Attenuating Effect of Vitamin E against Silver Nano Particles Toxicity in Submandibular Salivary Glands
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Groups
2.2. Preparation and Characterization of the Silver Nanoparticles:
- (a)
- Hematoxylin & Eosin.
- (b)
- Immunohistochemical examination.
- (c)
- Electron microscope.
2.3. Image and Statistical Analysis
3. Results
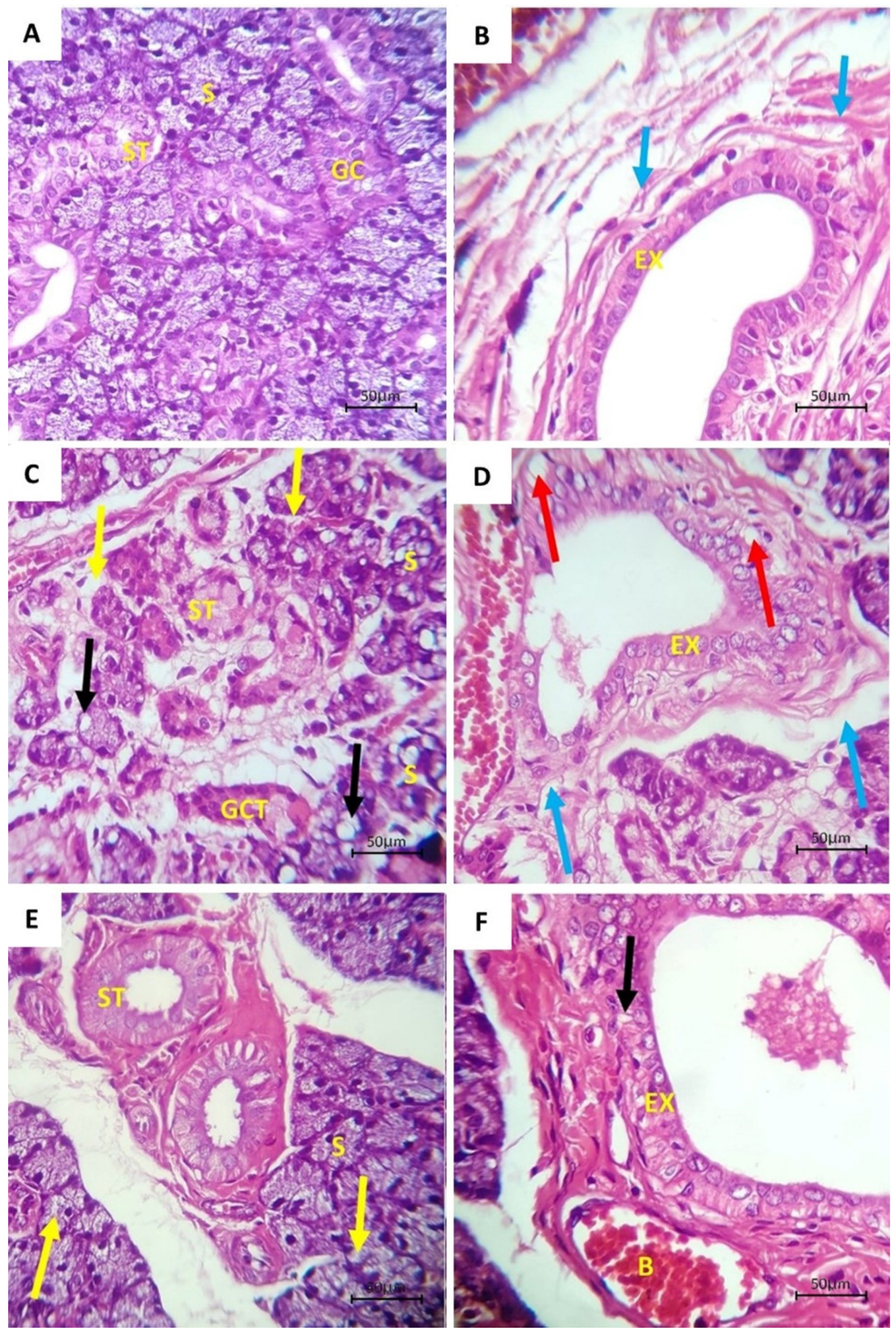
3.1. Histological Examination
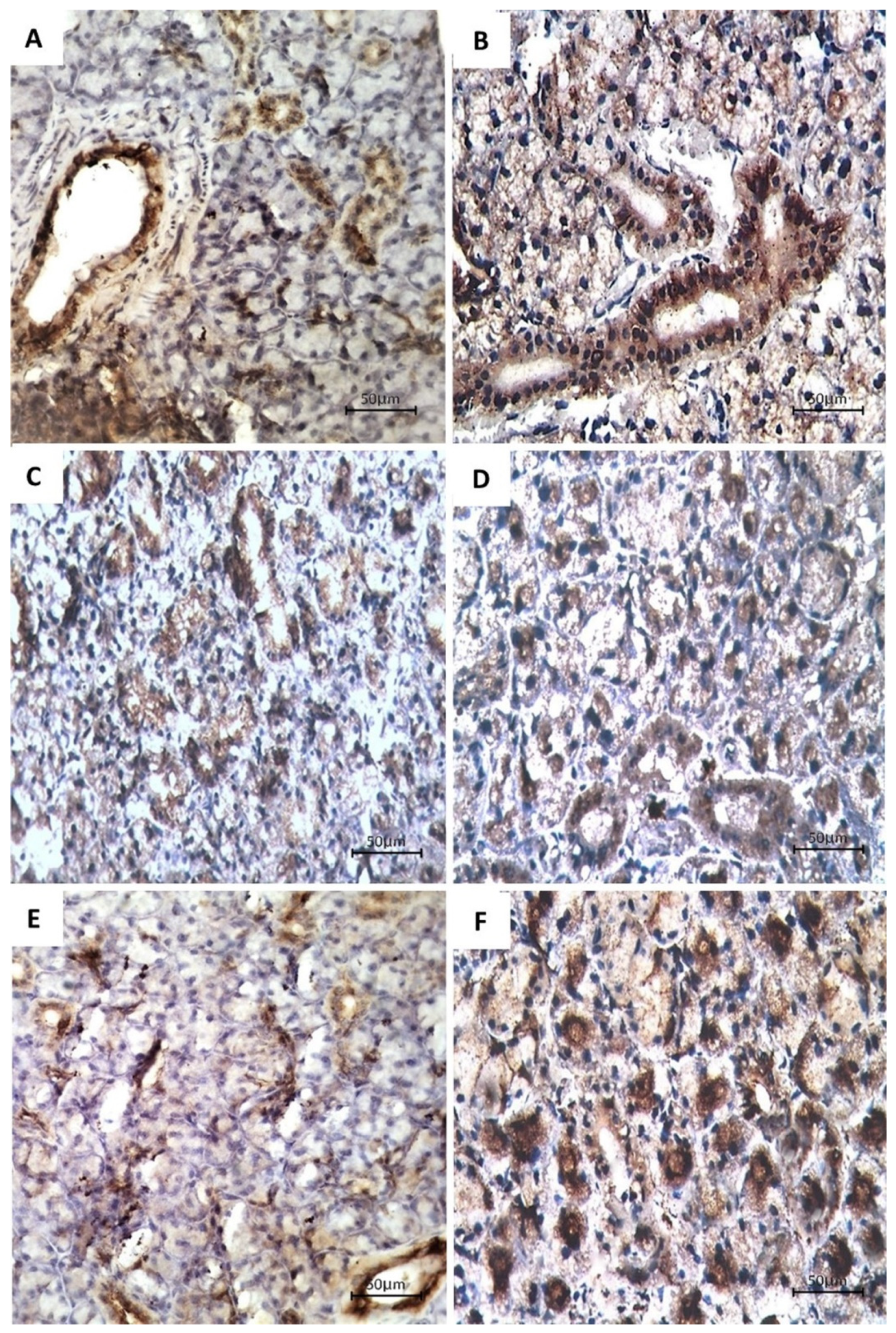
3.2. Immunohistochemical Examination
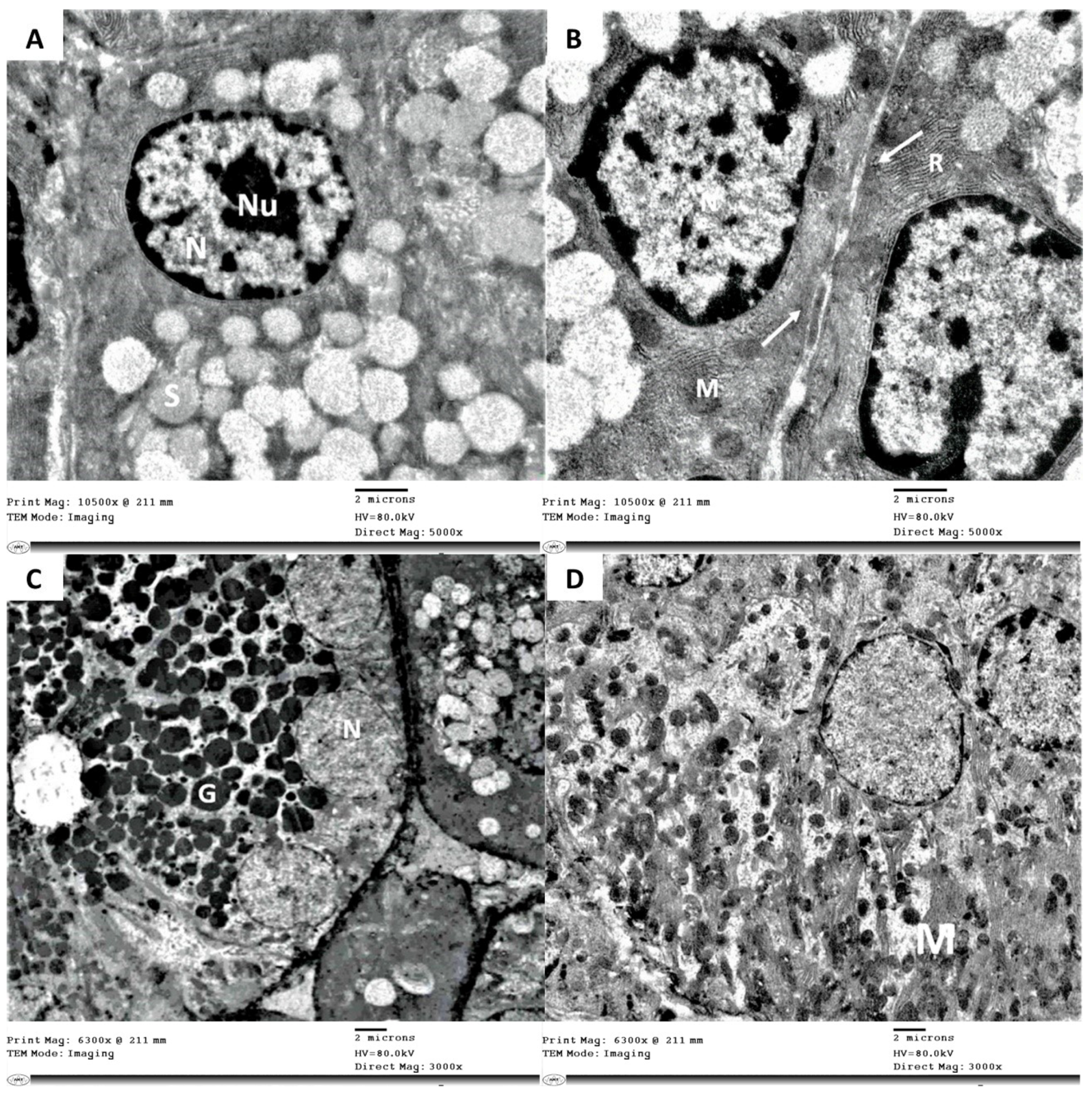
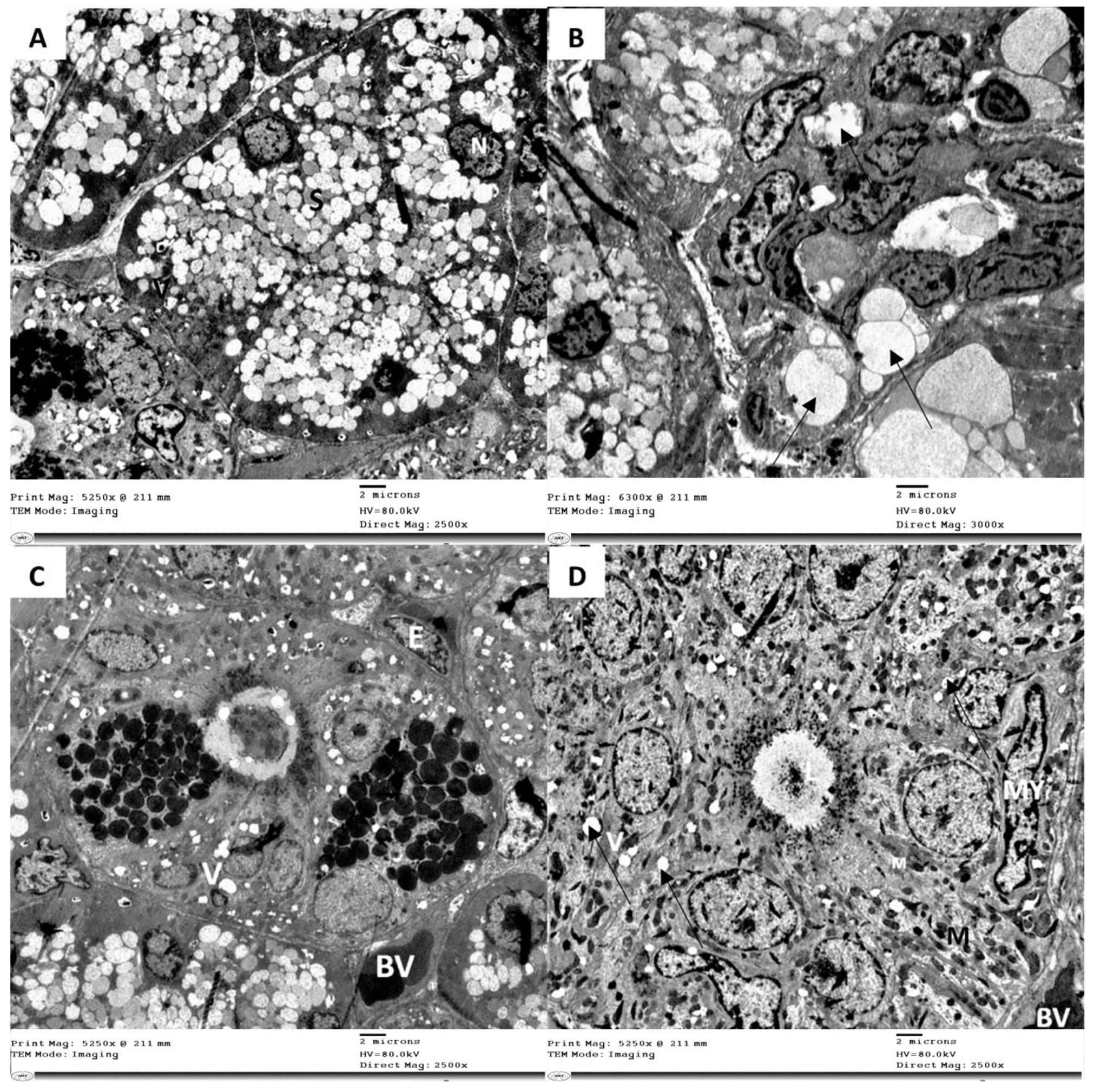
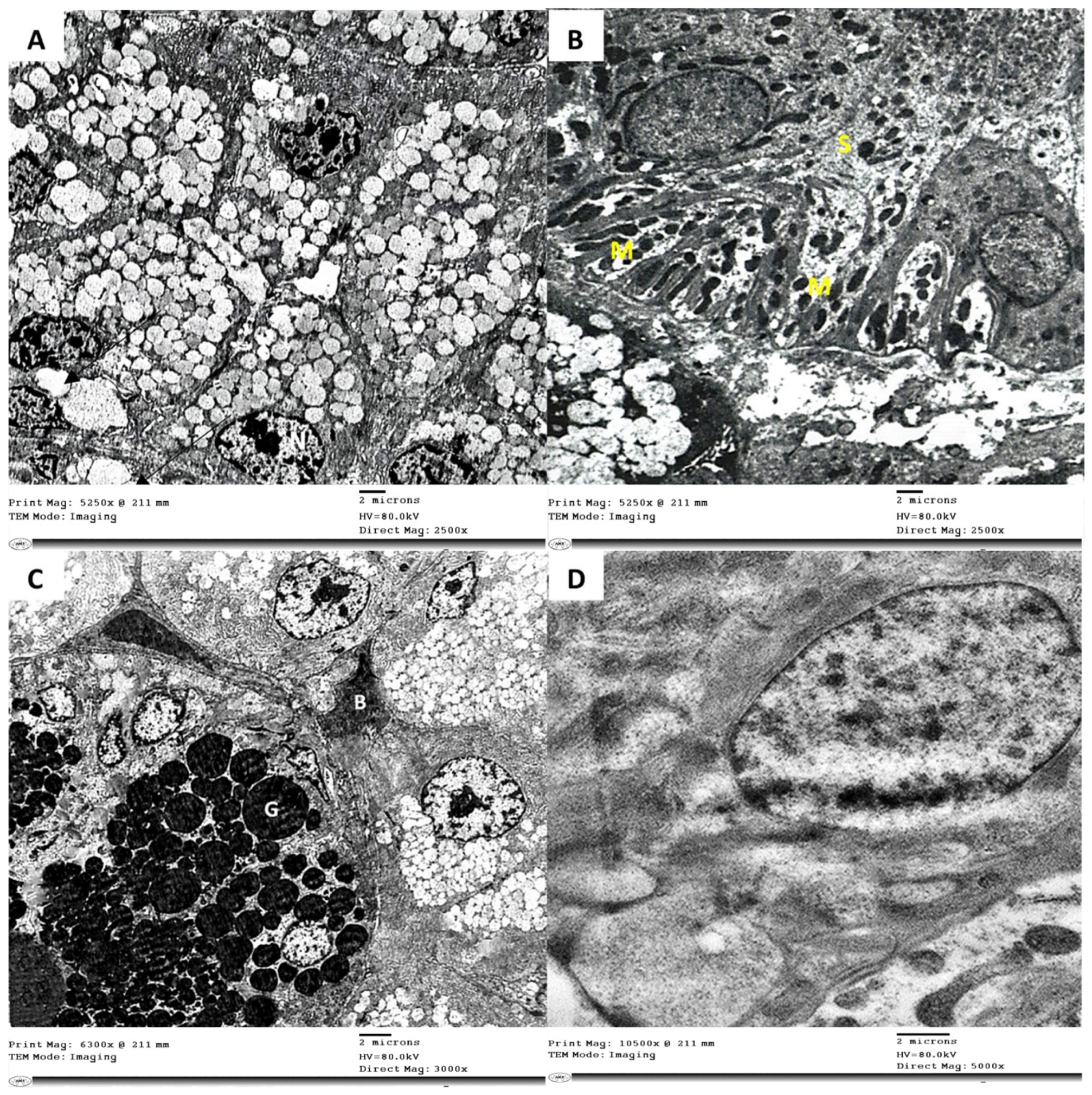
3.3. Electron Microscope Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Fathy, R.M.; Salem, M.S.E.-D.; Mahfouz, A.Y. Biogenic synthesis of silver nanoparticles using Gliocladium deliquescens and their application as household sponge disinfectant. Biol. Trace Elem. Res. 2019, 196, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Malarkodi, C. In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorg. Chem. Appl. 2014, 2014, 581890. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications–A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2016, 6, 1769–1780. [Google Scholar] [CrossRef]
- Roopan, S.M.; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crop. Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Islam, A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef] [PubMed]
- Farias, C.B.; Silva, A.F.; Rufino, R.D.; Luna, J.M.; Souza, J.E.G.; Sarubbo, L. Synthesis of silver nanoparticles using a biosurfactant produced in low-cost medium as stabilizing agent. Electron. J. Biotechnol. 2014, 17, 122–125. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Dos Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Hamouda, I.M. Current perspectives of nanoparticles in medical and dental biomaterials. J. Biomed. Res. 2012, 26, 143–151. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles—The real “silver bullet” in clinical medicine? Med. Chem. Comm. 2010, 1, 125–131. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. JBIC J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Talarska, P.; Boruczkowski, M.; Żurawski, J. Current Knowledge of Silver and Gold Nanoparticles in Laboratory Research—Application, Toxicity, Cellular Uptake. Nanomaterials 2021, 11, 2454. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Mansy, M.; Soliman, M.; Mubarak, R.; Shamel, M. The role of exogenous epidermal growth factor on Ki-67 proliferation marker expression in the submandibular salivary gland of albino rats receiving doxorubicin. F1000Research 2020, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Barcińska, E.; Wierzbicka, J.; Zauszkiewicz-Pawlak, A.; Jacewicz, D.; Dabrowska, A.; Inkielewicz-Stepniak, I. Role of Oxidative and Nitro-Oxidative Damage in Silver Nanoparticles Cytotoxic Effect against Human Pancreatic Ductal Adenocarcinoma Cells. Oxidative Med. Cell. Longev. 2018, 2018, 8251961. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Teixeira, A.L.; Dias, F.; Machado, V.; Medeiros, R.; Prior, J.A.V. Cytotoxic Effect of Silver Nanoparticles Synthesized by Green Methods in Cancer. J. Med. Chem. 2020, 63, 14308–14335. [Google Scholar] [CrossRef]
- Arindam, B. Cytotoxic effect of green synthesized silver nanoparticles in MCF7 and MDA-MB-231 human breast cancer cells in vitro. Nucleus 2020, 63, 191–202. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2010, 85, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Shamel, M.; Riad, D.; Al Ankily, M. Histological and Ultrastructural Study of Silver Nanoparticles Toxicity and the Possible Protective Effect of Vitamin C on Submandibular Salivary Glands of Albino Rats. Int. J. Dent. Oral. Sci. 2021, 8, 2166–2171. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Shinohara, Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem. Biophys. Res. Commun. 2009, 390, 733–737. [Google Scholar] [CrossRef]
- Veisi, S.; Johari, S.A.; Tyler, C.R.; Mansouri, B.; Esmaeilbeigi, M. Antioxidant properties of dietary supplements of free and nanoencapsulated silymarin and their ameliorative effects on silver nanoparticles induced oxidative stress in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2021, 28, 26055–26063. [Google Scholar] [CrossRef]
- Faedmaleki, F.; Shirazi, F.H.; Ejtemaeimehr, S.; Anjarani, S.; Salarian, A.-A.; Ashtiani, H.A.; Rastegar, H. Study of Silymarin and Vitamin E Protective Effects on Silver Nanoparticle Toxicity on Mice Liver Primary Cell Culture. Acta Med. Iran. 2016, 54, 85–95. [Google Scholar] [PubMed]
- Hedayati, S.A.; Farsani, H.G.; Naserabad, S.S.; Hoseinifar, S.H.; Van Doan, H. Protective effect of dietary vitamin E on immunological and biochemical induction through silver nanoparticles (AgNPs) inclusion in diet and silver salt (AgNO3) exposure on Zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 222, 100–107. [Google Scholar] [CrossRef]
- Yin, N.; Yao, X.; Zhou, Q.; Faiola, F.; Jiang, G. Vitamin E attenuates silver nanoparticle-induced effects on body weight and neurotoxicity in rats. Biochem. Biophys. Res. Commun. 2015, 458, 405–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giulia, C.; Simona, M.; Elena, M.; Daniela, C.; Lucia, M.; Ida, F.A.; Laura, M.; Gabriele, B.; Cesare, C. Oxidative and/or Inflammatory Thrust Induced by Silver Nanoparticles in Rabbits: Effect of Vitamin E or NSAID Administration on Semen Parameters. Mediat. Inflamm. 2020, 2020, 6664062. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free. Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- El Mahdy, M.M.; Eldin, T.A.S.; Aly, H.S.; Mohammed, F.F.; Shaalan, M. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp. Toxicol. Pathol. 2015, 67, 21–29. [Google Scholar] [CrossRef]
- Bashandy, S.A. Beneficial Effect of Combined Administration of Vitamin C and Vitamin E in Amelioration of Chronic Lead Hepatotoxicity. Egypt. J. Hosp. Med. 2006, 23, 371–384. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Arasu, M.V.; Vincent, S.; Oh, Y.-K.; Kim, K.H.; Choi, K.-C.; Choi, S.H.; Prakash, N.U. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: An in vitro study. Int. J. Nanomed. 2014, 9, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, M.J.; Yun, S.J.; Kim, K.; Choi, I.-H.; Park, S. Silver nanoparticles induce reactive oxygen species-mediated cell cycle delay and synergistic cytotoxicity with 3-bromopyruvate in Candida albicans, but not in Saccharomyces cerevisiae. Int. J. Nanomed. 2019, 14, 4801–4816. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.A.; Hossain, M.K.; Bin Lee, S.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M.; Moshtaghian, J.; Ghaedi, K.; Jafari, N.; Naderi, G. Effects of silver nanoparticle on the developing liver of rat pups after maternal exposure. Iran J. Pharm. Res. 2017, 16, 685–693. [Google Scholar] [PubMed]
- Naguib, M.; Mahmoud, U.M.; Mekkawy, I.A.; Sayed, A.E.-D.H. Hepatotoxic effects of silver nanoparticles on Clarias gariepinus; Biochemical, histopathological, and histochemical studies. Toxicol. Rep. 2020, 7, 133–141. [Google Scholar] [CrossRef]
- Li, J.; Tang, M.; Xue, Y. Review of the effects of silver nanoparticle exposure on gut bacteria. J. Appl. Toxicol. 2019, 39, 27–37. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Abdelrahman, S.A.; Shalaby, S.M. Evaluating the effect of silver nanoparticles on testes of adult albino rats (histological, immunohistochemical and biochemical study). J. Mol. Histol. 2017, 48, 9–27. [Google Scholar] [CrossRef]
- Zaki, N.T.; Ankily, A.; Mohamed, M.; Amin, R.M.; Halawa, A.M. The Possible Protective Role of Vitamin E on the Induced Silver Nanoparticles Toxicity on Filiform and Circumvallate Tongue Papillae of Albino Rats Histological and Immunohistochemical Study. J. Chem. Health Risks 2021, 11, 63–74. [Google Scholar]
- Taghyan, S.A.; Messiry, H.E.; Zainy, M.A.E. Evaluation of the toxic effect of silver nanoparticles and the possible protective effect of ascorbic acid on the parotid glands of albino rats: An in vivo study. Toxicol. Ind. Health 2020, 36, 446–453. [Google Scholar] [CrossRef]
- Asharani, P.V.; Low, K.M.G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Jing, L.; Valladares, A.; Mehta, S.L.; Wang, Z.; Li, P.A.; Bang, J.J. Silver Nanoparticle Exposure Induced Mitochondrial Stress, Caspase-3 Activation and Cell Death: Amelioration by Sodium Selenite. Int. J. Biol. Sci. 2015, 11, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Al-Anazi, M.; Sayed, A.Y.; Bhat, M.A. Potential cytotoxicity of silver nanoparticles: Stimulation of autophagy and mitochondrial dysfunction in cardiac cells. Saudi J. Biol. Sci. 2021, 28, 2762–2771. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Ashraf, B.; Ghazy, D.; Shamel, M. Effects of aflatoxin B1 on the submandibular salivary gland of albino rats and possible therapeutic potential of Rosmarinus officinalis: A light and electron microscopic study. F1000Research 2020, 9, 752. [Google Scholar] [CrossRef]
- Handajani, J.; Hanindriyo, L. Expression of Cytokeratin 19 in the epithelial cell of Azo-exposed buccal mucosa. Med. J. Islam. Repub. Iran 2018, 32, 132–135. [Google Scholar] [CrossRef][Green Version]
- Al-Ankily, M.M.; Shamel, M.; Bakr, M. Epidermal growth factor restores cytokeratin expression in rats with diabetes. J. Res. Med. Dent. Sci. 2018, 6, 196–203. [Google Scholar]
- Bakr, M.M.; Al-Ankily, M.M.; Shamel, M. Cytokeratin overexpression in Submandibular Salivary Glands of Rats Treated with Botulinum Toxin and Epidermal Growth Factor. Int. J. Dent. Oral. Sci. 2021, 8, 3491–3496. [Google Scholar]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Howard, A.C.; McNeil, A.K.; McNeil, P.L. Promotion of plasma membrane repair by vitamin E. Nat. Commun. 2011, 2, 597. [Google Scholar] [CrossRef]
- Lytvynenko, A.P.; Rieznichenko, L.S.; Sribna, V.A.; Stupchuk, M.I.; Grushka, N.G.; Shepel, A.A.; Voznesenska, T.Y.; Blashkiv, T.V.; Kaleynykova, O.N. Functional status of reproductive system under treatment of silver nanoparticles in female mice. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 1713. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Effect of the size and shape of silver nanoparticles on bacterial growth and metabolism by monitoring optical density and fluorescence intensity. Biotechnol. Bioprocess Eng. 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional antibacterial and cytotoxic potency of monodisperse greener AgNPs prepared under optimized pH and temperature. Sci. Rep. 2021, 11, 2866. [Google Scholar] [CrossRef] [PubMed]
- Bergin, I.L.; Wilding, L.A.; Morishita, M.; Walacavage, K.; Ault, A.; Axson, J.L.; Stark, D.I.; Hashway, S.A.; Capracotta, S.S.; Leroueil, P.R.; et al. Effects of particle size and coating on toxicologic parameters, fecal elimination kinetics and tissue distribution of acutely ingested silver nanoparticles in a mouse model. Nanotoxicology 2016, 10, 352–360. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Mai, M.; Thi Tam, L.; Vu, N.P.; Tien Khi, N.; Dinh Tam, P.; Quang Huy, T.; Le, A.-T.; Xuan Dinh, N.; Tran, V.-H. Functionalized-AgNPs for Long-Term Stability and Its Applicability in the Detection of Manganese Ions. Adv. Polym. Technol. 2020, 2020, 9437108. [Google Scholar] [CrossRef]
- Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; et al. The Impact of Surface Functionalization on the Biophysical Properties of Silver Nanoparticles. Nanomaterials 2019, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Bilek, O.; Fialova, T.; Otahal, A.; Adam, V.; Smerkova, K.; Fohlerova, Z. Antibacterial activity of AgNPs–TiO2 nanotubes: Influence of different nanoparticle stabilizers. RSC Adv. 2020, 10, 44601–44610. [Google Scholar] [CrossRef]
- Buszewski, B.; Rafiſska, K.; Pomastowski, P.; Walczak, J.; Rogowska, A. Novel aspects of silver nanoparticles functionalization. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 170–178. [Google Scholar] [CrossRef]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Kennedy, D.C.; Orts-Gil, G.; Lai, C.-H.; Müller, L.; Haase, A.; Luch, A.; Seeberger, P.H. Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake. J. Nanobiotechnol. 2014, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Filippo, E.; Re, M.; Palmisano, M.; Vittori-Antisari, M.; Buccolieri, A.; Manno, D. Non-functionalized silver nanoparticles for a localized surface plasmon resonance-based glucose sensor. Nanotechnology 2009, 20, 165501. [Google Scholar] [CrossRef]
- Pal, K.; Sarkar, P.; Anis, A.; Wiszumirska, K.; Jarzębski, M. Polysaccharide-Based Nanocomposites for Food Packaging Applications. Materials 2021, 14, 5549. [Google Scholar] [CrossRef] [PubMed]
- Murei, A.; Ayinde, W.B.; Gitari, M.W.; Samie, A. Functionalization and antimicrobial evaluation of ampicillin, penicillin and vancomycin with Pyrenacantha grandiflora Baill and silver nanoparticles. Sci. Rep. 2020, 10, 11596. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Rafińska, K.; Pomastowski, P.; Walczak, J.; Railean-Plugaru, V.; Buszewska-Forajta, M.; Buszewski, B. Silver nanoparticles functionalized with ampicillin. Electrophoresis 2017, 38, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Hajtuch, J.; Hante, N.; Tomczyk, E.; Wojcik, M.; Radomski, M.W.; Santos-Martinez, M.J.; Inkielewicz-Stepniak, I. Effects of functionalized silver nanoparticles on aggregation of human blood platelets. Int. J. Nanomed. 2019, 14, 7399–7417. [Google Scholar] [CrossRef]
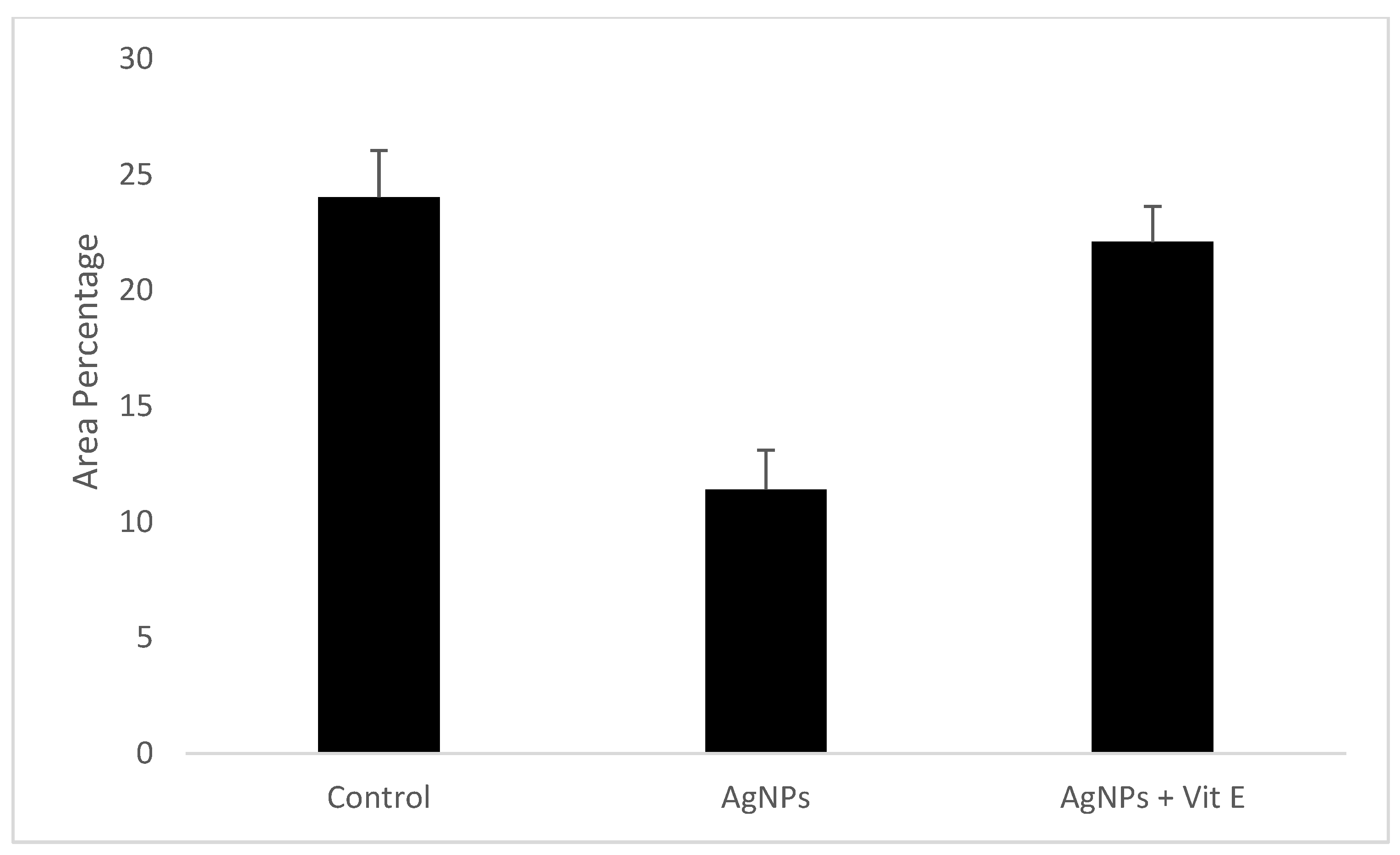
| Control | AgNPs | AgNPs + Vit E | p-Value | |
|---|---|---|---|---|
| Minimum | 19.48 | 7.95 | 18.42 | 0.001 |
| Median | 24.76 | 11.48 | 22.55 | |
| Maximum | 25.87 | 14.11 | 23.67 | |
| Mean | 24.03 | 11.41 | 22.1 | |
| Standard Deviation | 2.02 | 1.70 | 1.54 | |
| Standard Error | 0.74 | 0.6 | 0.916 |
| Tukey’s Multiple Comparison | Significance (p > 0.05) | Summary |
|---|---|---|
| Control vs. AgNPs | 0.001 | Significant |
| Control vs. AgNPs + Vit E | 0.055 | Not significant |
| AgNPs vs. AgNPs + Vit E | 0.001 | Significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakr, M.M.; Al-Ankily, M.M.; Shogaa, S.M.; Shamel, M. Attenuating Effect of Vitamin E against Silver Nano Particles Toxicity in Submandibular Salivary Glands. Bioengineering 2021, 8, 219. https://doi.org/10.3390/bioengineering8120219
Bakr MM, Al-Ankily MM, Shogaa SM, Shamel M. Attenuating Effect of Vitamin E against Silver Nano Particles Toxicity in Submandibular Salivary Glands. Bioengineering. 2021; 8(12):219. https://doi.org/10.3390/bioengineering8120219
Chicago/Turabian StyleBakr, Mahmoud M., Mahmoud M. Al-Ankily, Sara M. Shogaa, and Mohamed Shamel. 2021. "Attenuating Effect of Vitamin E against Silver Nano Particles Toxicity in Submandibular Salivary Glands" Bioengineering 8, no. 12: 219. https://doi.org/10.3390/bioengineering8120219
APA StyleBakr, M. M., Al-Ankily, M. M., Shogaa, S. M., & Shamel, M. (2021). Attenuating Effect of Vitamin E against Silver Nano Particles Toxicity in Submandibular Salivary Glands. Bioengineering, 8(12), 219. https://doi.org/10.3390/bioengineering8120219






