How Localized Z-Disc Damage Affects Force Generation and Gene Expression in Cardiomyocytes
Abstract
1. Introduction
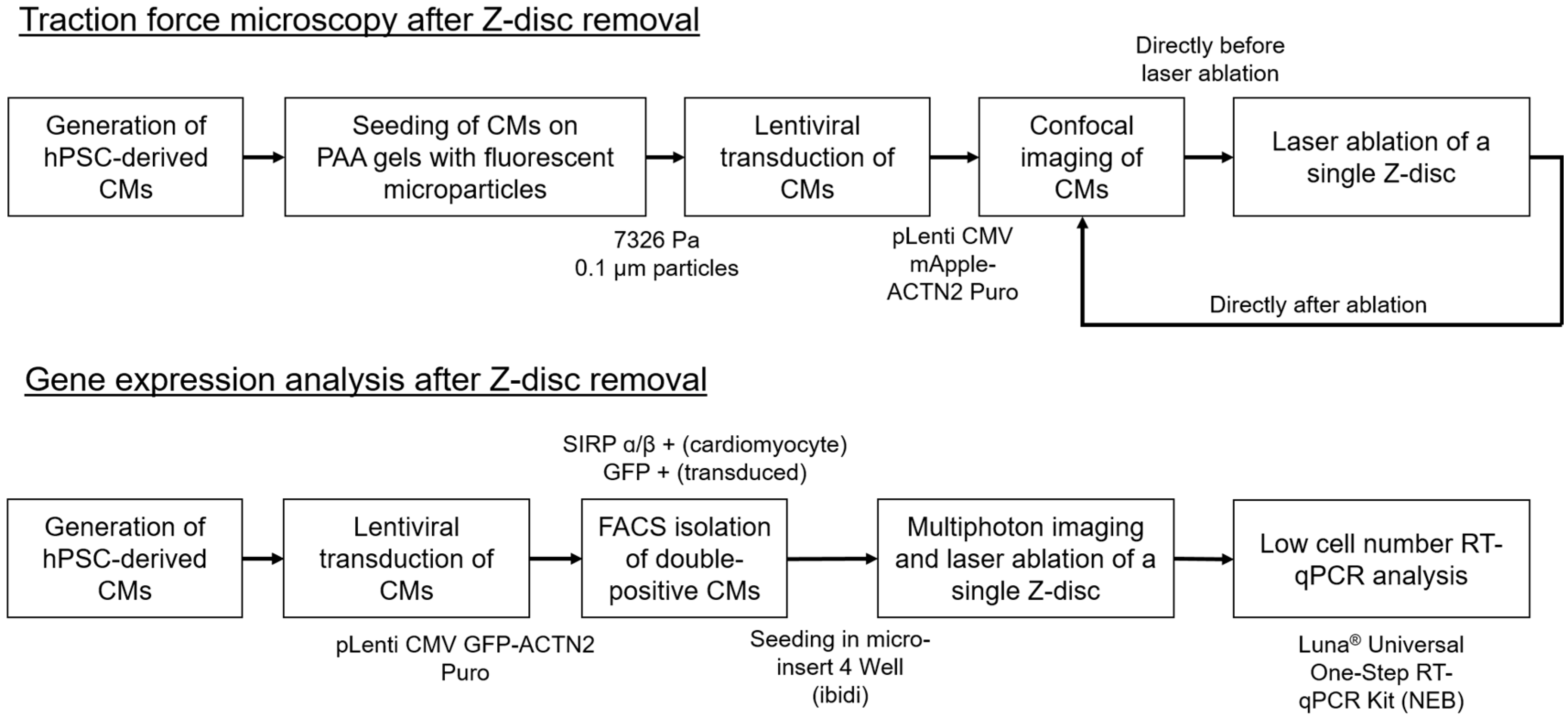
2. Materials and Methods
2.1. Generation of hPSC-Derived Cardiomyocytes
2.2. Polyacrylamide Gel Preparation and Traction Force Measurements
2.3. Traction Force Imaging and Laser Manipulation
2.4. Preparation of Cardiomyocytes for RT-qPCR Analysis
2.5. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.6. Data Analysis and Statistics
3. Results and Discussion
3.1. Single Z-Disc Removal Leads to Lowered Force Generation in Cardiomyocytes
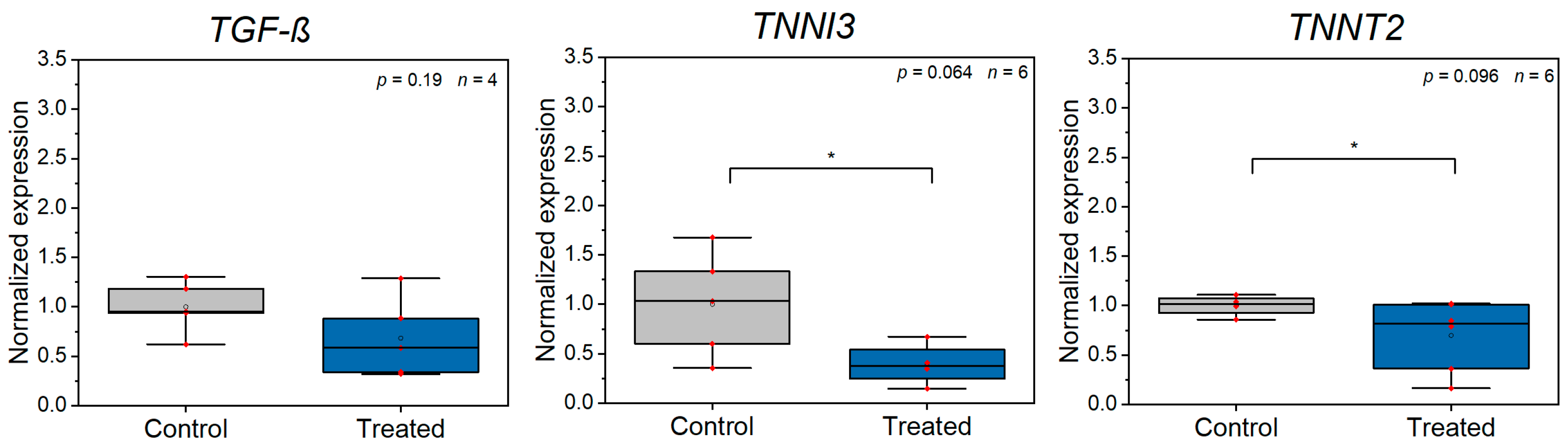
3.2. Z-Disc Removal Is Associated with Gene Expression Changes in Markers for Cell Stress, Injury, and Sarcomeric Cytoskeleton Remodeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Name | FW Primer 5′-3′ | REV Primer 5′-3′ | Function | Ref. |
|---|---|---|---|---|---|
| Genes related to cellular stress (general stress and cardiomyocyte specific, for example, strech induced) | |||||
| ANP | Natriuretic peptide A | CAGGATGGACAGGATTGGA | TGTCCTCCCTGGCTGTTATC | Strong connection to mechanical stretch of cardiomyocytes. | [43] |
| BNP | Natriuretic peptide B | TTGGAAACGTCCGGGTTAC | GGACTTCCAGACACCTGTGG | Strong connection to mechanical stretch of cardiomyocytes. | [43] |
| SP6 | Sp6 transcription factor | GAGGACCTGGAAAGCGACAG | GATGAAGGTCCCACCACGAG | Strong connection to mechanical stretch of cardiomyocytes. | [44] |
| FSTL3 | Follistatin like 3 | CACCCGGGGAACAAGATCAA | GTCGCACGAATCTTTGCAGG | Strong connection to mechanical stretch of cardiomyocytes. | [44] |
| NF-ΚB | Nuclear factor kappa B subunit 1 | AATTAACGGCGACAATCTGGAA | ACTTCACAAGCATAGCCATCAG | General regulator of stress reponse. | [46] |
| ZBTB17 | Zinc finger and BTB domain containing 17 | GTGTGATGTGCGGTAAGGC | TGGACTGGACGAATCTCTTGC | Can protect cardiomyocytes from apoptosis. | |
| Genes related to cardiac injury | |||||
| TGF-Β | Transforming growth factor beta | AAGATGACCGCTCTGACATCA | CTTATAGACCTCAGCAAAGCGAC | General marker of injury. | |
| TNNI3 | Troponin I, cardiac muscle | CCAACTACCGCGCTTATGC | CTCGCTCCAGCTCTTGCTTT | Involved in sarcomere assembly and contraction. Marker of myocardial injury. | [47] |
| TNNT2 | Cardiac muscle troponin T | TGGAGGCAGAGAAGTTCGAC | CCTGTTTCGGAGAACATTGAT | Involved in sarcomere assembly and contraction. Marker of myocardial injury. | [47] |
| Genes related to sarcomeric cytoskeletal organization | |||||
| ACTN2 | Actinin alpha 2 | CAAACCTGACCGGGGAAAAAT | CTGAATAGCAAAGCGAAGGATGA | Located at the Z-disc, cross-links actin and titin filaments. | [13] |
| CSRP3 | Cysteine and glycine-rich protein 3 | CCTGTGAAAAGACCGTCTACC | GTCGTGCTGTCAAGAGCCT | Involved in establishment and maintenance of the cardiomyocyte cytoskeleton. | [50] |
| DAAM1 | Disheveled associated activator of morphogenesis 1 | AGTATGCCAGCGAAAGGACC | TTCATCTCGATACCGCCCAGT | Located at the Z-disc. Regulates filamentous actin assembly. | [48] |
| MYH7 | Myosin heavy chain 7 | CGAAGGGCTTGAATGAGGAGT | TCCTCCCAAGGAGCTGTTAC | Major protein of the thick filament. | [51] |
| FLNC | Filamin C | CTGGGCGATGAGACAGACG | CGGATGGAACTTGCGGTA | Is involved in early stages of myofibrillar remodeling and repair. | [41] |
| FMNL2 | Formin like 2 encodes a formin-related protein | GCTATGAACCTACCTCCTGACA | AACACGCCGTCTGAATTTCTT | Required for myofibrillogenesis. | [49] |
| Housekeeper genes | |||||
| ATP5F1 | ATPase subunit b | AGGTCCAGGGGTATTGCAG | TCCTCAGGGATCAGTCCATAAC | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | AGCCACATCGCTCAGACACC | GTACTCAGCGCCAGCATCG | ||
Appendix B

References
- McCain, M.L.; Parker, K.K. Mechanotransduction: The role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflügers Arch.-Eur. J. Physiol. 2011, 462, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Knoell, R.; Hoshijima, M.; Chien, K. Cardiac mechanotransduction and implications for heart disease. J. Mol. Med. 2003, 81, 750–756. [Google Scholar] [CrossRef]
- De Tombe, P.P.; Mateja, R.D.; Tachampa, K.; Mou, Y.A.; Farman, G.P.; Irving, T.C. Myofilament length dependent activation. J. Mol. Cell. Cardiol. 2010, 48, 851–858. [Google Scholar] [CrossRef]
- Sequeira, V.; Nijenkamp, L.L.A.; Regan, J.A.; van der Velden, J. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 700–722. [Google Scholar] [CrossRef]
- Tardiff, J.C. Sarcomeric proteins and Familial Hypertrophic Cardiomyopathy: Linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail. Rev. 2005, 10, 237–248. [Google Scholar] [CrossRef]
- Hamdani, N.; Kooij, V.; Van Dijk, S.; Merkus, D.; Paulus, W.J.; Remedios, C.D.; Duncker, D.J.; Stienen, G.J.M.; Van Der Velden, J. Sarcomeric dysfunction in heart failure. Cardiovasc. Res. 2008, 77, 649–658. [Google Scholar] [CrossRef]
- Morimoto, S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc. Res. 2007, 77, 659–666. [Google Scholar] [CrossRef]
- Buyandelger, B.; Mansfield, C.; Knöll, R. Mechano-signaling in heart failure. Pflugers Arch. 2014, 466, 1093–1099. [Google Scholar] [CrossRef]
- Frank, D.; Frey, N. Cardiac Z-disc Signaling Network. J. Biol. Chem. 2011, 286, 9897–9904. [Google Scholar] [CrossRef]
- Knöll, R.; Hoshijima, M.; Hoffman, H.M.; Person, V.; Lorenzen-Schmidt, I.; Bang, M.-L.; Hayashi, T.; Shiga, N.; Yasukawa, H.; Schaper, W.; et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 2002, 111, 943–955. [Google Scholar] [CrossRef]
- Pyle, W.G.; Solaro, R.J. At the crossroads of myocardial signaling: The role of Z-discs in intracellular signaling and cardiac function. Circ. Res. 2004, 94, 296–305. [Google Scholar] [CrossRef]
- Zou, P.; Pinotsis, N.; Lange, S.; Song, Y.-H.; Popov, A.; Mavridis, I.; Mayans, O.M.; Gautel, M.; Wilmanns, M. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature 2006, 439, 229–233. [Google Scholar] [CrossRef]
- Knöll, R.; Buyandelger, B.; Lab, M. The sarcomeric Z-disc and Z-discopathies. J. Biomed. Biotechnol. 2011, 2011, 569628. [Google Scholar] [CrossRef]
- Knöll, R.; Buyandelger, B. Z-disc Transcriptional Coupling, Sarcomeroptosis and Mechanopoptosis. Cell Biochem. Biophys. 2013, 66, 65–71. [Google Scholar] [CrossRef][Green Version]
- Knöll, R.; Linke, W.A.; Zou, P.; Miočiċ, S.; Kostin, S.; Buyandelger, B.; Ku, C.-H.; Neef, S.; Bug, M.; Schäfer, K.; et al. Telethonin Deficiency Is Associated With Maladaptation to Biomechanical Stress in the Mammalian Heart. Circ. Res. 2011, 109, 758–769. [Google Scholar] [CrossRef]
- Purcell, N.H.; Darwis, D.; Bueno, O.F.; Müller, J.M.; Schüle, R.; Molkentin, J.D. Extracellular Signal-Regulated Kinase 2 Interacts with and Is Negatively Regulated by the LIM-Only Protein FHL2 in Cardiomyocytes. Mol. Cell. Biol. 2004, 24, 1081–1095. [Google Scholar] [CrossRef]
- Boateng, S.Y.; Belin, R.J.; Geenen, D.L.; Margulies, K.B.; Martin, J.L.; Hoshijima, M.; de Tombe, P.P.; Russell, B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am. J. Physiol. Circ. Physiol. 2007, 292, H259–H269. [Google Scholar] [CrossRef]
- Ecarnot-Laubriet, A.; De Luca, K.; Vandroux, D.; Moisant, M.; Bernard, C.; Assem, M.; Rochette, L.; Teyssier, J.-R. Downregulation and Nuclear Relocation of MLP During the Progression of Right Ventricular Hypertrophy Induced by Chronic Pressure Overload. J. Mol. Cell. Cardiol. 2000, 32, 2385–2395. [Google Scholar] [CrossRef]
- Halloin, C.; Schwanke, K.; Löbel, W.; Franke, A.; Szepes, M.; Biswanath, S.; Wunderlich, S.; Merkert, S.; Weber, N.; Osten, F.; et al. Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Stem Cell Rep. 2019, 13, 366–379. [Google Scholar] [CrossRef]
- Kempf, H.; Zweigerdt, R. Scalable Cardiac Differentiation of Pluripotent Stem Cells Using Specific Growth Factors and Small Molecules. In Advances in Biochemical Engineering/Biotechnology; Springer Nature: London, UK, 2017; pp. 39–69. [Google Scholar]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Weber, N.; Schwanke, K.; Greten, S.; Wendland, M.; Iorga, B.; Fischer, M.; Geers-Knörr, C.; Hegermann, J.; Wrede, C.; Fiedler, J.; et al. Stiff matrix induces switch to pure β-cardiac myosin heavy chain expression in human ESC-derived cardiomyocytes. Basic Res. Cardiol. 2016, 111, 68. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Kowalski, K.; Holler, T.; Radocaj, A.; Fischer, M.; Thiemann, S.; de la Roche, J.; Schwanke, K.; Piep, B.; Peschel, N.; et al. Advanced Single-Cell Mapping Reveals that in hESC Cardiomyocytes Contraction Kinetics and Action Potential Are Independent of Myosin Isoform. Stem Cell Rep. 2020, 14, 788–802. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Mummery, C.; Knollmann, B.C. Modelling sarcomeric cardiomyopathies in the dish: From human heart samples to iPSC cardiomyocytes. Cardiovasc. Res. 2015, 105, 424–438. [Google Scholar] [CrossRef]
- De la Roche, J.; Angsutararux, P.; Kempf, H.; Janan, M.; Bolesani, E.; Thiemann, S.; Wojciechowski, D.; Coffee, M.; Franke, A.; Schwanke, K.; et al. Comparing human iPSC-cardiomyocytes versus HEK293T cells unveils disease-causing effects of Brugada mutation A735V of NaV1.5 sodium channels. Sci. Rep. 2019, 9, 11173. [Google Scholar] [CrossRef]
- Di Pasquale, E.; Lodola, F.; Miragoli, M.; Denegri, M.; Avelino-Cruz, J.E.; Buonocore, M.; Nakahama, H.; Portararo, P.; Bloise, R.; Napolitano, C.; et al. CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 2013, 4, e843. [Google Scholar] [CrossRef]
- Bellin, M.; Casini, S.; Davis, R.P.; D’Aniello, C.; Haas, J.; Ward-van Oostwaard, D.; Tertoolen, L.G.J.; Jung, C.B.; Elliott, D.A.; Welling, A.; et al. Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long-QT syndrome. EMBO J. 2013, 32, 3161–3175. [Google Scholar] [CrossRef]
- Sheehy, S.P.; Pasqualini, F.; Grosberg, A.; Park, S.J.; Aratyn-Schaus, Y.; Parker, K.K. Quality metrics for stem cell-derived cardiac myocytes. Stem Cell Rep. 2014, 2, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Iorga, B.; Schwanke, K.; Weber, N.; Wendland, M.; Greten, S.; Piep, B.; dos Remedios, C.G.; Martin, U.; Zweigerdt, R.; Kraft, T.; et al. Differences in Contractile Function of Myofibrils within Human Embryonic Stem Cell-Derived Cardiomyocytes vs. Adult Ventricular Myofibrils Are Related to Distinct Sarcomeric Protein Isoforms. Front. Physiol. 2018, 8, 1111. [Google Scholar] [CrossRef]
- Müller, D.; Klamt, T.; Gentemann, L.; Heisterkamp, A.; Kalies, S.M.K. Evaluation of laser induced sarcomere micro-damage: Role of damage extent and location in cardiomyocytes. PLoS ONE 2021, 16, e0252346. [Google Scholar] [CrossRef]
- Müller, D.; Hagenah, D.; Biswanath, S.; Coffee, M.; Kampmann, A.; Zweigerdt, R.; Heisterkamp, A.; Kalies, S.M.K. Femtosecond laser-based nanosurgery reveals the endogenous regeneration of single Z-discs including physiological consequences for cardiomyocytes. Sci. Rep. 2019, 9, 3625. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Aratyn-Schaus, Y.; Oakes, P.W.; Stricker, J.; Winter, S.P.; Gardel, M.L. Preparation of Complaint Matrices for Quantifying Cellular Contraction. J. Vis. Exp. 2010, 46, e2173. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Kutys, M.L.; Zhang, K.; Polacheck, W.J.; Sheng, C.C.; Luu, R.J.; Eyckmans, J.; Hinson, J.T.; Seidman, J.G.; Seidman, C.E.; et al. Force Generation via β-Cardiac Myosin, Titin, and α-Actinin Drives Cardiac Sarcomere Assembly from Cell-Matrix Adhesions. Dev. Cell 2018, 44, 87–96.e5. [Google Scholar] [CrossRef]
- Tseng, Q. Study of Multicellular Architecture with Controlled Microenvironment. Ph.D. Thesis, Université de Grenoble, Grenoble, France, 2011. [Google Scholar]
- Tseng, Q.; Duchemin-Pelletier, E.; Deshiere, A.; Balland, M.; Guillou, H.; Filhol, O.; Thery, M. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proc. Natl. Acad. Sci. USA 2012, 109, 1506–1511. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Wheelwright, M.; Win, Z.; Mikkila, J.L.; Amen, K.Y.; Alford, P.W.; Metzger, J.M. Investigation of human iPSC-derived cardiac myocyte functional maturation by single cell traction force microscopy. PLoS ONE 2018, 13, e0194909. [Google Scholar] [CrossRef]
- Orfanos, Z.; Gödderz, M.P.O.; Soroka, E.; Gödderz, T.; Rumyantseva, A.; van der Ven, P.F.M.; Hawke, T.J.; Fürst, D.O. Breaking sarcomeres by in vitro exercise. Sci. Rep. 2016, 6, 19614. [Google Scholar] [CrossRef]
- Ribeiro, A.J.S.; Ang, Y.-S.; Fu, J.-D.; Rivas, R.N.; Mohamed, T.M.A.; Higgs, G.C.; Srivastava, D.; Pruitt, B.L. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 12705–12710. [Google Scholar] [CrossRef]
- Leber, Y.; Ruparelia, A.A.; Kirfel, G.; van der Ven, P.F.M.; Hoffmann, B.; Merkel, R.; Bryson-Richardson, R.J.; Fürst, D.O. Filamin C is a highly dynamic protein associated with fast repair of myofibrillar microdamage. Hum. Mol. Genet. 2016, 25, 2776–2788. [Google Scholar] [CrossRef]
- Ufford, K.; Friedline, S.; Tong, Z.; Tang, V.T.; Dobbs, A.S.; Tsan, Y.-C.; Bielas, S.L.; Liu, A.P.; Helms, A.S. Myofibrillar Structural Variability Underlies Contractile Function in Stem Cell-Derived Cardiomyocytes. Stem Cell Rep. 2021, 16, 470–477. [Google Scholar] [CrossRef]
- Deacon, D.C.; Happe, C.L.; Chen, C.; Tedeschi, N.; Manso, A.M.; Li, T.; Dalton, N.D.; Peng, Q.; Farah, E.N.; Gu, Y.; et al. Combinatorial interactions of genetic variants in human cardiomyopathy. Nat. Biomed. Eng. 2019, 3, 147–157. [Google Scholar] [CrossRef]
- Rysä, J.; Tokola, H.; Ruskoaho, H. Mechanical stretch induced transcriptomic profiles in cardiac myocytes. Sci. Rep. 2018, 8, 4733. [Google Scholar] [CrossRef]
- Ovchinnikova, E.; Hoes, M.; Ustyantsev, K.; Bomer, N.; de Jong, T.V.; van der Mei, H.; Berezikov, E.; van der Meer, P. Modeling Human Cardiac Hypertrophy in Stem Cell-Derived Cardiomyocytes. Stem Cell Rep. 2018, 10, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Murask, J.; Chen, Y.; Tsujita, Y.; Wall, J.; Glembotsk, C.C.; Schaefer, E.; Beckerle, M.; Sussman, M.A. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J. Clin. Investig. 2005, 115, 2716–2730. [Google Scholar] [CrossRef]
- Buyandelger, B.; Mansfield, C.; Kostin, S.; Choi, O.; Roberts, A.M.; Ware, J.S.; Mazzarotto, F.; Pesce, F.; Buchan, R.; Isaacson, R.L.; et al. ZBTB17 (MIZ1) Is Important for the Cardiac Stress Response and a Novel Candidate Gene for Cardiomyopathy and Heart Failure. Circ. Cardiovasc. Genet. 2015, 8, 643–652. [Google Scholar] [CrossRef]
- Hagenah, D.; Heisterkamp, A.; Kalies, S. Effects of cell state and staining on femtosecond laser nanosurgery. J. Biophotonics 2018, 11, e201700344. [Google Scholar] [CrossRef]
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y.R. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110. [Google Scholar] [CrossRef]
- Li, D.; Hallett, M.A.; Zhu, W.; Rubart, M.; Liu, Y.; Yang, Z.; Chen, H.; Haneline, L.S.; Chan, R.J.; Schwartz, R.J.; et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 2011, 138, 303–315. [Google Scholar] [CrossRef]
- Rosado, M.; Barber, C.F.; Berciu, C.; Feldman, S.; Birren, S.J.; Nicastro, D.; Goode, B.L. Critical roles for multiple formins during cardiac myofibril development and repair. Mol. Biol. Cell 2014, 25, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Vafiadaki, E.; Arvanitis, D.A.; Sanoudou, D. Muscle LIM Protein: Master regulator of cardiac and skeletal muscle functions. Gene 2015, 566, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Colegrave, M.; Peckham, M. Structural Implications of β-Cardiac Myosin Heavy Chain Mutations in Human Disease. Anat. Rec. 2014, 297, 1670–1680. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, D.; Donath, S.; Brückner, E.G.; Biswanath Devadas, S.; Daniel, F.; Gentemann, L.; Zweigerdt, R.; Heisterkamp, A.; Kalies, S.M.K. How Localized Z-Disc Damage Affects Force Generation and Gene Expression in Cardiomyocytes. Bioengineering 2021, 8, 213. https://doi.org/10.3390/bioengineering8120213
Müller D, Donath S, Brückner EG, Biswanath Devadas S, Daniel F, Gentemann L, Zweigerdt R, Heisterkamp A, Kalies SMK. How Localized Z-Disc Damage Affects Force Generation and Gene Expression in Cardiomyocytes. Bioengineering. 2021; 8(12):213. https://doi.org/10.3390/bioengineering8120213
Chicago/Turabian StyleMüller, Dominik, Sören Donath, Emanuel Georg Brückner, Santoshi Biswanath Devadas, Fiene Daniel, Lara Gentemann, Robert Zweigerdt, Alexander Heisterkamp, and Stefan Michael Klaus Kalies. 2021. "How Localized Z-Disc Damage Affects Force Generation and Gene Expression in Cardiomyocytes" Bioengineering 8, no. 12: 213. https://doi.org/10.3390/bioengineering8120213
APA StyleMüller, D., Donath, S., Brückner, E. G., Biswanath Devadas, S., Daniel, F., Gentemann, L., Zweigerdt, R., Heisterkamp, A., & Kalies, S. M. K. (2021). How Localized Z-Disc Damage Affects Force Generation and Gene Expression in Cardiomyocytes. Bioengineering, 8(12), 213. https://doi.org/10.3390/bioengineering8120213








