Pheno-SELEX: Engineering Anti-Metastatic Aptamers through Targeting the Invasive Phenotype Using Systemic Evolution of Ligands by Exponential Enrichment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Invasion Assay
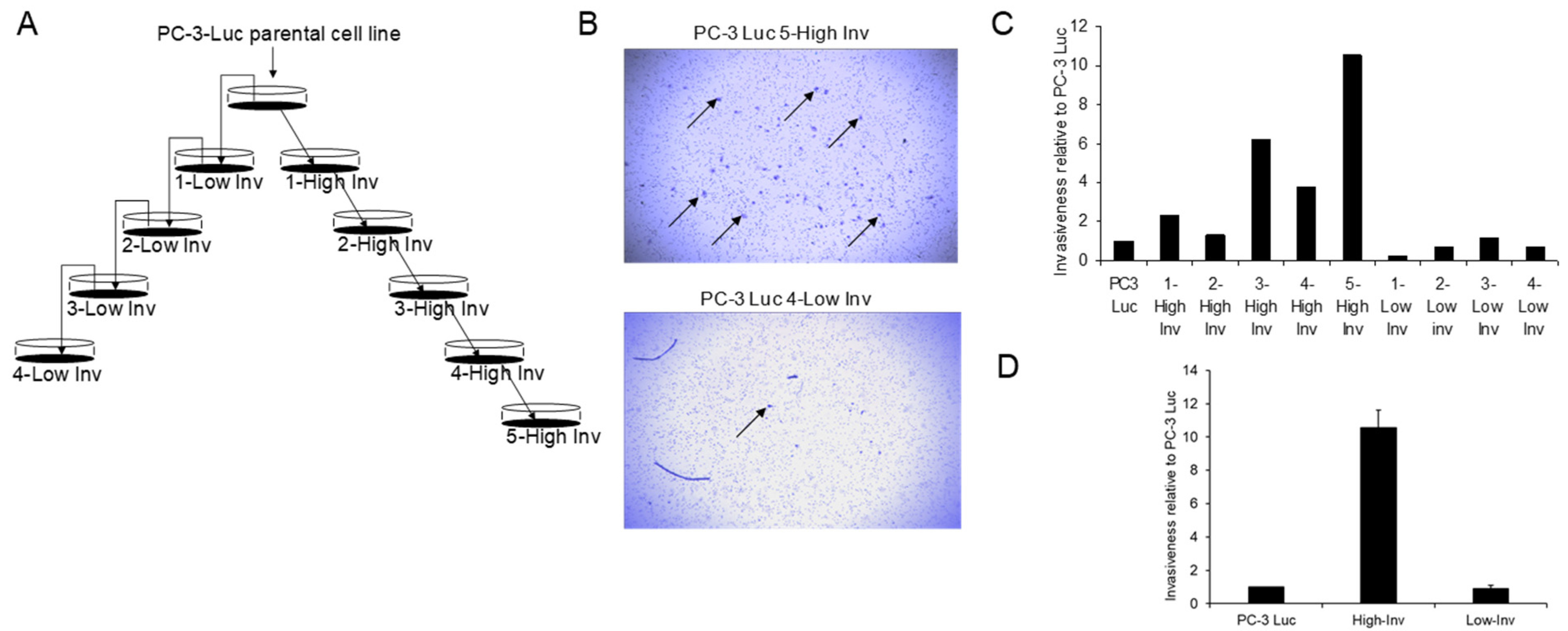
2.3. Generation of High and Low Invasive PCa Cell Lines
2.4. Cell Proliferation
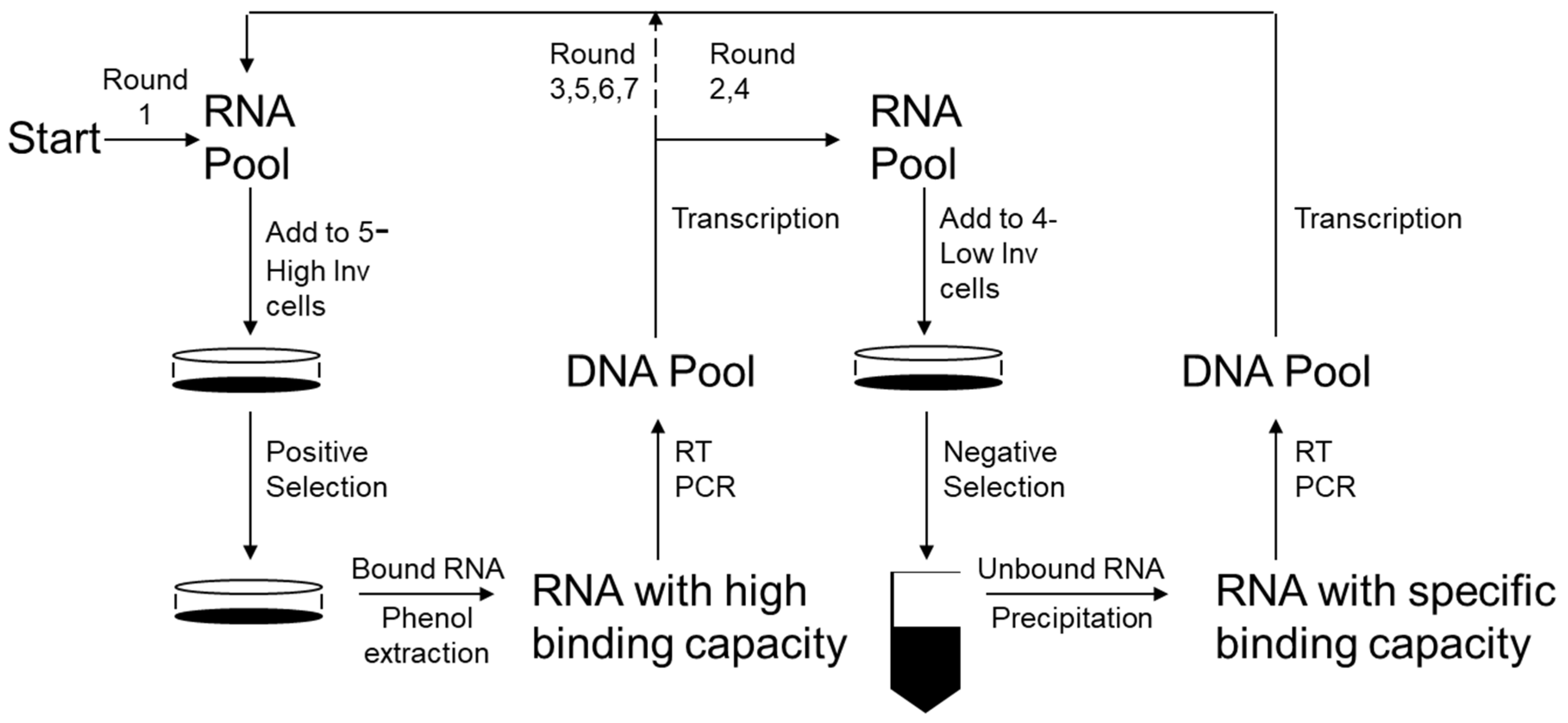
2.5. SELEX Procedures
2.6. Animals
2.7. Cellular Fluorescent Imaging
2.8. In Vivo Bioluminescent Imaging
2.9. Detection of Circulating Tumor Cells
2.10. Statistical Analysis
3. Results
3.1. Creation of High and Low Invasive PCa Cell Lines
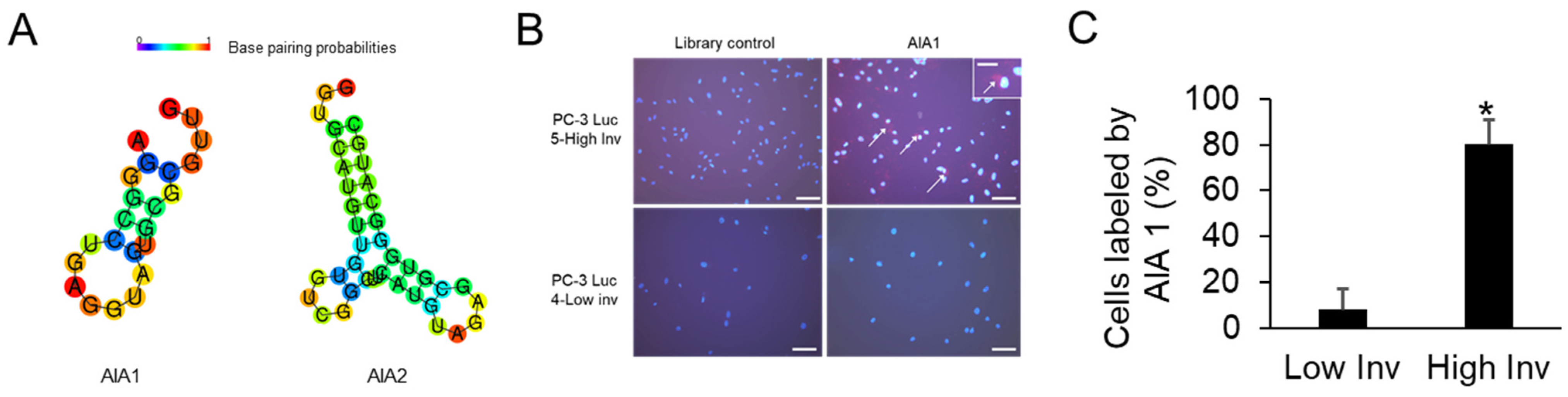
3.2. Selection of Anti-Invasive Aptamers
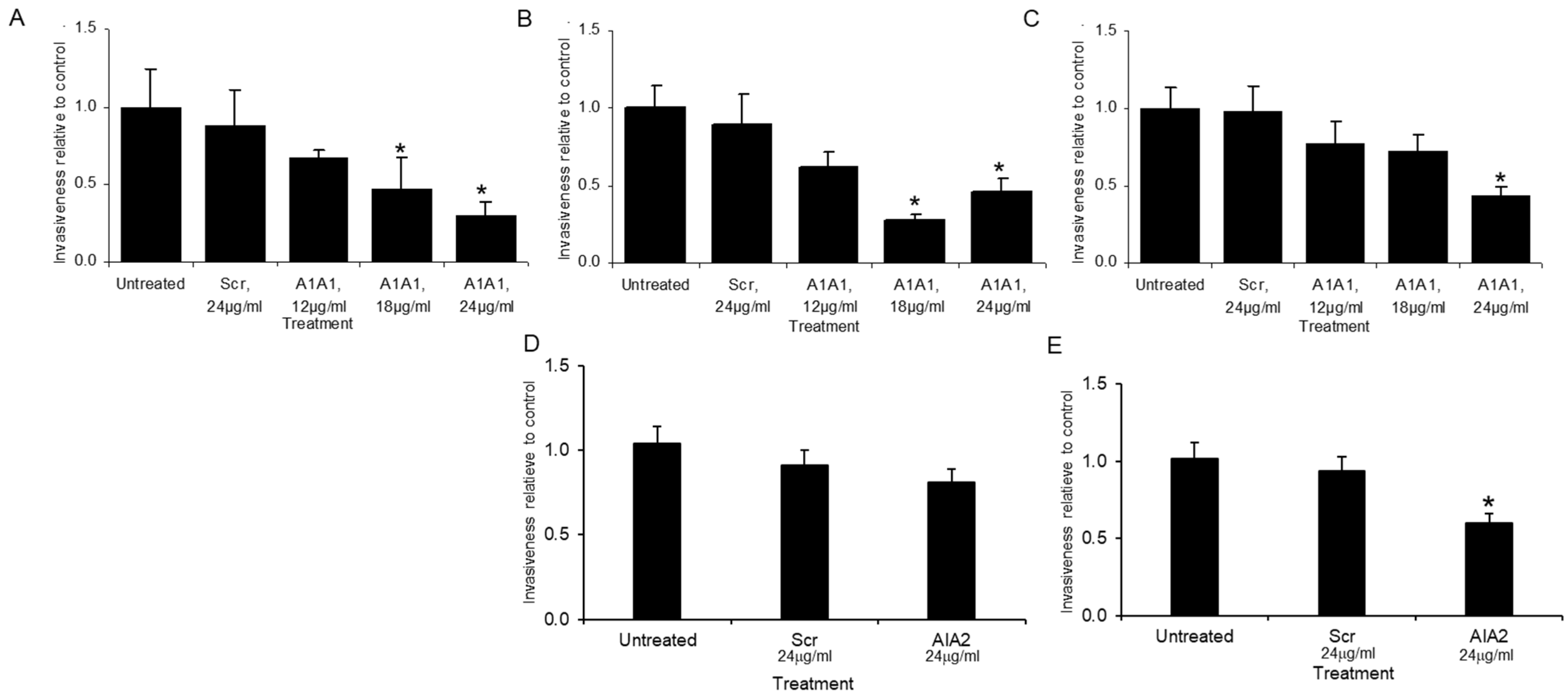
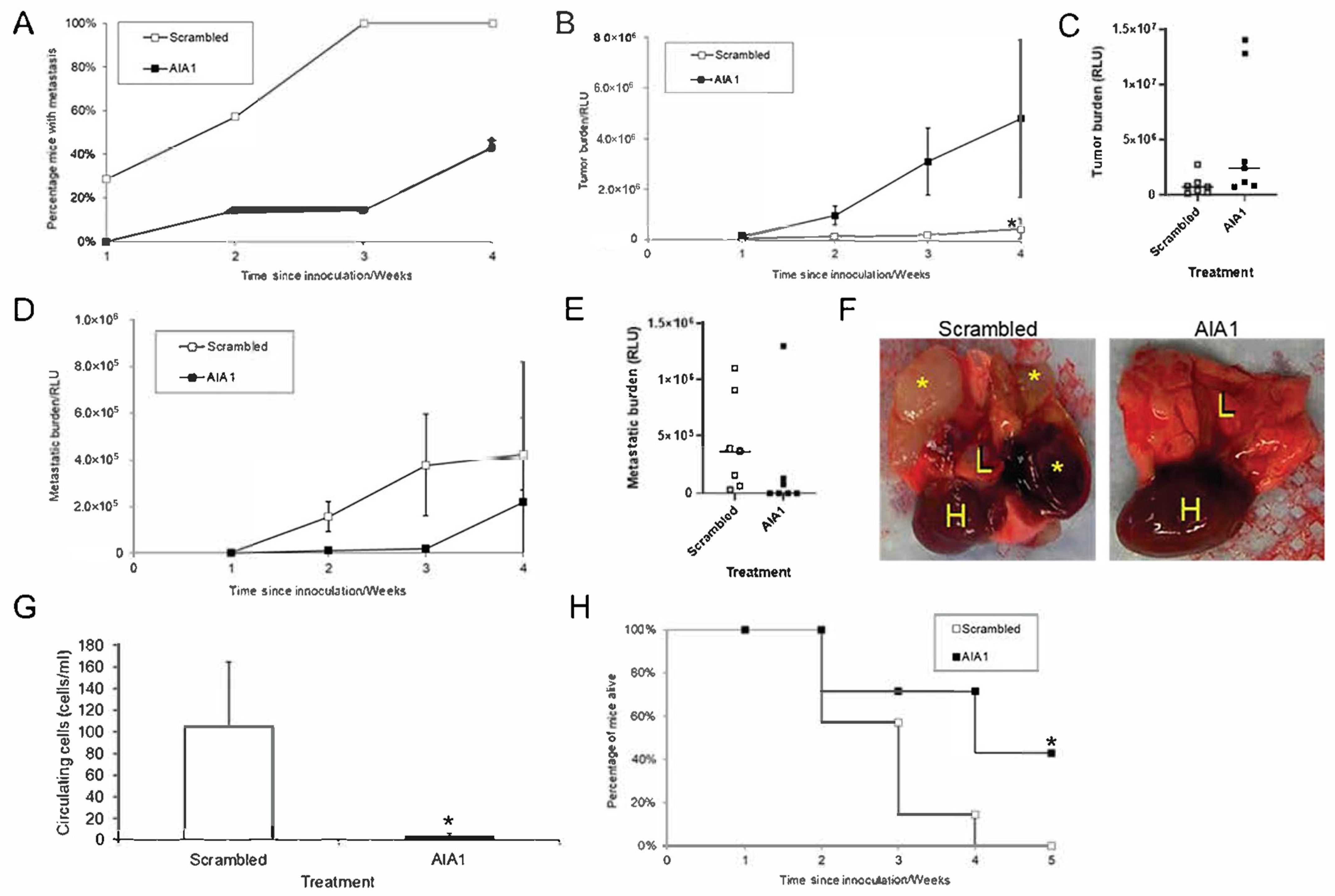
3.3. AIA1 Inhibits Metastasis In Vivo in a Murine Model
3.4. AIA1 Inhibits Development of Metastases in Osteosarcoma, a Non-Prostate Tumor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Clone | Sequence | Length | N |
|---|---|---|---|
| mod10 | GGTGCATGTTGTGTCGGCTTCATGTAGAGCGTGGGCATGC | 40 | 0 |
| mod12 | GGTGCATGTTGTGTCGGCTTCATGTAGAGCGTGGGCATGC | 40 | 0 |
| mod1 | AGGGCCTGAGGTAGTGCGCGTTG | 23 | 0 |
| mod13 | AGGGCCTGAGGTAGTGCGCGTTG | 23 | 0 |
| mod6 | TATTCGTGTCGAAGGCAGCCTCTTGCATATTTATTGGCGG | 40 | 0 |
| mod8 | GCTTATCACCAGCTGTTAGTCATATGCCGTGTGTTGGCC | 39 | 0 |
| mod3 | TAGCTGTCCTAACACGGTAATAGTACGCTTATGCTGCCCG | 40 | 0 |
| mod9 | TTCAGCGACCGCACGGT | 18 | 0 |
| mod5 | AGGTTAGGGCTGAAGCTATTCCTTAGTACCGTGGCCTCTGG | 41 | 0 |
| mod7 | GTGTACGGCTATTCCATTCTCCCTGTGGACATGTGCGGCG | 40 | 0 |
| mod2 | CAACATAATGTGGCGTATCGACGTTTTAGGTTGTGGACCG | 40 | 0 |
| mod18 | CTAGCACACTGACACTGCACGAGTGGGCTGCNCCGCTGGC | 41 | 0 |
| mod15 | GCNCNTGGNCTNAGTGCANGNTNCAGGGATGCCGCGNGCG | 40 | 7 |
| mod19 | GTTGCGTTGTGCTTGCTGTGTCGCAGAACAGGCGGGAAGG | 40 | 0 |
| mod16 | CCATTCATTAGTGTGCCTCCCCTTCTGCAATGGTTGTGGC | 40 | 0 |
| mod20 | CGACACGGTACATTGGGTTGGCTTACTTGTGAGTGGGCGG | 40 | 0 |
| mod14 | AGCGNGNTGTGCCGAGCNGCGNNGCCANGGTNTGGCGGCC | 40 | 7 |
| mod17 | CGCACANNGTGTNANCGCCATGNGGGCCTTGGNGGCGCGG | 40 | 6 |
| mod11 | CCATGTTTGATAAGTCACGGGGTCGCAAACGTAGGGGCCA | 40 | 0 |
| mod12 | GGTGCATGTTGTGTCGGCTTCATGTAGAGCGTGGGCATGC | 40 | 0 |
References
- Proske, D.; Blank, M.; Buhmann, R.; Resch, A. Aptamers-basic research, drug development, and clinical applications. Appl. Microbiol. Biotechnol. 2005, 69, 367–374. [Google Scholar] [CrossRef]
- Kim, Y.K. RNA Therapy: Current Status and Future Potential. Chonnam. Med. J. 2020, 56, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Qin, Z.; Zhu, Y.H.; He, Z.Y.; Xu, T. Current RNA-based Therapeutics in Clinical Trials. Curr. Gene Ther. 2019, 19, 172–196. [Google Scholar] [CrossRef] [PubMed]
- Pestourie, C.; Tavitian, B.; Duconge, F. Aptamers against extracellular targets for in vivo applications. Biochimie 2005, 87, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Brody, E.N.; Gold, L. Aptamers as therapeutic and diagnostic agents. J. Biotechnol. 2000, 74, 5–13. [Google Scholar] [CrossRef]
- Buglak, A.A.; Samokhvalov, A.V.; Zherdev, A.V.; Dzantiev, B.B. Methods and Applications of In Silico Aptamer Design and Modeling. Int. J. Mol. Sci. 2020, 21, 8420. [Google Scholar] [CrossRef]
- Byun, J. Recent Progress and Opportunities for Nucleic Acid Aptamers. Life 2021, 11, 193. [Google Scholar] [CrossRef]
- Yu, H.; Alkhamis, O.; Canoura, J.; Liu, Y.; Xiao, Y. Advances and Challenges in Small-Molecule DNA Aptamer Isolation, Characterization, and Sensor Development. Angew. Chem. Int. Ed. Engl. 2021, 60, 16800–16823. [Google Scholar] [CrossRef]
- Sun, S. Technology evaluation: SELEX, Gilead Sciences Inc. Curr. Opin. Mol. Ther. 2000, 2, 100–105. [Google Scholar]
- Almasi, F.; Mousavi Gargari, S.L.; Bitaraf, F.; Rasoulinejad, S. Development of a Single Stranded DNA Aptamer as a Molecular Probe for LNCap Cells Using Cell-SELEX. Avicenna J. Med. Biotechnol. 2016, 8, 104–111. [Google Scholar]
- Campos-Fernandez, E.; Alqualo, N.O.; Garcia, L.C.M.; Alves, C.C.H.; Vieira, T.D.F.A.; Moreira, D.C.; Alonso-Goulart, V. The use of aptamers in prostate cancer: A systematic review of theranostic applications. Clin. Biochem. 2021, 93, 9–25. [Google Scholar] [CrossRef]
- Cerchia, L.; Duconge, F.; Pestourie, C.; Boulay, J.; Aissouni, Y.; Gombert, K.; Tavitian, B.; de Franciscis, V.; Libri, D. Neutralizing aptamers from whole-cell SELEX inhibit the RET receptor tyrosine kinase. PLoS Biol. 2005, 3, e123. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Cui, C.; Wan, S.; Jiang, Y.; Zhang, L.; Xia, L.; Li, L.; Li, X.; Tan, W. Bioapplications of Cell-SELEX-Generated Aptamers in Cancer Diagnostics, Therapeutics, Theranostics and Biomarker Discovery: A Comprehensive Review. Cancers 2018, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.L.; Nascimento, I.C.; Santos, A.P.; Ogusuku, I.E.Y.; Lameu, C.; Mayer, G.; Ulrich, H. Aptamers: Novelty tools for cancer biology. Oncotarget 2018, 9, 26934–26953. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; He, J.; Zhong, L.; Huang, Y.; Zhao, Y. Production of aptamers by cell-SELEX and their applications in cancer biomarker identification. Discov. Med. 2020, 29, 159–167. [Google Scholar] [PubMed]
- Wu, T.T.; Sikes, R.A.; Cui, Q.; Thalmann, G.N.; Kao, C.; Murphy, C.F.; Yang, H.; Zhau, H.E.; Balian, G.; Chung, L.W. Establishing human prostate cancer cell xenografts in bone: Induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int. J. Cancer 1998, 77, 887–894. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). J. Urol. 1979, 17, 16–23. [Google Scholar]
- Fu, Z.; Smith, P.C.; Zhang, L.; Rubin, M.A.; Dunn, R.L.; Yao, Z.; Keller, E.T. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J. Natl. Cancer Inst. 2003, 95, 878–889. [Google Scholar] [CrossRef]
- Srisawat, C.; Engelke, D.R. Streptavidin aptamers: Affinity tags for the study of RNAs and ribonucleoproteins. RNA 2001, 7, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Hamada, M.; Asai, K.; Mituyama, T. CENTROIDFOLD: A web server for RNA secondary structure prediction. Nucleic Acids Res. 2009, 37, W277–W280. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.; Kalikin, L.M.; Mattos, A.C.; Keller, E.T.; Allen, M.J.; Pienta, K.J.; McCauley, L.K. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology 2005, 146, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Dai, J.; Qi, Y.; Lin, D.L.; Smith, P.; Strayhorn, C.; Mizokami, A.; Fu, Z.; Westman, J.; Keller, E.T. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J. Clin. Investig. 2001, 107, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Rehemtulla, A.; Stegman, L.D.; Cardozo, S.J.; Gupta, S.; Hall, D.E.; Contag, C.H.; Ross, B.D. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2000, 2, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Havens, A.M.; Pedersen, E.A.; Shiozawa, Y.; Ying, C.; Jung, Y.; Sun, Y.; Neeley, C.; Wang, J.; Mehra, R.; Keller, E.T.; et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia 2008, 10, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.M.; Go, M.J.; Lee, J.; Na, D.; Yoo, S.M. Recent Advances in Micro/Nanomaterial-Based Aptamer Selection Strategies. Molecules 2021, 26, 5187. [Google Scholar] [CrossRef]
- Zhu, J.; Liang, C.; Hua, Y.; Miao, C.; Zhang, J.; Xu, A.; Zhao, K.; Liu, S.; Tian, Y.; Dong, H.; et al. The metastasis suppressor CD82/KAI1 regulates cell migration and invasion via inhibiting TGF-beta 1/Smad signaling in renal cell carcinoma. Oncotarget 2017, 8, 51559–51568. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, Y.; Cao, L.; Zhang, J.; Wang, Y.; Xu, M. Metastasis suppressor protein 1 regulated by PTEN suppresses invasion, migration, and EMT of gastric carcinoma by inactivating PI3K/AKT signaling. J. Cell Biochem. 2019, 120, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Pal, M. Tumor metastasis suppressor functions of Ets transcription factor through integrin beta3-mediated signaling pathway. J. Cell Physiol. 2019, 234, 20266–20274. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.J.; Lee, M.S.; Jin, Y.J.; Jeoung, D.; Kim, Y.M.; Lee, H. The metastasis suppressor CD82/KAI1 inhibits fibronectin adhesion-induced epithelial-to-mesenchymal transition in prostate cancer cells by repressing the associated integrin signaling. Oncotarget 2017, 8, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hu, L.; Zhang, B.T.; Lu, A.; Wang, Y.; Yu, Y.; Zhang, G. Artificial Intelligence in Aptamer-Target Binding Prediction. Int. J. Mol. Sci. 2021, 22, 3605. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Lei, Y.; Ge, J.; He, X.; Cui, W.; Ye, X.; Liu, J.; Wang, K. A Simple, pH-Activatable Fluorescent Aptamer Probe with Ultralow Background for Bispecific Tumor Imaging. Anal. Chem. 2019, 91, 9154–9160. [Google Scholar] [CrossRef]
- Zhang, G.X.; Liu, Y.L.; Yang, M.; Huang, W.S.; Xu, J.H. An aptamer-based, fluorescent and radionuclide dual-modality probe. Biochimie 2020, 171–172, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Medley, C.D.; Sefah, K.; Shangguan, D.; Tang, Z.; Meng, L.; Smith, J.E.; Tan, W. Molecular recognition of small-cell lung cancer cells using aptamers. ChemMedChem 2008, 3, 991–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi, L.; Bagni, O.; Nervi, C. Aptamer-based technology for radionuclide targeted imaging and therapy: A promising weapon against cancer. Expert Rev. Med. Devices 2020, 17, 751–758. [Google Scholar] [CrossRef]
- Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, Y.; Tang, F. Nucleic Acid Aptamer: A Novel Potential Diagnostic and Therapeutic Tool for Leukemia. Onco Targets Ther. 2019, 12, 10597–10613. [Google Scholar] [CrossRef] [Green Version]
- Yazdian-Robati, R.; Bayat, P.; Oroojalian, F.; Zargari, M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020, 155, 1420–1431. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelley, G.; Dai, J.; Keller, J.M.; Keller, E.T. Pheno-SELEX: Engineering Anti-Metastatic Aptamers through Targeting the Invasive Phenotype Using Systemic Evolution of Ligands by Exponential Enrichment. Bioengineering 2021, 8, 212. https://doi.org/10.3390/bioengineering8120212
Shelley G, Dai J, Keller JM, Keller ET. Pheno-SELEX: Engineering Anti-Metastatic Aptamers through Targeting the Invasive Phenotype Using Systemic Evolution of Ligands by Exponential Enrichment. Bioengineering. 2021; 8(12):212. https://doi.org/10.3390/bioengineering8120212
Chicago/Turabian StyleShelley, Greg, Jinlu Dai, Jill M. Keller, and Evan T. Keller. 2021. "Pheno-SELEX: Engineering Anti-Metastatic Aptamers through Targeting the Invasive Phenotype Using Systemic Evolution of Ligands by Exponential Enrichment" Bioengineering 8, no. 12: 212. https://doi.org/10.3390/bioengineering8120212
APA StyleShelley, G., Dai, J., Keller, J. M., & Keller, E. T. (2021). Pheno-SELEX: Engineering Anti-Metastatic Aptamers through Targeting the Invasive Phenotype Using Systemic Evolution of Ligands by Exponential Enrichment. Bioengineering, 8(12), 212. https://doi.org/10.3390/bioengineering8120212






