Abstract
It is widely accepted that chondral defects in articular cartilage of adult joints are never repaired spontaneously, which is considered to be one of the major causes of age-related degenerative joint disorders, such as osteoarthritis. Since mobilization of subchondral bone (marrow) cells and addition of chondrocytes or mesenchymal stromal cells into full-thickness defects show some degrees of repair, the lack of self-repair activity in adult articular cartilage can be attributed to lack of reparative cells in adult joints. In contrast, during a fetal or embryonic stage, joint articular cartilage has a scar-less repair activity, suggesting that embryonic joints may contain cells responsible for such activity, which can be chondrocytes, chondroprogenitors, or other cell types such as skeletal stem cells. In this respect, the tendency of pluripotent stem cells (PSCs) to give rise to cells of embryonic characteristics will provide opportunity, especially for humans, to obtain cells carrying similar cartilage self-repair activity. Making use of PSC-derived cells for cartilage repair is still in a basic or preclinical research phase. This review will provide brief overviews on how human PSCs have been used for cartilage repair studies.
1. Background: Pros and Cons of Adult Chondrocyte- and Adult Stem Cell-Based Cartilage Repair
Joint articular cartilage lacks spontaneous repair activity in adult humans and large animals [1,2], which can be attributed to lack of proper reparative cells and lack of environment for endogenous reparative cells to perform proper repair in the adult joint. The potential lack of endogenous reparative cells in the adult joint would be compensated by addition of chondrocytes or chondrogenic stem/progenitor cells into the joint space or directly to the injury site. In fact, many clinical approaches have been developed to repair joint cartilage injury by providing endogenous or exogenous reparative cells to the injury site. For example, the microfracture [3] and autologous matrix-induced chondrogenesis [4] methods rely on mobilization of endogenous cells, and the autologous chondrocyte implantation (ACI) [5] and matrix-associated ACI (MACI) [6] methods rely on the addition of ex vivo expanded chondrocytes and lately, mesenchymal stromal cells (MSCs). These surgical methods have been successfully applied to repair restricted types of injury, such as focal cartilage injury. However, methods to repair a variety of types of articular cartilage damage, including osteoarthritic wear-and-tear cartilage, which reproducibly achieve long-lasting regeneration of hyaline articular cartilage and prevention of progression to osteoarthritis, have not been developed yet [7,8]. Thus, joint injuries still bring many elderly and even young adults to the path to degenerative joint disorders such as osteoarthritis.
The most natural source of cells to stimulate repair of damaged articular cartilage is articular cartilage itself: i.e., articular chondrocytes. However, isolation of articular chondrocytes needs surgical isolation of healthy articular cartilage pieces from patients, which risks morbidity of the patients, followed by expansion of obtained chondrocytes in culture to yield a clinical scale of cell numbers, which rapidly loses their chondrocytic phenotypes. In contrast, MSCs are mesenchymal cells that can be isolated from a variety of tissues of patients, such as bone, bone marrow, fat, and synovial membrane and fluid [9,10]. They are commonly defined in vitro by their ability to adhere to and grow on plastic, and differentiate into chondrocytes, as well as other lineages such as adipocytes and osteoblasts (tri-lineage potential) under conditions optimized for individual lineages. MSCs are expandable at least for several passages to yield large numbers of them for therapeutic purposes before they lose the chondrogenic potential. Therefore, many MSC-based therapies for damaged articular cartilage, either by injuries or degenerative disorders, are currently investigated in clinical trials [10,11].
Not all MSCs show the same cartilage repair capacity [12]. MSCs from bone marrow and synovium seemed to be better for focal cartilage injury repair than MSCs from fat and muscle. MSCs from bone marrow (and periosteum) are considered to be originated from skeletal stem cells (SSCs) that are bone/bone marrow-resident multipotent skeletogenic cells defined in a way similar for hematopoietic stem cells: i.e., by sub-fractionation of bone and bone marrow cells using fluorescence activated cell sorting (FACS) without expansion culture, and in vivo validation of fractionated cells (e.g., multi-lineage [bone, bone marrow stroma, cartilage, and fat] differentiation in kidney capsule), different from the way MSCs is defined: i.e., expansion culture followed by in vitro validation, as described above [13,14,15,16]. Synovial MSCs are implicated in cartilage homeostasis [17]: e.g., increase in their number in synovial fluid in response to joint destabilization and cartilage degeneration [18,19]. Recently, synovium-resident multipotent skeletogenic cells have also been defined by FACS without expansion culture, although biological validation was performed in vitro [20]. Thus, MSCs from bone marrow and synovium appear to be relevant to bone and cartilage homeostasis [16], but thus far no clear differences between synovial MSCs and bone marrow MSCs in their capacity to form articular-like permanent cartilage and repair focal cartilage injury have been reported [12,21]. Furthermore, the endogenous cartilage reparative cells mobilized by microfracture from the subchondral bone (marrow) are likely to be SSCs [22]. However, as indicated by the repair outcome of microfracture treatment, the repair tissues induced by such endogenous SSCs are generally fibrotic [22], which do not last long, since their physical property is different from that of normal articular cartilage surrounding the repair site. ACI/MACI-like treatment using bone marrow MSCs also tend to result in a similar repair outcome [23,24]. Therefore, no clear differences between bone marrow MSCs and SSCs in their capacity to form articular-like permanent cartilage and repair focal cartilage injury have been noted, either.
2. Biologics for Improving Local Milieu for Endogenous and Exogenous Chondrogenic Cells to Properly Repair Articular Cartilage Damages
One of the strategies toward improving the outcome of exogenous or endogenous reparative cell-based cartilage repair is to optimize the local milieu for such cells to properly rebuild the articular cartilaginous tissue in the damaged site, using factors that are known to directly or indirectly enhance chondrogenesis from MSCs and SSCs in vitro and in vivo. Such factors include transforming growth factor beta (TGF-β) [25,26,27], bone morphogenetic protein (BMP) [28,29,30,31], fibroblast growth factor 18 (FGF18) [32,33], insulin-like growth factor 1 (IGF1) [34,35], and stromal cell-derived factor 1 (SDF1/CXCL12) [36,37]. These factors have been preclinically and clinically tested and resulted in some positive outcomes [8]. However, they also show negative effects on repair outcomes, presumably when they are overexpressed. For example, TGF-β stimulates joint fibrosis [38], BMP induces ectopic ossification, hypertrophic differentiation of repair chondrocytes, and osteophyte formation [38,39], and SDF1 recruits inflammatory cells and is involved in osteoarthritic degeneration of cartilage [40]. Actually, microfracture in a TGF-β-inhibitory environment, created by losartan treatment, showed significant improvement in the repair outcome in rabbits: i.e., hyaline cartilage regeneration [41]. These observations imply that these factors may need to be provided at a proper timing and dose in the vicinity of repair site.
In addition, it has recently been reported that the local milieu suitable for microfracture-activated SSCs to regenerate hyaline cartilage tissue within osteochondral defects is a combination of the anti-angiogenic factor, sFLT1 (soluble form of the vascular endothelial growth factor [VEGF] receptor 1) and BMP2 [22]. The beneficial effect of sFLT1 on stem cell-based hyaline cartilage repair of full-thickness defects was first demonstrated using muscle-derived stem cells (MDSC) in the presence of BMP4 in rats [42,43]. Administration of a neutralizing monoclonal antibody against VEGF alone also resulted in repair of full-thickness injury of articular cartilage with a hyaline cartilage tissue [44], and slowed the degradation of anterior crucial ligament-transected joint cartilage [45] in rabbits. Furthermore, an anti-angiogenic environment controls the direction of differentiation of SSCs. For example, sFLT1 converted SSC’s fate from bony tissue formation to cartilaginous tissue formation in a kidney capsule [14,15], and sFLT1-dependent induction of spontaneous chondrogenesis was noted in MSCs and in adipose tissue [14,46] in mice. However, considering that side effects are anticipated by systemic anti-VEGF treatments [47], and that transient presence of endothelial cells specifically in the mesenchymal condensation stage facilitates subsequent chondrogenesis [48], the anti-angiogenic environment may need to be provided locally at a proper timing during the repair process [49].
Thus, attempts to manipulate local milieu for the endogenous or exogenous therapeutic cells using known biologics have led to significant benefits in regeneration of hyaline cartilage in repair sites, which are however mostly based on relatively short-term (4–12 weeks) observations. Therefore, some of the regenerated hyaline cartilaginous tissues may not be permanent cartilage in that in a longer term, chondrocytes in the repair site may die or be committed to endochondral ossification: i.e., hypertrophic differentiation, terminal maturation and mineralization, leading to the degradation of repair tissue [50] and the formation of osteophytes [51]. Interestingly, death of superficial chondrocytes protects articular cartilage from joint destabilization-induced degradation [52], but loss of maturation arrest in living articular chondrocytes is associated with the onset of osteoarthritis [53]. In this respect, use of parathyroid hormone-related peptide (PTHrP), preferred ligands for the FGF receptor 3 (FGFR3), such as FGF9 and FGF18, and sFLT1 may have a beneficial effect on the maturation arrest of articular chondrocytes, since PTHrP [54,55,56] and FGF18 [57] are expressed in articular cartilage, which are known to block chondrocyte hypertrophic differentiation [58], and VEGF that sFLT1 targets is essential for chondrocytes to terminally mature and mineralize [49,59,60]. These factors have been tested for their roles on protection of articular cartilage from osteoarthritic degeneration [45,57,61,62], as well as on repair of damaged articular cartilage [22,42,43,44,63,64,65], but their effects on permanent repair has not been demonstrated. Much improvements have also been made to scaffold or hydrogel technology to provide a suitable micromilieu, in which embedded MSCs survive and differentiate [66], but optimization has been aimed mostly at enhancing chondrogenesis, rather than at preventing terminal maturation, mineralization, and degradation of the resultant chondrocytes [67].
Thus, robust, biologics-induced chondrogenesis in vivo may be helpful in inducing hyaline cartilage repair tissue, instead of fibrotic repair tissue [68,69,70], but further optimization of the therapeutic strategy to develop articular-type permanent cartilage, for example by providing suppressive environment for chondrocyte maturation, has not been extensively investigated. It is worth mentioning that more extensive searches for therapeutic molecules, which involve comparative genetic, genomic, epigenomic and proteomic analyses of intrajoint tissues such as articular cartilage, ligament, synovium, and synovial fluid [71], have nominated a number of genes and proteins expressed in the intrajoint tissues in a disease condition-dependent manner. Validating effects of such genes and proteins on cartilage repair is still ongoing.
3. Potential Advantage of Embryonic Chondroprogenitors, as an Alternative Cell Type for Cartilage Repair
Another strategy to improve the outcome of cell-based cartilage repair is to take the age of reparative cells into account. In contrast to adult joint cartilage, embryonic/fetal joint cartilage possesses spontaneous scar-less repair activity for a chondral (partial-thickness) defect [72]. In small animals, such activity continues to the postnatal infant stage. For example, 3 week-old infant rats [73,74,75], and 12–14 week-old young rabbits [76,77,78] spontaneously repair partial-thickness defects of joint articular cartilage, but these activities disappear in the adulthood. Full-thickness and osteochondral defects show some spontaneous repair activity even in adult animals, but repair tissues are normally fibrotic, similar to the repair outcome of the microfracture method [3,22]. However, still the younger the better, since full-thickness and osteochondral defects in fetal [79] (Figure 1B) and adolescent animals (e.g., 6 week-old rats and 13–15 week-old rabbits) [77,78,80,81,82], show faster and hyaline cartilage repair, dependent on the size of injury.
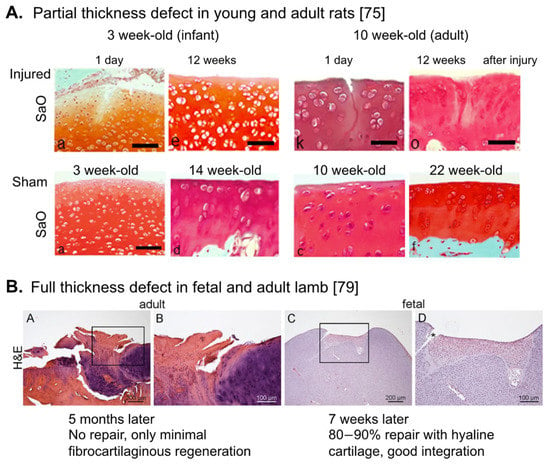
Figure 1.
Repair activity in the fetal, young and adult articular cartilage. Histological changes of articular cartilage after introducing partial-thickness injury (A) and full-thickness injury (B) in the fetal (B), young/infant (A), and adult (A,B) animals. (A) Taken from Akatsu, et al. [75]. Note that hyaline cartilage repair with good integration was observed in 3 week-old infant rats. (B) Taken from Ribitsch, et al. [79]. SaO: Safranin O, H&E: Haematoxylin-Eosin staining.
During embryogenesis, the entire synovial joint, including articular cartilage, ligament and synovium, develops de novo from a specific group of progenitor cells called interzone cells or joint progenitors, that are marked by the Growth and Differentiation Factor 5 (Gdf5) transcript [83,84]. These cells are distinct from the Sry-box (Sox) 9-expressing “general” chondroprogenitors [85], destined to primarily generate growth plate chondrocytes. GDF5 is a member of the BMP family, expressed in articular chondrocytes and needed for maintenance of articular cartilage [86,87,88,89]. Interestingly, the mouse Lgr5+Gdf5+ interzone cells facilitate repair of osteochondral defects of adult articular cartilage [90]. Recently, genetic lineage tracing experiments have demonstrated that progeny of the embryonic Gdf5+ interzone cells (‘Gdf5-lineage’ cells) remain in the synovium of the adult mouse joint, that synovial MSCs are mostly originated from such Gdf5-lineage cells, and that osteochondral defects of articular cartilage stimulate proliferation of and chondrogenesis from the Gdf5-lineage cells, and recruit them to the defect site [91]. These observations suggest that the cartilage self-repairing activity in the joint of embryonic and infant rodents and rabbits may be elicited by the synovial Gdf5-lineage cells.
Similar lineage tracing experiments have also demonstrated that Prg4 (Lubricin)+ cells of embryonic (E14.5) to newborn (P0-5) mouse articular cartilage appear to represent the articular cartilage stem/progenitor cells enriched in the superficial zone of articular cartilage [92], and contribute to articular cartilage growth by populating chondrocytes in all layers of articular cartilage till the infant stage in mice [93,94,95]. Therefore, Prg4+ embryonic and infant superficial zone chondrocytes can also contribute to the cartilage self-repairing activity in embryonic and infant joints, and may be able to “repopulate” articular chondrocytes in adult articular cartilage if provided exogenously.
While injury-induced micromilieu for endogenous reparative cells in embryonic and infant joints may be different from that in adult joints, and may play an important role on the spontaneous repair of articular cartilage defects, these observations suggest that SSCs [13,14,15,22,96], synovial Gdf5-lineage cells [91], Prg4+ superficial zone chondrocytes [92,93,94,95], and Gdf5+ interzone/joint progenitor cells [90] in embryonic and infant joints: i.e., young reparative cells, may be a better alternative to adult MSCs and SSCs to achieve the therapeutic goal: i.e., repair of articular cartilage injury with long-lasting hyaline cartilage tissues leading to prevention of injured cartilage from progressing to osteoarthritis.
4. Pluripotent Stem Cells as a Source for Embryonic Articular Chondroprogenitors for Humans
Thus, the embryonic and infant chondroprogenitors and chondrocytes may be interesting cell-types to test for their capability of long-term hyaline cartilage repair in adult articular cartilage, but these cells are not easily obtained from humans in a clinical quantity. In contrast, pluripotent stem cells (PSC) such as embryonic stem cells (ESC) and induced PSCs (iPSC) are capable of differentiating into all somatic cells, through processes that mimic early embryogenesis, and the resulting cells tend to carry embryonic characteristics. PSCs can be expanded in culture almost indefinitely, too. Therefore, for humans, PSCs are the only practical source for obtaining large numbers of embryonic/fetal cell-types. Methods to generate embryonic chondrocytes as well as embryonic chondroprogenitors from mouse (m) and human (h)PSCs have been established by many groups, which have been reviewed previously [97,98]. We have also previously established and refined signaling requirements for the differentiation of PSCs to three embryonic precursors of chondrocytes, namely lateral plate mesoderm, paraxial mesoderm, and (cranial) neural crest [99,100,101,102,103,104,105,106,107]. Interestingly, we and others have also shown that hPSC-derived chondrogenic cells of the mesodermal origin gave rise to hyaline cartilage pellets in vitro [104], which were maintained to some extent as an unmineralized state in vivo, especially when BMP signaling was limited in a late stage of the in vitro chondrogenesis culture [106,108,109]. These observations suggest that PSC-derived chondrogenic mesodermal cells may contain progeny that are committed to generate permanent chondrocytes: i.e., chondrocytes that resist endochondral ossification, the process which stimulates chondrocyte hypertrophy, terminal maturation, and mineralization to form bone as in the growth plate.
The PSC-derived chondrogenic mesenchymal cells can be expanded in a serum-containing medium or in a specialized serum-free medium (e.g., FGF2 + TGF-β receptor inhibitor) [98]. When expanded under the serum-free condition, hPSC-derived chondroprogenitors well maintain their hyaline chondrogenic activity for over 15 passages [106], but chondrocytes developed from such expanded cells acquire tendency to commit themselves to the endochondral ossification process: i.e., cartilage pellets developed with them express signs of hypertrophic differentiation (e.g., transcripts of the type X collagen and alkaline phosphatase genes) in vitro and readily form a bony tissue in vivo, similar to adult MSC-derived cartilage pellets [70,110,111]. These observation suggest that chondroprogenitors, generated in culture from mesodermal progeny of hPSCs and expanded in a way to maintain long-term their hyaline chondrogenic activity, somehow lose their capacity to form permanent chondrocytes.
5. Development and Isolation of Chondrogenic Cells from Pluripotent Stem Cells
There is a report that full-thickness defects of sheep articular cartilage were successfully repaired by providing undifferentiated sheep ES-like cells in a fibrin glue [112]. However, PSCs are tumorigenic, i.e., teratoma-forming cells, and the teratoma-forming activity has been the definition of pluripotency for hPSCs [113,114,115]. Therefore, lineage-restricted progenitor cells differentiated from PSCs are considered more suitable for therapeutic purposes than PSC themselves, but risk of contamination of tumor forming, undifferentiated PSCs in the differentiated PSC population remains [116,117]. In fact, when hPSCs, especially hiPSCs, are differentiated into chondrocytes or chondroprogenitors that are used without a step to purify them or their precursors by physical methods: e.g., FACS and magnetic-activated cell sorting (MACS), or by biological methods: e.g., expanding specifically the differentiated cell-type of interest in culture, immature teratoma-like tumor is developed in cartilage mass generated from them in vitro [118], and in an immunodeficient mouse knee after transplantation of them for 16 weeks [119]. Therefore, the safest way to regenerate cartilage using hPSCs is to include a step in the protocol to physically or biologically eliminate the tumor forming, undifferentiated PSCs, prior to transplantation.
In early studies, biological methods: e.g., selective expansion culture, were mainly employed for enriching or purifying chondrogenic mesenchymal cells or MSCs [98]. PSCs were differentiated by way of forming embryoid bodies (EB) in vitro. Then, mesenchymal cells growing out of EBs, called EB outgrowth cells, were selectively expanded in media similar to those developed for expanding bone marrow MSCs, prior to induction of chondrogenesis and use for cartilage repair analyses [98].
Recent studies tend to make use of antibody-based physical separation methods to enrich PSC-derived chondroprogenitor cells or their precursors such as PSC-derived mesoderm or neural crest (Figure 2). For example, FACS-isolated VEGF receptor 2 (FLK1/KDR)− platelet-derived growth factor receptor alpha (PDGFRα)+ EB cells are chondrogenic mesodermal progeny of PSCs [99,100,104,105]. The hPSC-derived mesodermal progeny, enriched by FACS-isolation of KDR−CD146+CD166+ BMP receptor 1B (BMPR1B)−/lo cells, or by MACS-depletion of contaminated epithelial endodermal, cardiovascular, and hematoendothelial mesodermal cells, as well as undifferentiated hPSCs, are chondrogenic [120,121]. Furthermore, FACS-isolated green fluorescence protein (GFP)+ cells from the type II collagen gene (Col2a1) promoter-GFP knocked-in PSCs are enriched in chondrogenic progeny [122,123], and FACS/MACS-purified CD271+ hPSC-derived neural crest cells generate chondrogenic ectomesenchymal cells [106,124,125].
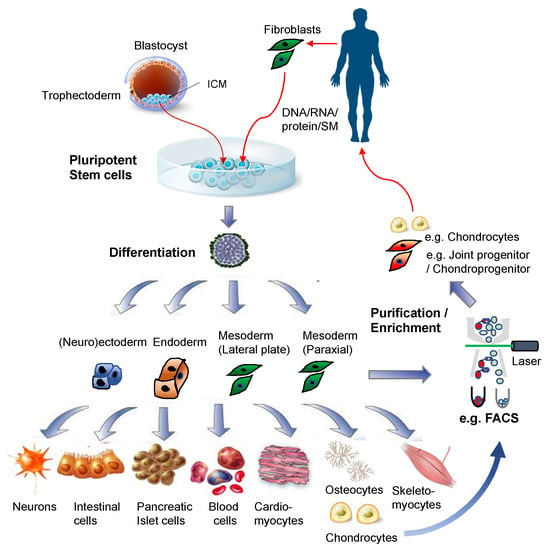
Figure 2.
Pluripotent stem cells for regenerative medicine. Illustrations for the blastocyst, pluripotent stem cells, and differentiated cells were purchased from Dreamstime.com.
As for hPSC-derived MSCs, surface markers such as CD73, CD24, CD105, and CD90 have been used for detecting and isolating them by FACS, as reviewed in [98]. However, since MSCs can be relatively easily generated via spontaneous differentiation of hPSCs, and enriched by expansion culture in the standard, serum-containing MSC medium, FACS/MACS is not widely employed for purifying or enriching PSC-derived MSCs. However, the developmental process of mesodermal MSCs from hPSCs was first defined by Slukvin’s group using FACS isolation of mesodermal progeny [126]. Their method generates Apelin receptor+ mesoderm (that is PDGFRα+KDR+ and Lin- [VE-cadherin-CD31−CD73−CD43−CD45−], and expresses T, MIXL1, and FOXF1: i.e., primitive streak and lateral plate mesoderm transcripts) from hPSCs, isolates them by MACS, and subjects them to mesenchymal colony forming culture to generate PDGFRβ+ CD271+Delta-like1(DLK1)+CD73− primitive mesenchymal cells (expressing PRRX1: i.e., limb bud mesenchyme transcript). Then, PDGFRβ+CD73+CD90+ MSCs are generated from them in the presence of FGF2 in a serum-free medium [127].
6. Cartilage Tissue Engineering Using Pluripotent Stem Cell-Derived Chondroprogenitors
Use of PSC-derived chondrogenic cells for articular cartilage repair has not been extensively performed. Many early studies employed methods to generate chondrogenic mesenchymal cells or MSCs (e.g., EB outgrowth cells) from spontaneously differentiated PSCs, expand and prime them, and then use them for repairing damaged articular cartilage. Hwang et al. [128] and Toh et al. [129,130] have convincingly demonstrated, using this strategy, that hESC-derived EB outgrowth cells are capable of repairing damaged articular cartilage at least up to 12 weeks, when the cells were either embedded in a hyaluronan-hydrogel followed by pre-differentiated toward chondrocytes for 4 weeks in the presence of BMP7 and TGF-β1 [129,130], or expanded in chondrocyte-conditioned medium, followed by pellet cultured for 3 days [128], prior to transplantation. Similarly, Gibson, et al. has demonstrated that use of MSCs, which had been generated by a 2-dimensional, spontaneous differentiation method of hESCs and pellet cultured with BMP2 for 2 days and then with WNT5a for 12 days, showed statistically significant improvements in the repair of damaged articular cartilage [131]. In contrast, EB outgrowth cell-derived MSCs that had been complexed with poly(lactic-co-glycolide) scaffold and transplanted to full-thickness defects of rabbit articular cartilage without any pre-treatments, such as chondrogenic differentiation or chondrocyte-conditioned medium treatment, showed only a weak repair [132]. Effects of various biomaterials have also been explored but mostly in vitro, which have been reviewed elsewhere [103,133].
More refined lineage-restricted (e.g., mesodermal) chondrogenic mesenchymal cells were also used for cartilage repair. Ferguson, et al. [120] identified cell surface markers that can be used for identifying and isolating chondrocytes from different locations in human fetal articular cartilage. The integrin alpha 4 (IGTA4)− BMPR1B+ chondrocytes that demonstrate the strongest matrix-depositing activity are form transitional zone, and the IGTA4+BMPR1B+ chondrocytes that show osteochondrogenic activity and PRG4 expression are from superficial zone. Interestingly, when mesodermal progeny of hPSCs generated based on the method of Wu, et al. [121] were purified by MACS-depletion of epithelial endodermal cells, cardiovascular and hematoendothelial mesodermal cells as well as undifferentiated hPSCs, and then differentiated by pellet culture for 60 days, the resulting cartilage pellets were enriched in IGTA4+BMPR1B- mesenchymal cells, with a minor population of IGTA4+BMPR1B+ superficial chondrocytes. These cartilage pellets were capable of repairing a focal lesion of rat articular cartilage in as soon as 30 days [120].
Similarly, Gardner, et al. [134] reported hPSC-derived mesodermal cartilage tissue also repair a focal osteochondral defects of articular cartilage in nude rats. They employed Craft et al.’s method of mesodermal differentiation of hPSCs [108], followed by EB outgrowth cell generation and expansion for 12 days in a serum-free medium to get chondrogenic mesenchymal cells. Then, these cells were subjected to TGF-β3-based micro-mass culture for 12–15 weeks to generate cartilage mass that was used to fill the osteochondral defects. The quantitative analyses of repair outcome based on the ICRSII scoring system showed statistically significant improvement 12 weeks, but not 6 weeks after transplantation of the hPSC-derived cartilage mass.
The first demonstration of significant cartilage repair by hPSC-derived chondrogenic progeny, without pre-differentiation or chondrocyte-condition medium treatment prior to transplantation, was reported by Cheng et al. [135]. Their method gives rise to SOX9+ chondroprogenitors and chondrocytes via mesodermal progeny of hESCs, based on Oldershaw et al.’s 2-dimmensional hESC differentiation method [136] that has been improved to bring the SOX9+ cell population up from 75 to 95%, by removing the day-12 obligated split during chondrogenesis stage of differentiation culture. These SOX9+ cells encapsulated in fibrin glue resulted in better repair outcome than spontaneous repair of a focal osteochondral defect of articular cartilage in nude rats from 4 to 12 weeks [135].
More direct roles of PSC-derived chondroprogenitors or chondrocytes on repairing a damage of articular cartilage were demonstrated by organ culture systems. Diekman, et al. [123] showed that Col2a1-GFP+ cells isolated from differentiating mPSCs by FACS and embedded in 1% agarose were capable of regenerating cartilage matrices within a chondral defect introduced in pig explant cartilage in 21 days of culture. In addition, Wu, et al. [121] demonstrated that FACS-purified CD166−/lo BMPR1B+ prechondrocytic cells, which had been generated by a 12–15-day chondrogenesis culture of CD166+CD146+KDR−/loEpCAM-BMPR1B−/lo hPSC-derived mesodermal cells in the presence of TGF-β1 and Leukemia Inhibitory Factor, contributed to repair defects introduced into a human fetal hip joint explant in 14 days of culture. These observations suggest the capacity of PSC-derived chondroprogenitors or chondrocytes to retain in defects sites of articular cartilage and regenerate cartilage matrices.
7. Conclusions and Future Prospective
PSC-derived MSCs, chondroprogenitors and chondrocytes have thus far given positive results in repairing focal full-thickness lesions in articular cartilage, using scaffold-free or scaffold/hydrogel-dependent methods, in small animal models and in organ culture models. However, their effects on age-related cartilage degenerative disorders such as osteoarthritis have not been extensively examined, while the beneficial effects of adult MSCs on osteoarthritis are now recognized, which is based on their trophic (e.g., anti-inflammatory) effects rather than their chondrogenic potential [10,137].
One of the major advantages of the iPSC technology on clinical application is to be able to get patient specific, “rejuvenated” cells [138,139,140,141] (Figure 3). Currently, it requires technically demanding two-step processes: i.e., gene/RNA/protein transfer or small molecule treatment to effectively reprogram adult cells to PSCs, followed by directed differentiation of the obtained PSCs to the cell-type of interest. In contrast, the “direct reprogramming” technology depends on one step process: i.e., gene/RNA/protein transfer, and is capable of inducing somatic cells, such as fibroblasts, to transdifferentiate to another type of somatic cells, such as neurons [142], osteoblasts [143], and chondrocytes [144], without going through the PSC stage. Therefore, this method will also eliminate the concern of contamination of teratoma-forming activity. However, such direct reprogramming technology seems to transfer the same problems or risks associated with the age of original somatic cells over to the reprogrammed cells [139,145]. The aging of adult stem cells has been noted, and attributed to the diminished regenerative activity in aged adult tissues [139,146,147]. Therefore, whenever rejuvenated stem cells are expected to show improved clinical outcome from a cell-based regenerative therapy, patient-specific hPSC-derived cells are likely suitable over cells isolated from patients or those directly reprogrammed. In this respect, while adult MSC-based therapies for injured or degenerated articular cartilage are currently being trialed [10,11], it would be of great interest in examining whether patient-specific hiPSC-derived embryonic MSCs and SSCs, or chondroprogenitors will result in better repair outcome than adult MSCs.
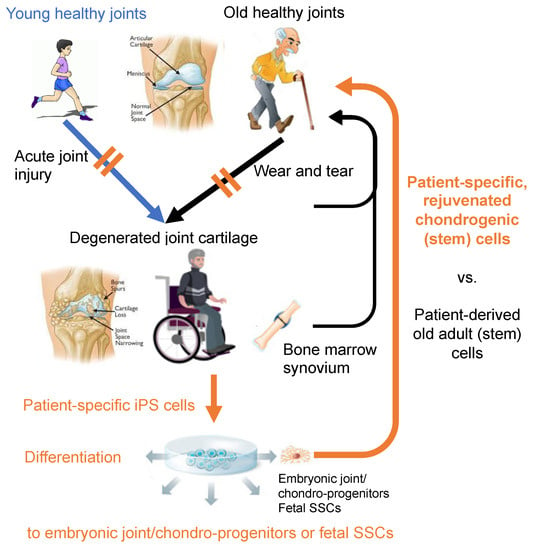
Figure 3.
In total, two sources of patient-specific cells for regeneration of injured and degenerated cartilage: adult cells vs. rejuvenated (i.e., iPSC-derived) cells. Does rejuvenation make difference in the outcome of joint cartilage repair? The joint illustrations are in courtesy of OrthoInfo © American Academy of Orthopaedic Surgeons. Other illustrations were purchased from Dreamstime.com.
Author Contributions
Conceptualization, writing and revision of the manuscript, N.N.; Review and editing, S.R. and J.H.; Funding acquisition, N.N. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
N.N. has been funded by Annie and Bob Graham Distinguished Chair in Stem Cell Biology, and NIH (1R01AR077045-01), and J.H. has been funded by NIH (1UG3AR077748-01, 1R21AR072870-01, 1R21AR073509-01, 1R01AR065445-01, 1R21AR075997-01, 1R21AR074132-01), and DOD (N00014-18-RFI-0014).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage repair and transplantation. Arthritis Rheum. 1998, 41, 1331–1342. [Google Scholar] [CrossRef]
- Mandelbaum, B.R.; Browne, J.E.; Fu, F.; Micheli, L.; Mosely, J.B., Jr.; Erggelet, C.; Minas, T.; Peterson, L. Articular cartilage lesions of the knee. Am. J. Sports Med. 1998, 26, 853–861. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef] [PubMed]
- Benthien, J.P.; Behrens, P. Autologous Matrix-Induced Chondrogenesis (AMIC): Combining Microfracturing and a Collagen I/III Matrix for Articular Cartilage Resurfacing. Cartilage 2010, 1, 65–68. [Google Scholar] [CrossRef]
- Brittberg, M. Autologous chondrocyte transplantation. Clin. Orthop. Relat. Res. 1999, 367, S147–S155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.H.; Willers, C.; Kirilak, L.; Yates, P.; Xu, J.; Wood, D.; Shimmin, A. Matrix-induced autologous chondrocyte implantation (MACI): Biological and histological assessment. Tissue Eng. 2007, 13, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Martin, A.R.; Patel, J.M.; Zlotnick, H.M.; Carey, J.L.; Mauck, R.L. Emerging therapies for cartilage regeneration in currently excluded ‘red knee’ populations. Npj Regen. Med. 2019, 4, 12. [Google Scholar] [CrossRef]
- Bianco, P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef]
- Barry, F. MSC Therapy for Osteoarthritis: An Unfinished Story. J. Orthop. Res. 2019, 37, 1229–1235. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Suire, C.; Brouard, N.; Hirschi, K.; Simmons, P.J. Isolation of the stromal-vascular fraction of mouse bone marrow markedly enhances the yield of clonogenic stromal progenitors. Blood 2012, 119, e86–e95. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and specification of the mouse skeletal stem cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Longaker, M.T.; Chan, C.K.F. A Revised Perspective of Skeletal Stem Cell Biology. Front. Cell Dev. Biol. 2019, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. 2020, 11, 381. [Google Scholar] [CrossRef]
- Morito, T.; Muneta, T.; Hara, K.; Ju, Y.J.; Mochizuki, T.; Makino, H.; Umezawa, A.; Sekiya, I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology 2008, 47, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Ojima, M.; Suzuki, S.; Yamaga, M.; Horie, M.; Koga, H.; Tsuji, K.; Miyaguchi, K.; Ogishima, S.; Tanaka, H.; et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J. Orthop. Res. 2012, 30, 943–949. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Koevoet, W.; Hakimiyan, A.A.; Sande, M.; Farrell, E.; Hoogduijn, M.J.; Verhaar, J.A.N.; Chubinskaya, S.; Buhring, H.J.; Van Osch, G. Cell-surface markers identify tissue resident multipotential stem/stromal cell subsets in synovial intimal and sub-intimal compartments with distinct chondrogenic properties. Osteoarthr. Cartil. 2019, 27, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004, 50, 142–150. [Google Scholar] [CrossRef]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okabe, T.; Ikawa, T.; Iida, T.; Yasuda, H.; Nakamura, H.; Wakitani, S. Articular cartilage repair with autologous bone marrow mesenchymal cells. J. Cell Physiol. 2010, 225, 291–295. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Kim, I.L.; Pfeifer, C.G.; Fisher, M.B.; Saxena, V.; Meloni, G.R.; Kwon, M.Y.; Kim, M.; Steinberg, D.R.; Mauck, R.L.; Burdick, J.A. Fibrous Scaffolds with Varied Fiber Chemistry and Growth Factor Delivery Promote Repair in a Porcine Cartilage Defect Model. Tissue Eng. Part A 2015, 21, 2680–2690. [Google Scholar] [CrossRef]
- Lee, C.H.; Cook, J.L.; Mendelson, A.; Moioli, E.K.; Yao, H.; Mao, J.J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet 2010, 376, 440–448. [Google Scholar] [CrossRef]
- Lee, B. Invossa, a first-in-class of cell and gene therapy for osteoarthritis treatment: The phase III trial. Osteoarthr. Cartil. 2018, 26, S43–S44. [Google Scholar] [CrossRef]
- Lu, S.; Lam, J.; Trachtenberg, J.E.; Lee, E.J.; Seyednejad, H.; Van den Beucken, J.; Tabata, Y.; Wong, M.E.; Jansen, J.A.; Mikos, A.G.; et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 2014, 35, 8829–8839. [Google Scholar] [CrossRef]
- Cheng, Z.; Landish, B.; Chi, Z.; Nannan, C.; Jingyu, D.; Sen, L.; Xiangjin, L. 3D printing hydrogel with graphene oxide is functional in cartilage protection by influencing the signal pathway of Rank/Rankl/OPG. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Crecente-Campo, J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M. New scaffolds encapsulating TGF-beta3/BMP-7 combinations driving strong chondrogenic differentiation. Eur. J. Pharm. Biopharm. 2017, 114, 69–78. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, H.; Awada, H.; Tang, Y.; Amra, S.; Lu, A.; Sun, X.; Lv, G.; Huard, C.; Wang, B.; et al. A comparison of BMP2 delivery by coacervate and gene therapy for promoting human muscle-derived stem cell-mediated articular cartilage repair. Stem Cell Res. 2019, 10, 346. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Hellot, S.; Dreher, D.; Krantz, E.F.; Kruger, D.S.; Guermazi, A.; Eckstein, F. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014, 66, 1820–1831. [Google Scholar] [CrossRef]
- Howard, D.; Wardale, J.; Guehring, H.; Henson, F. Delivering rhFGF-18 via a bilayer collagen membrane to enhance microfracture treatment of chondral defects in a large animal model. J. Orthop. Res. 2015, 33, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.K.; Cieply, R.D.; Gupta, G.; Milbrandt, T.A.; Puleo, D.A. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J. Tissue Eng. Regen. Med. 2015, 9, E202–E209. [Google Scholar] [CrossRef] [PubMed]
- Florine, E.M.; Miller, R.E.; Liebesny, P.H.; Mroszczyk, K.A.; Lee, R.T.; Patwari, P.; Grodzinsky, A.J. Delivering heparin-binding insulin-like growth factor 1 with self-assembling peptide hydrogels. Tissue Eng. Part A 2015, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Brouillette, M.J.; Seol, D.; Zheng, H.; Buckwalter, J.A.; Martin, J.A. Use of recombinant human stromal cell-derived factor 1alpha-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol. 2015, 67, 1274–1285. [Google Scholar] [CrossRef]
- Zhang, F.; Leong, W.; Su, K.; Fang, Y.; Wang, D.A. A transduced living hyaline cartilage graft releasing transgenic stromal cell-derived factor-1 inducing endogenous stem cell homing in vivo. Tissue Eng. Part A 2013, 19, 1091–1099. [Google Scholar] [CrossRef]
- Steinert, A.F.; Ghivizzani, S.C.; Rethwilm, A.; Tuan, R.S.; Evans, C.H.; Noth, U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. 2007, 9, 213. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Villalvilla, A.; Gomez, R.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. SDF-1 signaling: A promising target in rheumatic diseases. Expert Opin. Targets 2014, 18, 1077–1087. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Gao, X.; Deng, Z.; Cheng, H.; Nakama, G.; Scibetta, A.C.; Ravuri, S.K.; Goldman, J.L.; Lowe, W.R.; Rodkey, W.G.; et al. Biologically Regulated Marrow Stimulation by Blocking TGF-beta1 With Losartan Oral Administration Results in Hyaline-like Cartilage Repair: A Rabbit Osteochondral Defect Model. Am. J. Sports Med. 2020, 48, 974–984. [Google Scholar] [CrossRef]
- Kubo, S.; Cooper, G.M.; Matsumoto, T.; Phillippi, J.A.; Corsi, K.A.; Usas, A.; Li, G.; Fu, F.H.; Huard, J. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009, 60, 155–165. [Google Scholar] [CrossRef]
- Matsumoto, T.; Cooper, G.M.; Gharaibeh, B.; Meszaros, L.B.; Li, G.; Usas, A.; Fu, F.H.; Huard, J. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009, 60, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Sato, M.; Kutsuna, T.; Kokubo, M.; Ebihara, G.; Ohta, N.; Mochida, J. Intravenous administration of anti-vascular endothelial growth factor humanized monoclonal antibody bevacizumab improves articular cartilage repair. Arthritis Res. 2010, 12, R178. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Sato, M.; Kobayashi, M.; Yokoyama, M.; Tani, Y.; Mochida, J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res. 2014, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Marsano, A.; Medeiros da Cunha, C.M.; Ghanaati, S.; Gueven, S.; Centola, M.; Tsaryk, R.; Barbeck, M.; Stuedle, C.; Barbero, A.; Helmrich, U.; et al. Spontaneous In Vivo Chondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells by Blocking Vascular Endothelial Growth Factor Signaling. Stem Cells Transl. Med. 2016, 5, 1730–1738. [Google Scholar] [CrossRef]
- Wu, J.M.; Staton, C.A. Anti-angiogenic drug discovery: Lessons from the past and thoughts for the future. Expert Opin. Drug Discov. 2012, 7, 723–743. [Google Scholar] [CrossRef]
- Takebe, T.; Kobayashi, S.; Suzuki, H.; Mizuno, M.; Chang, Y.M.; Yoshizawa, E.; Kimura, M.; Hori, A.; Asano, J.; Maegawa, J.; et al. Transient vascularization of transplanted human adult-derived progenitors promotes self-organizing cartilage. J. Clin. Investig. 2014, 124, 4325–4334. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Min. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Wang, J.; Caldwell, K.L.; Lu, Q.; Feng, Y.; Barnthouse, N.C.; Miller, A.H. NFAT1 deficiency provokes hypertrophic repair of articular cartilage defects and progression of posttraumatic osteoarthritis. Osteoarthr. Cartil. 2016, 24, S19. [Google Scholar] [CrossRef]
- Steinert, A.F.; Noth, U.; Tuan, R.S. Concepts in gene therapy for cartilage repair. Injury 2008, 39 (Suppl. 1), S97–S113. [Google Scholar] [CrossRef]
- Zhang, M.; Mani, S.B.; He, Y.; Hall, A.M.; Xu, L.; Li, Y.; Zurakowski, D.; Jay, G.D.; Warman, M.L. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J. Clin. Investig. 2016, 126, 2893–2902. [Google Scholar] [CrossRef]
- Minguzzi, M.; Cetrullo, S.; D’Adamo, S.; Silvestri, Y.; Flamigni, F.; Borzì, R.M. Emerging Players at the Intersection of Chondrocyte Loss of Maturational Arrest, Oxidative Stress, Senescence and Low-Grade Inflammation in Osteoarthritis. Oxid. Med. Cell Longev. 2018, 2018, 3075293. [Google Scholar] [CrossRef]
- Tsukazaki, T.; Ohtsuru, A.; Enomoto, H.; Yano, H.; Motomura, K.; Ito, M.; Namba, H.; Iwasaki, K.; Yamashita, S. Expression of parathyroid hormone-related protein in rat articular cartilage. Calcif. Tissue Int. 1995, 57, 196–200. [Google Scholar] [CrossRef]
- Chen, X.; Macica, C.M.; Nasiri, A.; Broadus, A.E. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008, 58, 3788–3797. [Google Scholar] [CrossRef]
- Fischer, J.; Dickhut, A.; Rickert, M.; Richter, W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010, 62, 2696–2706. [Google Scholar] [CrossRef]
- Mori, Y.; Saito, T.; Chang, S.H.; Kobayashi, H.; Ladel, C.H.; Guehring, H.; Chung, U.I.; Kawaguchi, H. Identification of fibroblast growth factor-18 as a molecule to protect adult articular cartilage by gene expression profiling. J. Biol. Chem. 2014, 289, 10192–10200. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef]
- Carlevaro, M.F.; Cermelli, S.; Cancedda, R.; Descalzi Cancedda, F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: Auto-paracrine role during endochondral bone formation. J. Cell Sci. 2000, 113, 59–69. [Google Scholar] [PubMed]
- Wang, D.; Taboas, J.M.; Tuan, R.S. PTHrP overexpression partially inhibits a mechanical strain-induced arthritic phenotype in chondrocytes. Osteoarthr. Cartil. 2011, 19, 213–221. [Google Scholar] [CrossRef][Green Version]
- Nagao, M.; Hamilton, J.L.; Kc, R.; Berendsen, A.D.; Duan, X.; Cheong, C.W.; Li, X.; Im, H.J.; Olsen, B.R. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci. Rep. 2017, 7, 13027. [Google Scholar] [CrossRef]
- Moore, E.E.; Bendele, A.M.; Thompson, D.L.; Littau, A.; Waggie, K.S.; Reardon, B.; Ellsworth, J.L. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartil. 2005, 13, 623–631. [Google Scholar] [CrossRef]
- Kudo, S.; Mizuta, H.; Takagi, K.; Hiraki, Y. Cartilaginous repair of full-thickness articular cartilage defects is induced by the intermittent activation of PTH/PTHrP signaling. Osteoarthr. Cartil. 2011, 19, 886–894. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Zhang, S.; Ouyang, H.W. Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: The implication for articular cartilage repair. Arthritis Res. 2012, 14, 221. [Google Scholar] [CrossRef]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cell Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Madry, H.; Cucchiarini, M. Hydrogel-Based Controlled Delivery Systems for Articular Cartilage Repair. BioMed Res. Int. 2016, 2016, 1215263. [Google Scholar] [CrossRef]
- Sun, M.M.; Beier, F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res. C Embryo Today 2014, 102, 74–82. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Cong, R.; Wu, H.; Pei, M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015, 2, 76–95. [Google Scholar] [CrossRef]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef]
- Reynard, L.N.; Barter, M.J. Osteoarthritis year in review 2019: Genetics, genomics and epigenetics. Osteoarthr. Cartil. 2020, 28, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Namba, R.S.; Meuli, M.; Sullivan, K.M.; Le, A.X.; Adzick, N.S. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J. Bone Jt. Surg. Am. 1998, 80, 4–10. [Google Scholar] [CrossRef]
- Tsuruoka, H.; Sasho, T.; Yamaguchi, S.; Ikegawa, N.; Saito, M.; Akagi, R.; Ochiai, N.; Nakagawa, K.; Nakajima, A.; Fallouh, L.; et al. Maturation-dependent spontaneous healing of partial thickness cartilage defects in infantile rats. Cell Tissue Res. 2011, 346, 263–271. [Google Scholar] [CrossRef]
- Mukoyama, S.; Sasho, T.; Akatsu, Y.; Yamaguchi, S.; Muramatsu, Y.; Katsuragi, J.; Fukawa, T.; Endo, J.; Hoshi, H.; Yamamoto, Y.; et al. Spontaneous repair of partial thickness linear cartilage injuries in immature rats. Cell Tissue Res. 2015, 359, 513–520. [Google Scholar] [CrossRef]
- Akatsu, Y.; Enomoto, T.; Yamaguchi, S.; Tahara, M.; Fukawa, T.; Endo, J.; Hoshi, H.; Yamamoto, Y.; Sasaki, T.; Takahashi, K.; et al. Age-dependent differences in response to partial-thickness cartilage defects in a rat model as a measure to evaluate the efficacy of interventions for cartilage repair. Cell Tissue Res. 2019, 375, 425–435. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Rosenberg, L.C. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Jt. Surg. Am. 1996, 78, 721–733. [Google Scholar] [CrossRef]
- Wei, X.; Gao, J.; Messner, K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J. BioMed Mater. Res. 1997, 34, 63–72. [Google Scholar] [CrossRef]
- Wei, X.; Messner, K. Maturation-dependent durability of spontaneous cartilage repair in rabbit knee joint. J. BioMed Mater. Res. 1999, 46, 539–548. [Google Scholar] [CrossRef]
- Ribitsch, I.; Mayer, R.L.; Egerbacher, M.; Gabner, S.; Kandula, M.M.; Rosser, J.; Haltmayer, E.; Auer, U.; Gultekin, S.; Huber, J.; et al. Fetal articular cartilage regeneration versus adult fibrocartilaginous repair: Secretome proteomics unravels molecular mechanisms in an ovine model. Dis. Model. Mech. 2018, 11, dmm033092. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. 1993, 75, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Anraku, Y.; Mizuta, H.; Sei, A.; Kudo, S.; Nakamura, E.; Senba, K.; Hiraki, Y. Analyses of early events during chondrogenic repair in rat full-thickness articular cartilage defects. J. Bone Min. Metab. 2009, 27, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Lalor, P.A.; Aberman, H.M.; Simon, T.M. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J. Bone Jt. Surg. Am. 2001, 83, 53–64. [Google Scholar] [CrossRef]
- Koyama, E.; Shibukawa, Y.; Nagayama, M.; Sugito, H.; Young, B.; Yuasa, T.; Okabe, T.; Ochiai, T.; Kamiya, N.; Rountree, R.B.; et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008, 316, 62–73. [Google Scholar] [CrossRef]
- Decker, R.S.; Koyama, E.; Pacifici, M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014, 39, 5–10. [Google Scholar] [CrossRef]
- De Crombrugghe, B.; Akiyama, H. Transcriptional control of chondrocyte differentiation. In The Skeletal System; Pourquié, O., Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; pp. 147–170. [Google Scholar]
- Rountree, R.B.; Schoor, M.; Chen, H.; Marks, M.E.; Harley, V.; Mishina, Y.; Kingsley, D.M. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004, 2, e355. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Mabuchi, A.; Shi, D.; Kubo, T.; Takatori, Y.; Saito, S.; Fujioka, M.; Sudo, A.; Uchida, A.; Yamamoto, S.; et al. A functional polymorphism in the 5’ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat. Genet. 2007, 39, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Southam, L.; Rodriguez-Lopez, J.; Wilkins, J.M.; Pombo-Suarez, M.; Snelling, S.; Gomez-Reino, J.J.; Chapman, K.; Gonzalez, A.; Loughlin, J. An SNP in the 5’-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum. Mol. Genet. 2007, 16, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Takahashi, A.; Meulenbelt, I.; Watson, C.; Rodriguez-Lopez, J.; Egli, R.; Tsezou, A.; Malizos, K.N.; Kloppenburg, M.; Shi, D.; et al. A meta-analysis of European and Asian cohorts reveals a global role of a functional SNP in the 5’ UTR of GDF5 with osteoarthritis susceptibility. Hum. Mol. Genet. 2008, 17, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chan, W.C.W.; Lam, Y.; Wang, X.; Chen, P.; Niu, B.; Ng, V.C.W.; Yeo, J.C.; Stricker, S.; Cheah, K.S.E.; et al. Lgr5 and Col22a1 Mark Progenitor Cells in the Lineage toward Juvenile Articular Chondrocytes. Stem Cell Rep. 2019, 13, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.K.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef] [PubMed]
- Chagin, A.S.; Medvedeva, E.V. Regenerative medicine: Cartilage stem cells identified, but can they heal? Nat. Rev. Rheumatol. 2017, 13, 522–524. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.; Warman, M.L.; Lassar, A.B. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015, 67, 1261–1273. [Google Scholar] [CrossRef]
- Decker, R.S.; Um, H.B.; Dyment, N.A.; Cottingham, N.; Usami, Y.; Enomoto-Iwamoto, M.; Kronenberg, M.S.; Maye, P.; Rowe, D.W.; Koyama, E.; et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 2017, 426, 56–68. [Google Scholar] [CrossRef]
- Li, L.; Newton, P.T.; Bouderlique, T.; Sejnohova, M.; Zikmund, T.; Kozhemyakina, E.; Xie, M.; Krivanek, J.; Kaiser, J.; Qian, H.; et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017, 31, 1067–1084. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G. Skeletal stem cells. Development 2015, 142, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Lee, J.Y.; Matthias, N.; Umeda, K.; Yan, Q.; Huard, J. Cartilage regeneration using pluripotent stem cell-derived chondroprogenitors: Promise and challenges. In Pluripotent Stem Cells; Tomizawa, M., Ed.; Intech Open: Rijeka, Croatia, 2016; pp. 385–425. [Google Scholar]
- Nakayama, N.; Pothiawala, A.; Lee, J.Y.; Matthias, N.; Umeda, K.; Ang, B.K.; Huard, J.; Huang, Y.; Sun, D. Human pluripotent stem cell-derived chondroprogenitors for cartilage tissue engineering. Cell Mol. Life Sci. 2020, 77, 2543–2563. [Google Scholar] [CrossRef]
- Nakayama, N.; Duryea, D.; Manoukian, R.; Chow, G.; Han, C.Y. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J. Cell Sci. 2003, 116, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Jokubaitis, V.; Wood, C.; Wang, Y.; Brouard, N.; Pera, M.; Hearn, M.; Simmons, P.; Nakayama, N. BMP inhibition stimulates WNT-dependent generation of chondrogenic mesoderm from embryonic stem cells. Stem Cell Res. 2009, 3, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakayama, N. WNT and BMP signaling are both required for hematopoietic cell development from human ES cells. Stem Cell Res. 2009, 3, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Umeda, K.; Nakayama, N. Collaboration between WNT and BMP signaling promotes hemoangiogenic cell development from human fibroblast-derived iPS cells. Stem Cell Res. 2010, 4, 223–231. [Google Scholar] [CrossRef][Green Version]
- Nakayama, N.; Umeda, K. From pluripotent stem cells to lineage-specific chondrocytes: Essential signalling and cellular intermediates. In Embryonic Stem Cells: The Hormonal Regulation of Pluripotency and Embryogenesis; Atwood, C., Ed.; Intech Open: Vienna, Austria, 2011; pp. 621–648. [Google Scholar]
- Umeda, K.; Zhao, J.; Simmons, P.; Stanley, E.; Elefanty, A.; Nakayama, N. Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Sci. Rep. 2012, 2, 455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, S.; Trilok, S.; Tanaka, M.; Jokubaitis-Jameson, V.; Wang, B.; Niwa, H.; Nakayama, N. Small molecule-directed specification of sclerotome-like chondroprogenitors and induction of a somitic chondrogenesis program from embryonic stem cells. Development 2014, 141, 3848–3858. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Oda, H.; Yan, Q.; Matthias, N.; Zhao, J.; Davis, B.R.; Nakayama, N. Long-Term Expandable SOX9 (+) Chondrogenic Ectomesenchymal Cells from Human Pluripotent Stem Cells. Stem Cell Rep. 2015, 4, 712–726. [Google Scholar] [CrossRef]
- Lee, J.Y.; Matthias, N.; Pothiawala, A.; Ang, B.K.; Lee, M.; Li, J.; Sun, D.; Pigeot, S.; Martin, I.; Huard, J.; et al. Pre-transplantational Control of the Post-transplantational Fate of Human Pluripotent Stem Cell-Derived Cartilage. Stem Cell Rep. 2018, 11, 440–453. [Google Scholar] [CrossRef]
- Craft, A.M.; Rockel, J.S.; Nartiss, Y.; Kandel, R.A.; Alman, B.A.; Keller, G.M. Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep. 2015, 4, 404–418. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Weiss, S.; Hennig, T.; Bock, R.; Steck, E.; Richter, W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J. Cell Physiol. 2010, 223, 84–93. [Google Scholar] [CrossRef]
- Pilichi, S.; Rocca, S.; Dattena, M.; Pool, R.R.; Mara, L.; Sanna, D.; Masala, G.; Manunta, M.L.; Dore, S.; Manunta, A.; et al. Sheep embryonic stem-like cells engrafted into sheep femoral condyle osteochondral defects: 4-year follow-up. BMC Vet. Res. 2018, 14, 213. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Hong, S.G.; Winkler, T.; Wu, C.; Guo, V.; Pittaluga, S.; Nicolae, A.; Donahue, R.E.; Metzger, M.E.; Price, S.D.; Uchida, N.; et al. Path to the clinic: Assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep. 2014, 7, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 2009, 27, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Liu, S.; Woltjen, K.; Thomas, B.; Meng, G.; Hotta, A.; Takahashi, K.; Ellis, J.; Yamanaka, S.; Rancourt, D.E. Cartilage tissue engineering identifies abnormal human induced pluripotent stem cells. Sci. Rep. 2013, 3, 1978. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yano, F.; Mori, D.; Kawata, M.; Hoshi, K.; Takato, T.; Masaki, H.; Otsu, M.; Eto, K.; Nakauchi, H.; et al. Hyaline cartilage formation and tumorigenesis of implanted tissues derived from human induced pluripotent stem cells. Biomed. Res. 2015, 36, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.B.; Van Handel, B.; Bay, M.; Fiziev, P.; Org, T.; Lee, S.; Shkhyan, R.; Banks, N.W.; Scheinberg, M.; Wu, L.; et al. Mapping molecular landmarks of human skeletal ontogeny and pluripotent stem cell-derived articular chondrocytes. Nat. Commun. 2018, 9, 3634. [Google Scholar] [CrossRef]
- Wu, L.; Bluguermann, C.; Kyupelyan, L.; Latour, B.; Gonzalez, S.; Shah, S.; Galic, Z.; Ge, S.; Zhu, Y.; Petrigliano, F.A.; et al. Human developmental chondrogenesis as a basis for engineering chondrocytes from pluripotent stem cells. Stem Cell Rep. 2013, 1, 575–589. [Google Scholar] [CrossRef]
- Adkar, S.S.; Wu, C.L.; Willard, V.P.; Dicks, A.; Ettyreddy, A.; Steward, N.; Bhutani, N.; Gersbach, C.A.; Guilak, F. Step-Wise Chondrogenesis of Human Induced Pluripotent Stem Cells and Purification Via a Reporter Allele Generated by CRISPR-Cas9 Genome Editing. Stem Cells 2019, 37, 65–76. [Google Scholar] [CrossRef]
- Diekman, B.O.; Christoforou, N.; Willard, V.P.; Sun, H.; Sanchez-Adams, J.; Leong, K.W.; Guilak, F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19172–19177. [Google Scholar] [CrossRef]
- Menendez, L.; Yatskievych, T.A.; Antin, P.B.; Dalton, S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci. USA 2011, 108, 19240–19245. [Google Scholar] [CrossRef]
- Lee, G.; Chambers, S.M.; Tomishima, M.J.; Studer, L. Derivation of neural crest cells from human pluripotent stem cells. Nat. Protoc. 2010, 5, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Slukvin, I.I.; Kumar, A. The mesenchymoangioblast, mesodermal precursor for mesenchymal and endothelial cells. Cell Mol. Life Sci. 2018, 75, 3507–3520. [Google Scholar] [CrossRef]
- Vodyanik, M.A.; Yu, J.; Zhang, X.; Tian, S.; Stewart, R.; Thomson, J.A.; Slukvin, I.I. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 2010, 7, 718–729. [Google Scholar] [CrossRef]
- Hwang, N.S.; Varghese, S.; Lee, H.J.; Zhang, Z.; Ye, Z.; Bae, J.; Cheng, L.; Elisseeff, J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc. Natl. Acad. Sci. USA 2008, 105, 20641–20646. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lee, E.H.; Guo, X.M.; Chan, J.K.; Yeow, C.H.; Choo, A.B.; Cao, T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials 2010, 31, 6968–6980. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Guo, X.M.; Choo, A.B.; Lu, K.; Lee, E.H.; Cao, T. Differentiation and enrichment of expandable chondrogenic cells from human embryonic stem cells in vitro. J. Cell Mol. Med. 2009, 13, 3570–3590. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.D.; O’Sullivan, M.B.; Alaee, F.; Paglia, D.N.; Yoshida, R.; Guzzo, R.M.; Drissi, H. Regeneration of Articular Cartilage by Human ESC-Derived Mesenchymal Progenitors Treated Sequentially with BMP-2 and Wnt5a. Stem Cells Transl. Med. 2017, 6, 40–50. [Google Scholar] [CrossRef]
- Xu, X.; Shi, D.; Liu, Y.; Yao, Y.; Dai, J.; Xu, Z.; Chen, D.; Teng, H.; Jiang, Q. In vivo repair of full-thickness cartilage defect with human iPSC-derived mesenchymal progenitor cells in a rabbit model. Exp. Ther. Med. 2017, 14, 239–245. [Google Scholar] [CrossRef]
- Bertucci, T.B.; Dai, G. Biomaterial Engineering for Controlling Pluripotent Stem Cell Fate. Stem Cells Int. 2018, 2018, 9068203. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.; Juneja, S.C.; Whetstone, H.; Nartiss, Y.; Sieker, J.T.; Veillette, C.; Keller, G.M.; Craft, A.M. Effective repair of articular cartilage using human pluripotent stem cell-derived tissue. Eur. Cell Mater. 2019, 38, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Kapacee, Z.; Peng, J.; Lu, S.; Lucas, R.J.; Hardingham, T.E.; Kimber, S.J. Cartilage repair using human embryonic stem cell-derived chondroprogenitors. Stem Cells Transl. Med. 2014, 3, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Wahlestedt, M.; Norddahl, G.L.; Sten, G.; Ugale, A.; Frisk, M.A.; Mattsson, R.; Deierborg, T.; Sigvardsson, M.; Bryder, D. An epigenetic component of hematopoietic stem cell aging amenable to reprogramming into a young state. Blood 2013, 121, 4257–4264. [Google Scholar] [CrossRef]
- Schultz, M.B.; Sinclair, D.A. When stem cells grow old: Phenotypes and mechanisms of stem cell aging. Development 2016, 143, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Studer, L.; Vera, E.; Cornacchia, D. Programming and Reprogramming Cellular Age in the Era of Induced Pluripotency. Cell Stem Cell 2015, 16, 591–600. [Google Scholar] [CrossRef]
- Spitzhorn, L.S.; Megges, M.; Wruck, W.; Rahman, M.S.; Otte, J.; Degistirici, O.; Meisel, R.; Sorg, R.V.; Oreffo, R.O.C.; Adjaye, J. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res. Ther. 2019, 10, 100. [Google Scholar] [CrossRef]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Sudhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kishida, T.; Sato, Y.; Nishioka, K.; Ejima, A.; Fujiwara, H.; Kubo, T.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proc. Natl. Acad. Sci. USA 2015, 112, 6152–6157. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Sasagawa, S.; Outani, H.; Nakagawa, K.; Yoshikawa, H.; Tsumaki, N. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J. Clin. Investig. 2011, 121, 640–657. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Reid, D.; Lau, S.; Kim, Y.; Gage, F.H. Aging in a Dish: iPSC-Derived and Directly Induced Neurons for Studying Brain Aging and Age-Related Neurodegenerative Diseases. Annu. Rev. Genet. 2018, 52, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef]
- Lavasani, M.; Robinson, A.R.; Lu, A.; Song, M.; Feduska, J.M.; Ahani, B.; Tilstra, J.S.; Feldman, C.H.; Robbins, P.D.; Niedernhofer, L.J.; et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat. Commun. 2012, 3, 608. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).