Notes in Mechanical Engineering; Spinger: Berlin, Germany, 2017; pp. 261–270. [Google Scholar]
- Scardulla, F.; Rinaudo, A.; Pasta, S.; Scardulla, C. Mechanics of pericardial effusion: A simulation study. Proc. Inst. Mech. Eng. H 2015, 229, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Scardulla, F.; Agnese, V.; Romano, G.; Di Gesaro, G.; Sciacca, S.; Bellavia, D.; Clemenza, F.; Pilato, M.; Pasta, S. Modeling Right Ventricle Failure After Continuous Flow Left Ventricular Assist Device: A Biventricular Finite-Element and Lumped-Parameter Analysis. Cardiovasc. Eng. Technol. 2018, 9, 427–437. [Google Scholar] [CrossRef]
- Scardulla, F.; Bellavia, D.; Vitulo, P.; Romano, G.; Mina, C.; Gentile, G.; Clemenza, F.; Pasta, S. Biomechanical Determinants of Right Ventricular Failure in Pulmonary Hypertension. ASAIO J. 2018, 64, 557–564. [Google Scholar] [CrossRef]
- Scardulla, F.; Rinaudo, A.; Pasta, S.; Scardulla, C. Evaluation of ventricular wall stress and cardiac function in patients with dilated cardiomyopathy. Proc. Inst. Mech. Eng. H 2016, 230, 71–74. [Google Scholar] [CrossRef]
- Fratini, L.; Macaluso, G.; Pasta, S. Residual stresses and FCP prediction in FSW through a continuous FE model. J. Mater. Process. Technol. 2009, 209, 5465–5474. [Google Scholar] [CrossRef]
- Fratini, L.; Pasta, S. Residual stresses in friction stir welded parts of complex geometry. Int. J. Adv. Manuf. Technol. 2012, 59, 547–557. [Google Scholar] [CrossRef]
- Ingrassia, T.; Nalbone, L.; Nigrelli, V.; Pisciotta, D.; Ricotta, V. Influence of the metaphysis positioning in a new reverse shoulder prosthesis. In Advances on Mechanics, Design Engineering and Manufacturing; Lecture Notes in Mechanical Engineering; Spinger: Berlin, Germany, 2017; pp. 469–478. [Google Scholar]
- Bruse, J.L.; for the Modeling of Congenital Hearts Alliance (MOCHA) Collaborative Group; McLeod, K.; Biglino, G.; Ntsinjana, H.N.; Capelli, C.; Hsia, T.-Y.; Sermesant, M.; Pennec, X.; Taylor, A.M.; et al. A statistical shape modelling framework to extract 3D shape biomarkers from medical imaging data: Assessing arch morphology of repaired coarctation of the aorta. BMC Med. Imaging 2016, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Linzbach, A.J. Heart failure from the point of view of quantitative anatomy. Am. J. Cardiol. 1960, 5, 370–382. [Google Scholar] [CrossRef]
- Khanna, S.; Bhat, A.; Chen, H.H.; Tan, J.W.; Gan, G.C.; Tan, T.C. Left Ventricular Sphericity Index is a reproducible bedside echocardiographic measure of geometric change between acute phase Takotsubo’s syndrome and acute anterior myocardial infarction. Int. J. Cardiol. Heart Vasc. 2018, 29, 100547. [Google Scholar] [CrossRef]
- Wong, S.P.; French, J.K.; Lydon, A.-M.; Manda, S.O.; Gao, W.; Ashton, N.G.; White, H.D. Relation of left ventricular sphericity to 10-year survival after acute myocardial infarction. Am. J. Cardiol. 2004, 94, 1270–1275. [Google Scholar] [CrossRef]
- Wall, S.T.; Walker, J.C.; Healy, K.E.; Ratcliffe, M.B.; Guccione, J.M. Theoretical impact of the injection of material into the myocardium: A finite element model simulation. Circulation 2006, 114, 2627–2635. [Google Scholar] [CrossRef]
- Lew owski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A.; et al. Preterm heart in adult life: Cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013, 127, 197–206. [Google Scholar]
- Zhang, X.; Cowan, B.R.; Bluemke, D.A.; Finn, J.P.; Fonseca, C.G.; Kadish, A.H.; Lee, D.C.; Lima, J.A.C.; Suinesiaputra, A.; Young, A.A.; et al. Atlas-based quantification of cardiac remodeling due to myocardial infarction. PLoS ONE 2014, 9, e110243. [Google Scholar] [CrossRef]
- Ardekani, S.; Jain, S.; Sanzi, A.; Corona-Villalobos, C.P.; Abraham, T.P.; Abraham, M.R.; Zimmerman, S.L.; Wu, K.C.; Winslow, R.L.; Miller, M.I.; et al. Shape analysis of hypertrophic and hypertensive heart disease using MRI-based 3D surface models of left ventricular geometry. Med. Image Anal. 2016, 29, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Piras, P.; Evangelista, A.; Gabriele, S.; Nardinocchi, P.; Teresi, L.; Torromeo, C.; Schiariti, M.S.M.; Varano, V.; Puddu, P.E. 4D-analysis of left ventricular heart cycle using procrustes motion analysis. PLoS ONE 2014, 9, e86896. [Google Scholar]
- Bruse, J.L.; Cervi, E.; McLeod, K.; Biglino, G.; Sermesant, M.; Pennec, X.; Taylor, A.M.; Schievano, S.; Hsia, T.-Y.; Khambadkone, S.; et al. Looks Do Matter! Aortic Arch Shape After Hypoplastic Left Heart Syndrome Palliation Correlates with Cavopulmonary Outcomes. Ann. Thorac. Surg. 2017, 103, 645–654. [Google Scholar] [CrossRef]

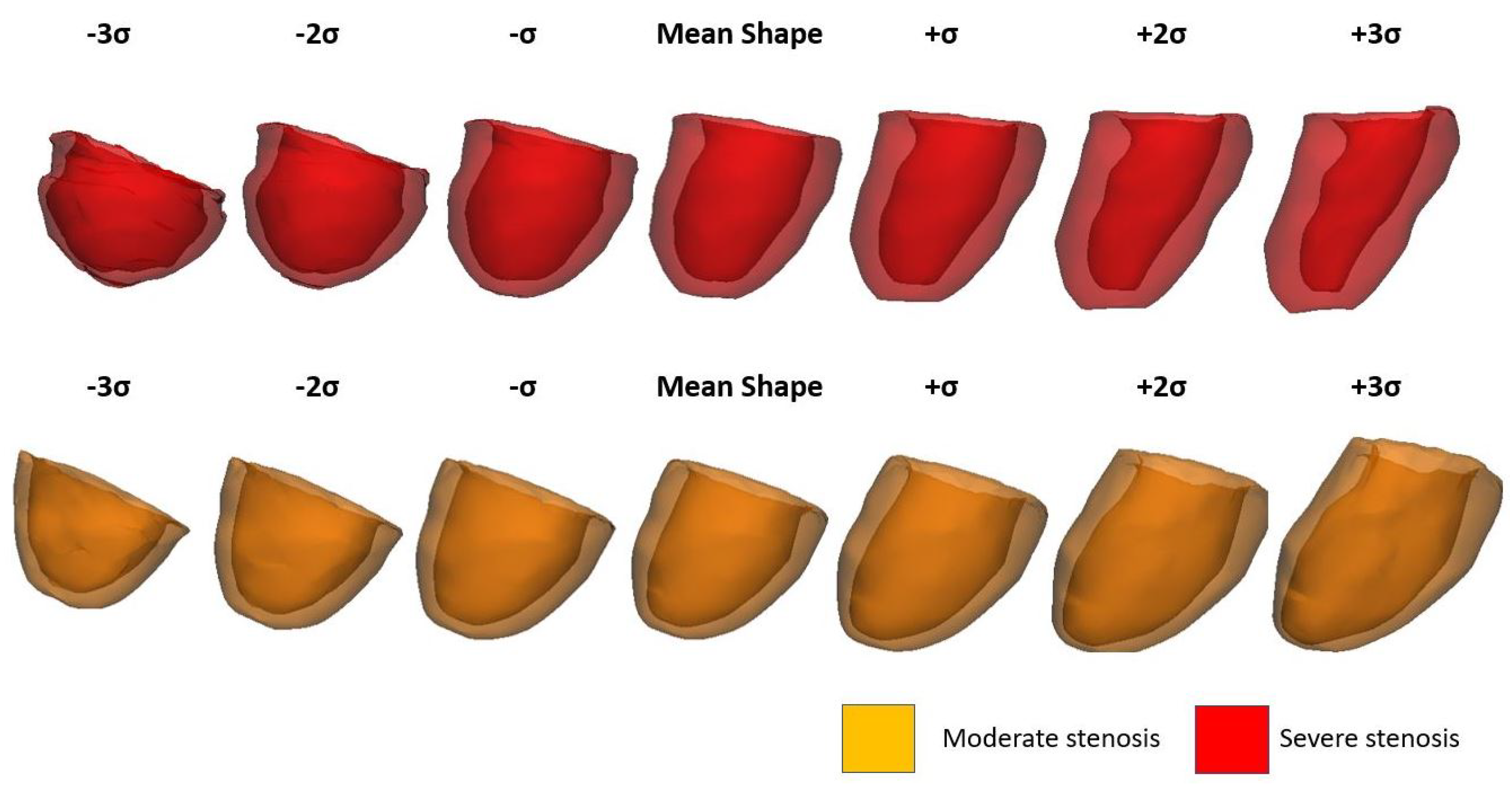
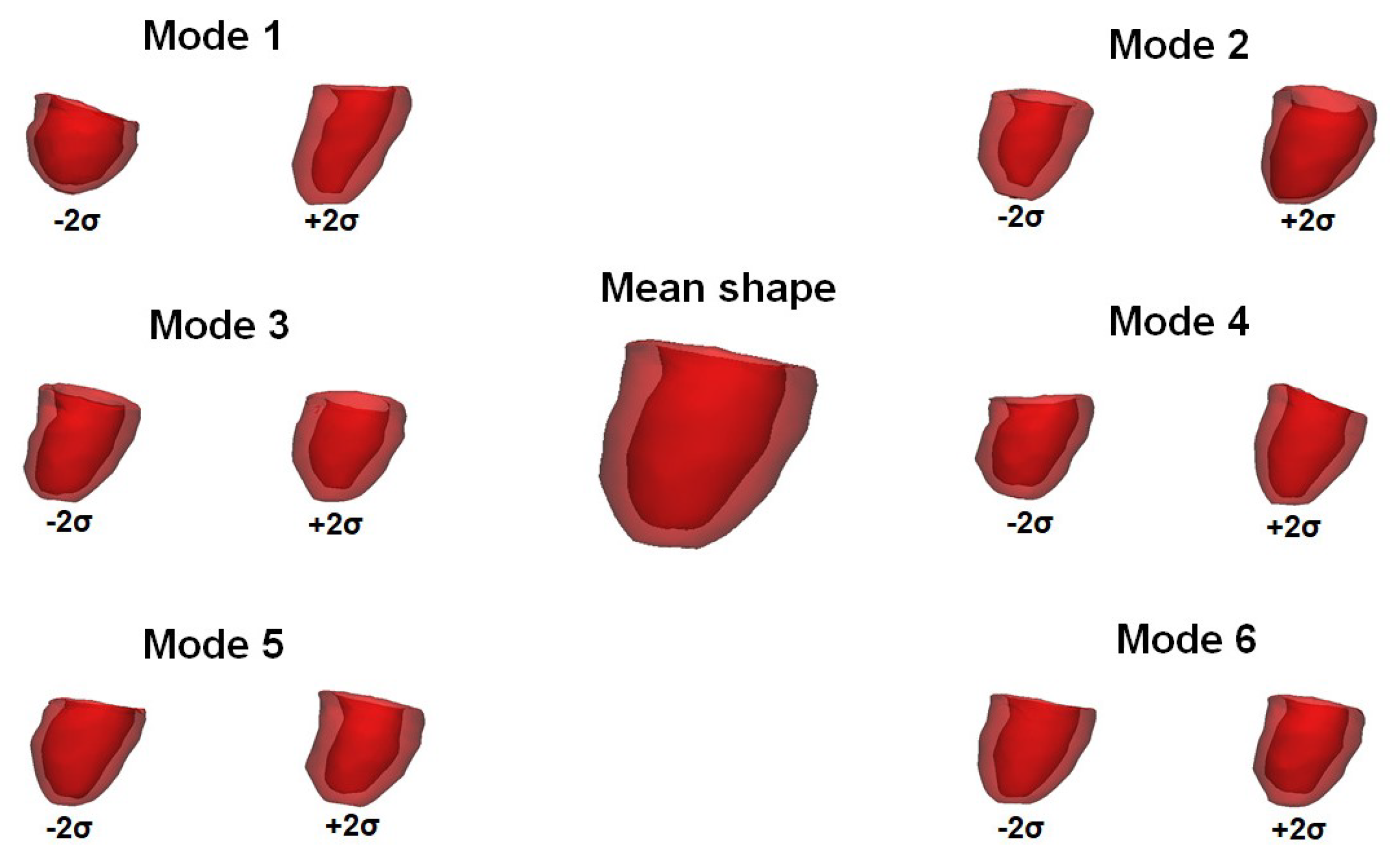
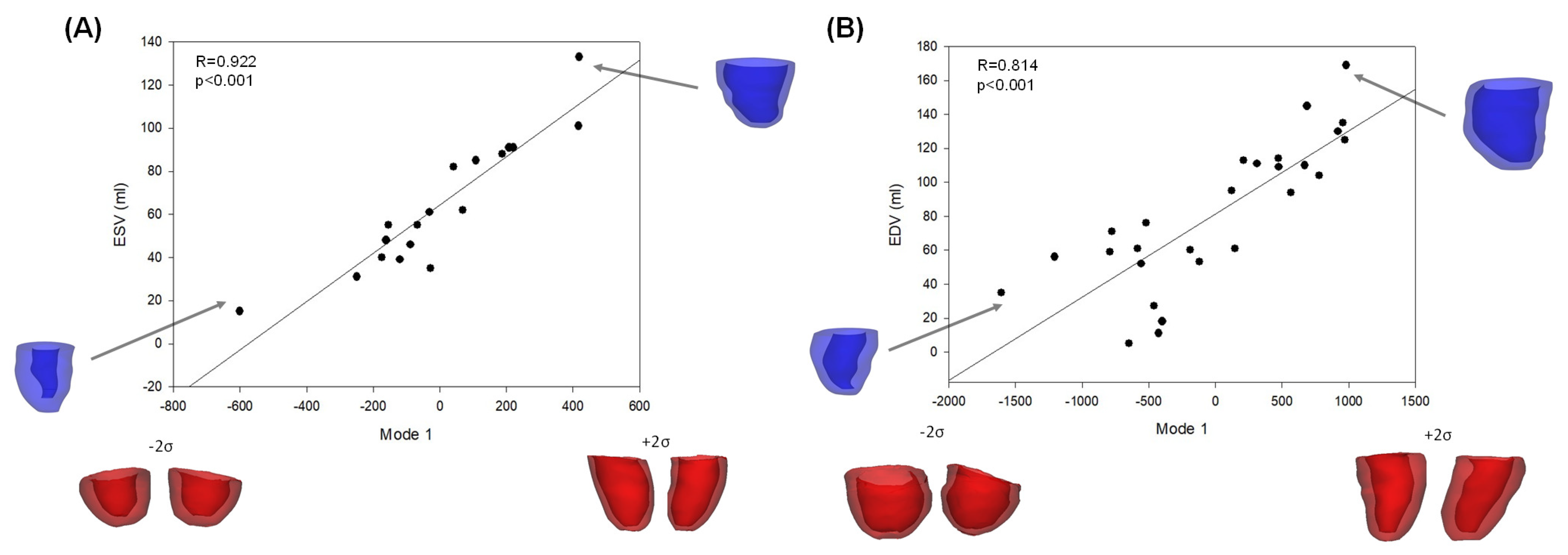
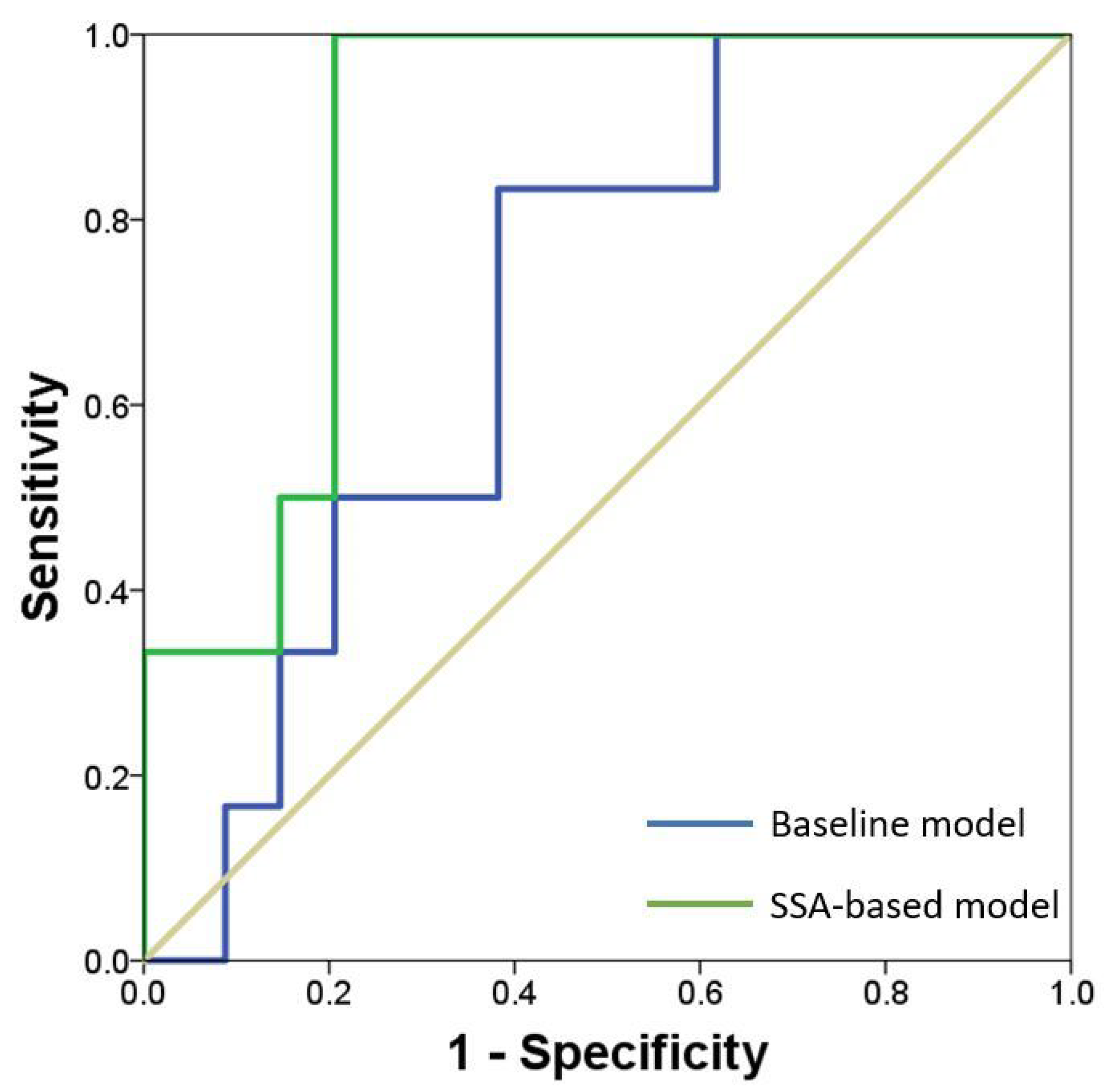
| No Stenosis | Moderate Stenosis | Severe Stenosis | p-Value | |
|---|---|---|---|---|
| Age (years) | * | |||
| BSA () | ||||
| BMI () | ||||
| Heart Rate (bpm) | ||||
| SP (mmHg) | ||||
| DP (mmHg) | ||||
| MAP (mmHg) | ||||
| SV (ml) | ||||
| CO (ml/min) | ||||
| TA flow (m/s) | <0.001 * | |||
| LVMI () | ||||
| EDV (ml) | ||||
| ESV (ml) | ||||
| EF (%) | ||||
| (mmHg) | <0.001 * | |||
| Sample size | 30 | 29 | 27 |
| Parameter | Coefficient | Standard Error | SC | OR | OR 95%CI | |
|---|---|---|---|---|---|---|
| Constant | −9.507 | 2.278 | ||||
| Age | 0.130 | 0.330 | 0.356 | 0.799 | 0.260 | 2.264 |
| Sex | 2.180 | 1.031 | 0.651 | 1.039 | 0.986 | 1.082 |
| Mode 1 * | 0.024 | 0.002 | 2.65 | 1.024 | 1.019 | 1.035 |
| Mode 2 * | −0.012 | 0.003 | −0.589 | 1.011 | 0.984 | 0.992 |
| Mode 3 | −0.002 | 0.004 | −0.091 | 1.038 | 1.035 | 1.038 |
| Mode 4 * | 0.056 | 0.002 | 0.228 | 1.007 | 1.001 | 1.013 |
| Mode 5 | 0.019 | 0.007 | 0.257 | 1.021 | 1.006 | 1.034 |
| Mode 6 * | −0.012 | 0.008 | −0.145 | 0.978 | 0.961 | 1.003 |
| Mode 7 | −0.019 | 0.007 | −0.247 | 1.020 | 1.066 | 1.034 |
| Mode 8 | −0.031 | 0.005 | −0.913 | 1.010 | 1.000 | 1.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutugno, S.; Ingrassia, T.; Nigrelli, V.; Pasta, S. On the Left Ventricular Remodeling of Patients with Stenotic Aortic Valve: A Statistical Shape Analysis. Bioengineering 2021, 8, 66. https://doi.org/10.3390/bioengineering8050066
Cutugno S, Ingrassia T, Nigrelli V, Pasta S. On the Left Ventricular Remodeling of Patients with Stenotic Aortic Valve: A Statistical Shape Analysis. Bioengineering. 2021; 8(5):66. https://doi.org/10.3390/bioengineering8050066
Chicago/Turabian StyleCutugno, Salvatore, Tommaso Ingrassia, Vincenzo Nigrelli, and Salvatore Pasta. 2021. "On the Left Ventricular Remodeling of Patients with Stenotic Aortic Valve: A Statistical Shape Analysis" Bioengineering 8, no. 5: 66. https://doi.org/10.3390/bioengineering8050066
APA StyleCutugno, S., Ingrassia, T., Nigrelli, V., & Pasta, S. (2021). On the Left Ventricular Remodeling of Patients with Stenotic Aortic Valve: A Statistical Shape Analysis. Bioengineering, 8(5), 66. https://doi.org/10.3390/bioengineering8050066







